Abstract
Non-native species cause several impacts on freshwater biodiversity, but studies focusing on the Neotropical stream’s biota are still incipient. We used a data set of 586 headwater stream’s fish assemblages from the Brazilian Upper Paraná ecoregion to test whether the presence/absence of non-native species affect: species richness (S), functional diversity (MPD) and taxonomic diversity (Δ+). We compared diversity patterns of fish assemblages formed only by native species against those of assemblages formed by native and non-native species (Scenario 1); then, we removed non-native species from their original assemblages and recalculated their diversity values to compare them with those of fish assemblages formed only by native species again (Scenario 2). We also investigated: (1) whether non-native’s fish assemblages are associated with land use, topographic and watercourse connectivity variables; (2) fish ecological traits-environment relationship. In Scenario 1, S was higher in assemblages with the presence of non-native species, while in Scenario 2, both S and MPD were higher in assemblages where non-native species were removed. Non-native species were not directly related to land use, topographic or connectivity variables and most of them had a similar response to the environment when compared with native species. Findings show that non-native fish species are related to high-rich assemblages in headwaters, and they increase species richness and the functional redundancy of assemblages, decreasing functional diversity. Moreover, in most cases, native and non-native species seem to respond similarly to the environmental influence on their occurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human activities are promoting the degradation of natural ecosystems so quickly that a sixth process of mass extinction may be in action (Ceballos et al. 2015, 2020). Freshwater ecosystems are among the most threatened habitats, and fish assemblages are subject to high extinction rates in the last decades because of climatic changes, loss and fragmentation of habitats, overexploitation of natural resources, changes in the water flux, and the introduction of non-native species (Hermoso et al. 2009; Dudgeon et al. 2006; Dias et al. 2017; Reid et al. 2019; Garcia et al. 2021; Pelicice et al. 2021; Su et al. 2021).
The introduction of non-native fish species in freshwater systems is potentialized by changes at local and global scales, and it promotes several negative impacts on species, populations, communities, and ecosystems (Vitule 2009; Jeschke et al. 2014; Doria et al. 2021; Latini et al. 2021). This introduction is often associated to human activities that facilitate their entrance in the ecosystems, such as: aquiculture, sport fishing, fishkeeping, fish farming, biocontrol programs, and dams’ construction (Britton and Orsi 2012; Frehse et al. 2016; Liew et al. 2016; Latini et al. 2021; Muniz et al. 2021; Pelicice et al. 2022). Once introduced, non-native fish species compete with or predate native fishes, hybridize with other species, and disseminate several pathogens, promoting changes in niche dimensions and species loss via extinction (Vitule 2009; Vitule and Prodocimo 2012; Enders et al. 2020). Considering an ecological context, non-native fish species may promote the loss of important ecological traits/functions, driving communities to functional homogenization (Olden 2006; Rocha et al. 2011; Daga et al. 2015; Toussaint et al. 2018), or causing changes in the functional patterns of communities (Blanchet et al. 2010; Toussaint et al. 2018). In the first case, the presence of non-native fish species can cause the erosion of provisioning (fisheries), regulating (seed dispersal, decomposition), supporting (nutrient cycling, ecosystem engineering), and cultural (recreation, tourism, education) services (Pelicice et al. 2022). In the latter case, the inclusion of non-native species in fish assemblages changes the functional space in direction to species with larger and less elongated bodies (Blanchet et al. 2010; Toussaint et al. 2018). Despite that, measuring the effects of non-native species on diversity patterns of native assemblages remains a great challenge (Gurevitch and Padilla 2004), especially for fishes of megadiverse tropical regions where studies are still incipient (Dudgeon et al. 2006; Frehse et al. 2016). Ironically, despite tropical regions encompass a great number of world’s freshwater fish species, they present an elevated number of non-native species (Agostinho et al. 2007; Alb Magalhães and Jacobi 2013; Frehse et al. 2016; Nelson et al. 2016; Reis et al. 2016; Toussaint et al. 2016; Vitule et al. 2017; Garcia et al. 2021).
Fish assemblages can be described by different components of diversity, such as species richness, taxonomic diversity, and functional diversity. Species richness is measured as the number of species found in a local community, whereas taxonomic diversity considers taxonomic relationships among species within assemblages (Warwick and Clarke 1998), and functional diversity quantifies the variation of species functional traits within assemblages (Díaz and Cabido 2001). Intuitively, one may expect that a decrease in fish species richness caused by the introduction of non-native species may be followed by a reduction of taxa and sets of functional traits causing biotic homogenization and a diversity decrease (Olden 2006; Vitule 2009). However, the relationship among different components of diversity at local scales is not always clear and constant in freshwater habitats (Carvalho and Tejerina-Garro 2015a), and even high-rich communities may be formed by species that are taxonomically and functionally similar (Casatti et al. 2015). Moreover, in poor sites and highly altered freshwater habitats even non-native fish species may increase species richness and present relevant ecological roles in maintaining community and ecosystem process in freshwater habitats (Matsuzaki et al. 2013; Reid et al. 2019; Rosa et al. 2021). Thusly, studies that explore the role of non-native species on fish assemblage’s structure and diversity are relevant to improve our understanding about the spatial patterns of fish assemblage’s diversity and the consequences of fish species introductions on them.
In headwater streams, the variability and unpredictability of environmental factors may lead to fish assemblages composed by (i) species with unique identities and ecological traits (Altermatt 2013; Borges et al. 2020; Carvalho et al. 2020), or (ii) redundant species regarding their phylogenetic heritage and ecological functions (Carvalho and Tejerina-Garro 2015b). In the first case, the introduction of non-native fish species could promote biotic homogenization through the loss of unique species and sets of ecological traits (Altermatt 2013; Borges et al. 2020; Garcia et al. 2021), even when only one species is lost (Olden et al. 2006; Vitule 2009; Naeem et al. 2012). In the second case, the loss of one or more species could not lead to significant reductions in the diversity of taxa and ecological traits since species are phylogenetically closely related and ecologically similar (Carvalho and Tejerina-Garro 2015b). For both cases, another possibility is the occurrence of changes in the taxonomic and functional space within assemblages in different spatial/temporal scales without extinction events and according to the characteristics of introduced species. For example, introduced species may increase the redundancy within assemblages if they have similar traits regarding native species, or promote biotic differentiation if they have distinct ecological traits (Vitule et al. 2012; Pool and Olden 2012; Villéger et al. 2014; Daga et al. 2020). Given the relevance of headwater streams for the conservation of freshwater ecosystems (Moulton 2008; Altermatt 2013; Carvalho et al. 2020), it is important to investigate how the diversity patterns of headwater stream’s fish assemblages are affected by the presence of non-native species.
In this study, we investigated how the spatial patterns of fish assemblage’s diversity in headwater streams are affected by non-native fish species. First, we hypothesized that fish assemblages including the presence of non-native fish species have lower values of species richness, taxonomic diversity, and functional diversity than assemblages formed solely by native species. We expect that the presence of non-native fish species is related to assemblages that passed through the homogenization process via extinction of local native species (species richness reduction), loss of different taxa (taxonomic diversity reduction) and sets of ecological traits (functional diversity reduction), or simply by changes in taxonomic and functional space increasing similarity among species. To test the hypotheses, we compared patterns of fish assemblage’s diversity encompassing solely native species against the patterns of fish assemblages encompassing native and non-native species (hereafter mentioned as Scenario 1). After that, we investigated the relative importance of non-native species for diversity patterns by simulating their removal from fish assemblages. To that, we removed non-native species from fish assemblages and compared the new diversity patterns of these assemblages against the diversity patterns of assemblages composed solely by native species (hereafter mentioned as Scenario 2).
Additionally, we investigated how fish assemblages (considering the presence or absence of non-native fish species) are associated with land use, topographic (altitude and slope) and watercourse connectivity (betweenness centrality) variables. In this case, we expect that: (i) fish assemblages associated with highly impacted areas are prone to fish introductions; and (ii) fish assemblages located in watercourses at lower altitude and slope and central regions of the hydrographic basin will facilitate the dispersal and occurrence of non-native fish species. Finally, we tested the relationship between fish ecological traits and environmental variables to investigate whether: (i) native and non-native fish species have similar functional traits; and (ii) native and non-native fish species are similarly related to the environment.
We used a dataset of 586 headwater streams distributed along the Upper Paraná ecoregion in Brazil (Borges et al. 2020). This ecoregion encompasses a high fish diversity (± 310–340 species; Langeani et al. 2007; www.feow.org), and it is influenced by different human activities that facilitate fish introduction (Agostinho et al. 2007, 2008; Garcia et al. 2018; Borges et al. 2020), being a good model to test the influence of non-native species on the native ichthyofauna (Garcia et al. 2021).
Material and methods
Study area and sampling sites
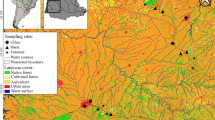
The study was conducted in the Brazilian section of the Upper Paraná freshwater ecoregion (sensu Abell et al. 2008; FEOW 2021), a region that encompass five Brazilian states (Fig. 1). This region is under the influence of urban areas with high population density and industrial activities that jeopardize freshwater ecosystems and facilitate the introduction of non-native fish species (Garcia et al. 2018; Santana et al. 2021).
Initially, we accessed freshwater fish species occurrence for 1,136 headwater stream’s assemblages. This dataset was revised and standardized according to the criteria used by Borges et al. (2020): (i) maintenance of streams occurring inside the Upper Paraná freshwater ecoregion; (ii) removal of streams with spatial coordinates suspected of error (not georeferenced or occurring distant from watercourses); (iii) maintenance of streams from first, second, and third orders (Strahler 1957); (iv) maintenance of streams with only one sampling collection and with information for species composition; (v) removal of streams with sampling collections in stretches lower than 50 m; and (vi) the maintenance of streams sampled with seine-nets (Carvalho et al. 2017), or electrofishing (Mazzoni et al. 2000; Peressin et al. 2018) methods. After that, the final dataset was composed by 586 headwater streams (Fig. 1), and the final list of fish species was evaluated by fish experts (Dr. Francisco Langeani, and Dr. Fernando Carvalho) to confirm taxonomic species identification, including non-native fish species.
Fish data and diversity measures
We collected, for each species, data related to its genus, family, and order from the Eschmeyer’s Catalog of Fishes (Fricke et al. 2023) to calculate fish assemblages’ taxonomic diversity represented by the taxonomic distinctiveness index (Δ+). It represents the average distance among all pair of species present in the community/assemblage (Warwick and Clarke 1998). Values of Δ+ indicate a high (closer to zero) or low (closer to 100) taxonomic relationship among species in the assemblage (García-Martínez et al. 2015), and, in these cases, the index indicates a set of species with high or low taxonomic diversity, respectively.
To calculate fish assemblages’ functional diversity, we used 15 morphological/functional traits related to five ecomorphological characteristics (body size, head, mouth, eyes, and fins) which are linked to habitat use and trophic ecology and describe different relationships related to the structure and functioning of communities and ecosystems (Table 1). Functional data for each functional trait was obtained from photographic images (one to five fish specimens of each species) using the software ImageJ (Leitão et al. 2018; Toussaint et al. 2016). Fish photographic images were deposited in the scientific collections of the Universidade Estadual Paulista "Júlio de Mesquita Filho" (DZSJRP). We measured functional diversity using the Mean Pairwise Distance (MPD) that considers the average distances among all pair of species present in the community (Webb et al. 2002). Given that this index was initially constructed to measure phylogenetic diversity, we took the following steps to its calculation: (i) construction of a functional distance matrix using a modification of the Gower’s distance (Pavoine et al. 2009); (ii) conversion of this functional distance matrix into a functional dendrogram using the Unweighted Pair Group Method with Arithmetic Means (UPGMA); (iii) transformation of the functional dendrogram into an object with phylogenetic properties (phylogeny); (iv) MPD calculation (Pavoine and Bonsall 2010). The use of a dendrogram to calculate the MPD index is possible because both dendrogram and phylogeny have similar properties (Pavoine and Bonsall 2010). It was not possible to calculate Δ+ and MPD for fish assemblages with a single species; therefore, headwater streams with a single species were used only to compose the species pool to calculate fish assemblage’s diversity but they were excluded from statistical analyses (see below). Diversity measures were calculated in the software R (R Development Core Team 2020) using the packages: vegan (Oksanen et al. 2022), picante (Kembel et al. 2010), and ade4 (Dray and Dufour 2007).
Land use, topographic and watercourse connectivity variables
To obtain land use variables, we delimited a buffer of 1 km for each sampling site related to each fish assemblage. Then, using the MapBiomas database (http://mapbiomas.org) of 2016, we extracted the percentage of anthropogenic land use (the sum of pasture, annual crops, semi-perennial crops, agriculture or pasture, and non-vegetated areas) and natural formation (represented by the sum of natural forest formation and non-forest natural humid areas and fields). Data for topography (altitude and slope) were obtained using earthenv database (http://www.earthenv.org/topography) with cells of 1 km of resolution. Finally, the betweenness centrality of a fish assemblage was measured as the number of connections with other fish assemblages, and it was calculated using the pairwise distance between streams via watercourse.
Environmental data
We used 19 bioclimatic variables related to temperature and precipitation (Table 2) as environmental variables. These were obtained from CHELSA (Climatologies at High Resolution for the Earth’s Land Surface Areas) database (http://chelsa-climate.org/) that provides information for grid cells with 1 km of resolution. According to Frederico et al. (2014), macroscale variables can reflect local conditions of aquatic environments and function as surrogates for local variables.
Statistical analyses
To perform the comparisons of the Scenario 1, we divided the dataset in two groups: fish assemblages composed solely by native species and fish assemblages encompassing both native and non-native fish species. The values of species richness, taxonomic diversity and functional diversity of each group were tested to evaluate the existence of spatial autocorrelation effects, homogeneity of variance, and normal distribution. Spatial autocorrelation was tested with the method of the semivariogram (Cressie 1993), homogeneity of variance with the Levene test (Levene 1960), and normal distribution with the Shapiro–Wilk test (Shapiro and Wilk 1965). These tests indicated the existence of a spatial autocorrelation structure and heterogeneity of variance (see the results in the Supplementary File), then we choose to use the Generalized Least Squares method for all diversity metrics (GLS; Menke 2015), instead of a traditional and univariate method (t-test), to compare the values of species richness, taxonomic diversity, and functional diversity between both groups. Using the spatial coordinates of sampled streams, we inserted in the GLS analysis different structures of spatial autocorrelation to estimate the parameters of the model and find the spatial autocorrelation model that better describes the observed structured. The GLS compared the diversity values for both groups considering the Scenario 1. To perform the comparisons contemplating the Scenario 2, we removed non-native species from their original assemblages and recalculated the values of species richness, functional diversity, and taxonomic diversity for all fish assemblages. Then, these diversity values of assemblages where non-native species were removed were compared with the values found for assemblages with only native species to test how non-native fish species affect the diversity patterns of fish assemblages. Analyses for the second scenario followed the same procedures described for the first one. Analytical processes were performed in the software R (R Development Core Team 2020) using the packages: pgirmess (Giraudoux 2018), nlme (Pinheiro et al. 2020) e AICcmodavg (Mazerolle 2020).
To investigate the association of fish assemblages with land use, topographic and watercourse connectivity variables, we performed a Principal Coordinate Analysis (PCoA) considering the presence/absence of native and non-native species. If the PCoA displayed different associations of the variables with fish assemblages composed solely by native species and those with native and non-native species, we will perform a classification tree (De’ath and Fabricius 2000) to explore specific relationships between explanatory variables (land use, topographic, and watercourse connectivity) and response variable (presence or absence of non-native species).
We tested the relationship between fish species functional traits and environmental variables with the RLQ method, a multivariate analysis-based on the ordination of three distinct data matrices (Dolédec et al. 1996): environmental matrix (R, sampled streams vs. environmental variables), species matrix (L, sampled streams vs. species occurrence), and functional traits matrix (Q, species vs. functional traits). The analysis submits both matrices R and Q to a Principal Components Analysis (PCA), and their results were used as restrictions in a Correspondence Analysis with Matrix L (Dray et al. 2003). Therefore, matrix L works as a link between R and Q matrices. The significance of the Co-inertia provided by the RLQ analysis was tested with the permutation of matrices and the combination of two models that test the relationship between species distribution and environmental configuration among sites (model 2, permutation of rows of the matrix L), and the relationship between species distribution and functional attributes (model 4, permutation of columns of the matrix L; ter Braak et al. 2012). Permutations were performed with 999 iterations and using the package ade4 (Thioulouse et al. 2018) available in the software R.
Results
We found 159 fish species distributed among 31 families and 7 orders in the 586 headwater streams (see Table S1 in the Supplementary File), and 27 of the 159 fish species were identified as non-native of the upper Paraná River basin (Table 3). Non-native fish species occurred in 285 of the 586 streams, representing 48.63% of the fish assemblages studied. For the Scenario 1, species richness ranged from 1 to 23 species, Δ+ between 15.16 and 100.00, and MPD between 0.03 and 0.41 in fish assemblages composed by native species, whereas species richness ranged from 1 to 30 species, Δ+ between 50.08 and 100.00, and MPD between 0.04 and 0.48 in fish assemblages including native and non-native fish species. For the Scenario 2, when non-native species were removed and diversity values were recalculated, species richness ranged from 1 to 23 species, Δ+ between 16.82 and 100.00, and MPD between 0.06 and 0.45 in fish assemblages formed by native species, whereas species richness ranged from 1 to 27, Δ+ between 50.93 and 100.00, and MPD between 0.04 and 0.46 in fish assemblages where non-native species were removed.
For the first scenario, GLS models demonstrated that species richness of fish assemblages with native and non-native species was higher than species richness of fish assemblages composed solely by native species (Table 4, Scenario 1), while no differences were observed for Δ+ and MPD. For the second scenario, when non-native species were removed from fish assemblages, species richness of these assemblages continued to be higher than species richness of fish assemblages formed solely by native species, but the effect size decreased (Table 4, Scenario 2). In this second scenario, we also detected that MPD of fish assemblages composed solely by native species was lower than the MPD of fish assemblages where non-native species were removed. Despite the small effect size (Table 4, Scenario 2), this result suggests that the presence of non-natives species changes the functional space increasing the functional similarity among fishes within assemblages in headwater streams, given that MPD increased when non-native species were removed. For taxonomic diversity, there was no significant difference between the two groups in both scenarios (Table 4).
The PCoA result indicated that the first axis explained 51.00% of the variation among fish assemblages and it was mainly related to land use (natural formation and anthropogenic use) and topographic (altitude and slope) variables (Fig. 2). The second axis explained 21.00% of the variance and it was mostly related to betweenness centrality (Fig. 2). Despite that, it was not possible to identify any clear trend between the set of variables considered and fish assemblages composed by native species or by native and non-native species, i.e., composition was not determined by land use, topographic and watercourse connectivity variables.
Fish assemblages’ ordination according to landscape, topographic and watercourse connectivity variables. White dots—fish assemblages composed by native species, black triangles—fish assemblages formed by native and non-native species, Alt—Altitude, Bt—Betweenness centrality, LU—land use, RE—remaining vegetation
RLQ results indicated that the distribution of native and non-native fish species responds to the variation of the environment among streams (model 2, p = 0.001), but this distribution is not related to their functional traits (model 4, p = 0.442). Regarding the relationship between species distribution and environment, the cumulative projected inertia of the first two axes of the RLQ was 98.55% (axis 1 = 87.94%, and axis 2 = 10.61%), and they indicated that native and non-native species respond to the environment in a similar way (Fig. 3A, B). Despite the absence of relationship between functional traits and environmental variables and the similar response of native and non-native fish species to environment, it is important to notice that some of the non-native fish species (Clarias gariepinus—Clagar, Coptodon rendalli—Copren, Cyprinus carpio —Cypcar, Cichla kelberi—Cickel, and Oreochromis niloticus—Orenil) seem to present a different distribution of other native and non-native species and have notably different attributes than others such as the body size (Fig. 3A, C).
Discussion
The introduction of non-native fish species may alter local diversity patterns of freshwater assemblages through biotic homogenization process via species extinction (Pool and Olden 2012; Daga et al. 2015; Garcia et al. 2021), or simply changing taxonomic and functional characteristics of local assemblages (Blanchet et al. 2010; Toussaint et al. 2018; Daga et al. 2020). Our central hypothesis was constructed on the expectation that fish assemblages of headwater streams with the presence of non-native species would have lower values of species richness, Δ+ and MPD than fish assemblages formed only by native species, because non-native species can promote species extinction and/or changes in the taxonomic and functional space within assemblages increasing the redundancy among species. Contrary, our findings revealed that species richness was higher in headwater streams formed by fish assemblages with native and non-native species than in headwater streams with assemblages formed solely by native species (results of the Scenario 1). Considering that the first group of assemblages continued to present higher levels of species richness, even when non-native species were removed from them, it seems that non-native species are associated with local fish assemblages that naturally have more species (results of the Scenario 2). In the first scenario, we also found that the MPD of fish assemblages with native and non-native species was not lower (or higher) than the observed MPD of fish assemblages formed solely by native species; however, when non-native species were removed, this group of assemblages had higher values of MPD than fish assemblages composed by native species (Scenario 2). It suggests that non-native species are at least capable of changing the functional space of assemblages increasing the similarity among species within assemblages and decreasing local functional diversity. We did not find any clear evidence that fish assemblages with non-native species are more associated to central sites with low altitude and slope, and high human impact by land use.
Headwater streams are often structured by environmental factors that are highly variable through space and time. Then, environmental variables act as selective filters to species (Poff 1997) and limit their number in local fish assemblages (Jackson et al. 2001; Costa et al. 2018; Rodrigues-Filho et al. 2018). Therefore, this scenario contributes to the formation of fish assemblages with a low number of species, where the addition of a new species capable of surviving in new environmental conditions may lead to an increase in species richness, even if it is a non-native fish species (Weyl et al. 2016). The increase of fish species richness because of the introduction process in low-diversity assemblages was already observed for higher spatial scales at temperate regions (Matsuzaki et al. 2013). Our results indicate that a similar phenomenon may occur at a local scale. On the other hand, this showed that the presence of non-native species in fish assemblages of headwater streams seems to increase the functional redundancy among species and decrease the local MPD. Despite the small effect size found, it is important to notice that fish assemblages of headwater streams have a natural tendency to functional clustering (Carvalho and Tejerina-Garro 2015b); therefore, even a small difference can be highly significant in this kind of freshwater habitat. This is reinforced by the results of the RLQ showing that most non-native fish species have similar functional attributes to native species and demonstrate a similar response to environment. In this context, the view that non-native fish species increase local species richness of headwater stream’s assemblages must be considered with caution since their presence also changes local functional diversity (decreasing the MPD in this case), and negative impacts of non-native fish species on freshwater populations, communities and ecosystems are extensively described by several studies worldwide (Olden 2006; Vitule 2009; Vitule and Prodocimo 2012; Jeschke et al. 2014; Dias et al. 2017; Pereira et al. 2017; Toussaint et al. 2018; Pelicice et al. 2022).
Environment has been an important factor explaining the distribution patterns of freshwater fish species and their functional traits in headwater streams (Carvalho and Tejerina-Garro 2015b, 2018; Ribeiro et al. 2016; Rodrigues-Filho et al. 2017). Our results point out the existence of a significant relationship between species distribution and the environmental spatial structure, but species functional traits were not necessarily preponderant to determine species distribution among streams. Despite macroscale environmental variables are considered as good substitutes of local environmental variables (Frederico et al. 2014), other studies have demonstrated that instream habitat features have explain more variability in fish assemblages than riparian, catchment, or natural covariates (Junqueira et al. 2016; Leal et al. 2018). Therefore, it is possible that their use is more efficient to detect the variation related to distribution/environment, and finer relationships such as those between traits and environment should be better detected using local environmental variables. Still, it is important to notice that Teresa and Casatti (2017) found that taxonomic and functional diversity of stream fish assemblages are weakly related to the environment. These authors suggest that the history of degradation of the upper Paraná River region possibly reduces the pool of species and the influence of the environment. Future studies combining the effects of land used and the addition of new traits and environmental variables could provide more powerful models to explain these relationships with the non-native fauna.
Despite functional traits were not directly related to species distribution, it is important to notice that a group of non-native fish species (Clarias gariepinus, Coptodon rendalli, Cyprinus carpio, Cichla kelberi, and Oreochromis niloticus) stands out. Clarias gariepinus (African catfish), Cyprinus carpio (common carp), Oreochromis niloticus and Coptodon rendalli (tilapias) are native from Africa, they generally have larger bodies and are known as voracious predators and competitors (Pelicice and Agostinho 2008; Weyl et al. 2016; Casimiro et al. 2018; Frota et al. 2019; Jorissen et al. 2020). Besides, these species can support hostile conditions as those found in low quality headwater streams (see the revision about Clarias gariepinus in Weyl et al. 2016, and studies with tilapias such as Vitule et al. 2009; Araújo et al. 2009; and Padial et al. 2017). This context could explain why these species are functionally distinguished from other native and non-native fish species. Other non-native species, such as Poecilia reticulata and Knodus moenkhausii, seem to present similar traits when compared to native species from the upper Paraná River basin.
The Upper Paraná Ecoregion has a history of intense degradation by human activities (Garcia et al. 2018) that threatens the habitat integrity in these headwater streams and seems to favor the formation of poor-rich fish assemblages, where non-native species introduction may increase the number of species. Conversely, our study demonstrated that the presence of non-native species increases the functional redundancy of headwater stream’s fish assemblages and causes the decrease of functional diversity (MPD in this case). For Brazil, particularly for the Upper Paraná ecoregion, the development of studies focusing on (i) the identity and distribution of non-native species, (ii) factors promoting species introduction, (iii) stages of species introduction, and (iv) the description of native and non-native species ecology is essential to a better understanding of the impacts of species introduction (Garcia et al. 2021). Concomitantly, developing environmental policies to avoid and monitoring species introduction, and initiatives to educate and inform human populations are a central challenge to preserve biodiversity.
Data availability
All data used in the development of this study are available upon reasonable requests to corresponding author or FBT.
Code availability
Analyses were performed with R software (version 4.1.1) and all packages and codes are available in R software library. Scripts used to perform analyses are available upon requests from the corresponding author.
References
Abell R, Thieme ML, Revenga C et al (2008) Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience 58:403–414. https://doi.org/10.1641/B580507
Agostinho AA, Pelicice FM, Petry AC, Gomes LC, Júlio Fr HF (2007) Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquat Ecosyst Health 10(2):174–186. https://doi.org/10.1080/14634980701341719
Agostinho AA, Pelicice FM, Gomes LC (2008) Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol 68:1119–1132. https://doi.org/10.1590/S1519-69842008000500019
Alb Magalhães, Jacobi CM (2013) Ornamental exotic fish introduced into Atlantic Forest water bodies, Brazil. Neotrop Biol Conserv 3:73–77
Altermatt F (2013) Diversity in riverine metacommunities: a network perspective. Aquat Ecol 47:365–377. https://doi.org/10.1007/s10452-013-9450-3
Araújo FG, Pinto BCT, Teixeira TP (2009) Longitudinal patterns of fish assemblages in a large tropical river in southeastern Brazil: evaluating environmental influences and some concepts in river ecology. Hydrobiologia 618:89–107. https://doi.org/10.1007/s10750-008-9551-5
Blanchet S, Grenouillet G, Beauchard O et al (2010) Non-native species disrupt the worldwide patterns of freshwater fish body size: implications for Bergmann’s rule. Ecol Lett 13:421–431. https://doi.org/10.1111/j.1461-0248.2009.01432.x
Borges PP, Dias MS, Carvalho FR et al (2020) Stream fish metacommunity organisation across a Neotropical ecoregion: the role of environment, anthropogenic impact and dispersal-based processes. PLoS ONE 15(5):e0233733. https://doi.org/10.1371/journal.pone.0233733
Britton JR, Orsi ML (2012) Non-native fish in aquaculture and sport fishing in Brazil: economic benefits versus risks to fish diversity in the upper River Parana Basin. Rev Fish Biol Fish 22:555–565. https://doi.org/10.1007/s11160-012-9254-x
Carvalho RA, Tejerina-Garro FL (2015a) Relationships between taxonomic and functional components of diversity: implications for conservation of tropical freshwater fishes. Freshw Biol 60:1854–1862. https://doi.org/10.1111/fwb.12616
Carvalho RA, Tejerina-Garro FL (2015b) Environmental and spatial processes: What controls the functional structure of fish assemblages in tropical rivers and headwater streams? Ecol Freshw Fish 24:317–328. https://doi.org/10.1111/eff.12152
Carvalho RA, Tejerina-Garro FL (2018) Headwater–river gradient: trait-based approaches show functional dissimilarities among tropical fish assemblages. Mar Freshw Res 69:574–584. https://doi.org/10.1071/MF17149
Carvalho DR, Leal CG, Junqueira NT et al (2017) A fish-based multimetric index for Brazilian savanna streams. Ecol Indic 77:386–396. https://doi.org/10.1016/j.ecolind.2017.02.032
Carvalho RA, Teresa FB, Tejerina-Garro FL (2020) The effect of riverine networks on fish β-diversity patterns in a Neotropical system. Hydrobiologia 848:515–529. https://doi.org/10.1007/s10750-020-04459-9
Casatti L, Teresa FB, Zeni JO, Ribeiro MD, Brejão GL, Ceneviva-Bastos M (2015) More of the same: high functional redundancy in stream fish assemblages from tropical agroecosystems. Environ Manag 55:1300–1314. https://doi.org/10.1007/s00267-015-0461-9
Casimiro et al (2018) Escapes of non-native fish from flooded aquaculture facilities: the case of Paranapanema River, southern Brazil. Zoologia 35:1–6. https://doi.org/10.3897/zoologia.35.e14638
Ceballos G, Ehrlich PR, Barnosky AD, Garcia A, Pringle RM, Palmer TM (2015) Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci Adv 1:e1400253. https://doi.org/10.1126/sciadv.1400253
Ceballos G, Ehrlich PR, Raven PH (2020) Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc Natl Acad Sci 117:13596–13602. https://doi.org/10.1073/pnas.1922686117
Costa ID et al (2018) Responses of fish assemblages to subtle elevations in headwater streams in southwestern Amazonia. Hydrobiologia 809:175–184. https://doi.org/10.1007/s10750-017-3463-1
Cressie NAC (1993) Statistics for spatial data. Wiley, New York
Daga VS, Skóra F, Padial AA, Abilhoa V, Gubiani EA, Vitule JRS (2015) Homogenization dynamics of the fish assemblages in Neotropical reservoirs: comparing the roles of introduced species and their vectors. Hydrobiologia 746:327–347. https://doi.org/10.1007/s10750-014-2032-0
Daga VS, Olden JD, Gubiani EA, Piana PA, Padial AA, Vitule JRS (2020) Scale-dependent patterns of fish faunal homogenization in Neotropical reservoirs. Hydrobiologia 847:3759–3772. https://doi.org/10.1007/s10750-019-04145-5
De’ath G, Fabricius KE (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178–3192. https://doi.org/10.1890/0012-9658(2000)081[3178:CARTAP]2.0.CO;2
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655. https://doi.org/10.1016/S0169-5347(01)02283-2
Dias MS et al (2017) Anthropogenic stressors and riverine fish extinctions. Ecol Indic 79:37–46. https://doi.org/10.1016/j.ecolind.2017.03.053
Dolédec S, Chessel D, ter Braak CJF, Champely S (1996) Matching species traits to environmental variables: a new three-table ordination method. Env Ecol Stat 3:143–166. https://doi.org/10.1007/BF02427859
Doria CRC et al (2021) The silent threat of non-native fish in the Amazon: ANNF database and review. Front Ecol Evol 9:1–11
Dray S, Dufour A (2007) The ade4 Package: Implementing the Duality Diagram for Ecologists. J Stat Softw 22(4):1–20. https://doi.org/10.18637/jss.v022.i04
Dray S, Chessel D, Thioulouse J (2003) Co-inertia analysis and the linking of ecological data tables. Ecology 84:3078–3089. https://doi.org/10.1890/03-0178
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc 81:163–182. https://doi.org/10.1017/S1464793105006950
Enders M et al (2020) A conceptual map of invasion biology: Integrating hypotheses into a consensus network. Glob Ecol Biogeogr 29:978–991. https://doi.org/10.1111/geb.13082
FEOW (2021) Freshwater Ecoregions of the world. https://www.feow.org/ecoregions/details/344. Accessed 14 Sept 2021
Frederico RG, De Marco P, Zuanon J (2014) Evaluating the use of macroscale variables as proxies for local aquatic variables and to model stream fish distributions. Freshw Biol 59:2303–2314. https://doi.org/10.1111/fwb.12432
Frehse FA, Braga RR, Nocera GA, Vitule JRS (2016) Non-native species and invasion biology in a megadiverse country: scientometric analysis and ecological interactions in Brazil. Biol Invas 18:3713–3725. https://doi.org/10.1007/s10530-016-1260-9
Fricke R, Eschmeyer WN, van der Laan R (eds) (2023) Eschmeyer’s catalog of fishes: genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Frota A, Message HJ, Oliveira RC, Benedito E, Graça WJ (2019) Ichthyofauna of headwater streams from the rio Ribeira de Iguape basin, at the boundaries of the Ponta Grossa Arch, Paraná, Brazil. Biota Neotrop 19:e20180666. https://doi.org/10.1590/1676-0611-BN-2018-0666
Garcia DAZ, Britton JR, Vidotto-Magnoni AP, Orsi ML (2018) Introductions of non-native fishes into a heavily modified river: rates, patterns and management issues in the Paranapanema River (Upper Paraná ecoregion, Brazil). Biol Invas 20:1229–1241. https://doi.org/10.1007/s10530-017-1623-x
Garcia DAZ, Pelicice FM, Brito MFG, Orsi ML, Magalhães ALB (2021) Peixes não-nativos em riachos no Brasil: estado da arte, lacunas de conhecimento e perspectivas. Oecol Aust 25:565–587
García-Martínez MA, Martínez-Tlapa DL, Pérez-Toledo GR, Quiroz-Robledo LN, Castaño-Meneses G, Laborde J, Valenzuela-González JE (2015) Taxonomic, species and functional group diversity of ants in a tropical anthropogenic landscape. Trop Conserv Sci 8:1017–1032. https://doi.org/10.1177/194008291500800412
Giraudoux P (2018) pgirmess: spatial analysis and data mining for field ecologists. R package version 1.6.9. https://CRAN.R-project.org/package=pgirmess
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19:470–474. https://doi.org/10.1016/j.tree.2004.07.005
Hermoso V, Linke S, Prenda J (2009) Identifying priority sites for the conservation of freshwater fish biodiversity in a Mediterranean basin with a high degree of threatened species. Hydrobiologia 623:127–140. https://doi.org/10.1007/s10750-008-9653-0
Jackson DA, Peres-Neto PR, Olden KD (2001) What controls who is where in freshwater fish communities: the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170. https://doi.org/10.1139/cjfas-58-1-157
Jeschke JM, Bacher S, Blackburn TM et al (2014) Defining the impact of non-native species. Conserv Biol 28:1188–1194. https://doi.org/10.1111/cobi.12299
Jorissen MWP, Huyse T, Pariselle A et al (2020) Historical museum collections help detect parasite species jumps after tilapia introductions in the Congo Basin. Biol Invas 22:2825–2844. https://doi.org/10.1007/s10530-020-02288-4
Junqueira NT, Macedo DR, Souza RCRD, Hughes RM, Callisto M, Pompeu PS (2016) Influence of environmental variables on stream fish fauna at multiple spatial scales. Neotrop Ichthyol 14(3):e150116. https://doi.org/10.1590/1982-0224-20150116
Kembel SW et al (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L (2007) Diversidade da ictiofauna do Alto Rio Paraná: composição atual e perspectivas futuras. Biota Neotrop 7:181–197. https://doi.org/10.1590/S1676-06032007000300020
Latini AO et al (2021) Brazil’s new fish farming decree threatens freshwater conservation in South America. Biol Conserv 263:109353. https://doi.org/10.1016/j.biocon.2021.109353
Leal CG et al (2018) Is environmental legislation conserving tropical stream faunas? A large scale assessment of local, riparian and catchment scale influences on Amazonian fish. J Appl Ecol 55(3):1312–1326. https://doi.org/10.1111/1365-2664.13028
Leitão RP, Zuanon J, Villéger S, Willians SE, Baraloto C, Fortunel C, Mendonça FP, Mouillot D (2018) Rare species contribute disproportionately to the functional structure of species assemblages. Proc R Soc Lond 283:1–9. https://doi.org/10.1098/rspb.2016.0084
Levene H (1960) Robust tests for equality of variances. In: Gleser LJ, Pearlman MD, Press SJ, Sampson AR (eds) Contributions to probability and statistics. Palo Alto, pp 278–292
Liew JH, Tan HH, Yeo DCJ (2016) Dammed rivers: impoundments facilitate fish invasions. Freshw Biol 61(9):1421–1429. https://doi.org/10.1111/fwb.12781
Matsuzaki SS, Sasaki T, Akasaka M (2013) Consequences of the introduction of exotic and translocated species and future extirpations on the functional diversity of freshwater fish assemblages. Glob Ecol Biogeogr 22(9):1071–1082. https://doi.org/10.1111/geb.12067
Mazerolle MJ (2020) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.3-1. https://cran.r-project.org/package=AICcmodavg.
Mazzoni R, Fenerich-Verani N, Caramaschi EP (2000) Electrofishing as a sampling technique for coastal stream fish populations and communities in the southeast of Brazil. Braz J Biol 60:205–216. https://doi.org/10.1590/s0034-71082000000200003
Menke W (2015) Review of the generalized least squares method. Surv Geophys 36:1–25. https://doi.org/10.1007/s10712-014-9303-1
Mindel BL, Neat FC, Trueman, CN, Webb TJ, Blanchard JL (2016) Functional, size and taxonomic diversity of fish along a depth gradient in the deep sea. PeerJ 4:e2387. https://doi.org/10.7717/peerj.2387
Moulton TP (2008) Defying water’s end: do we need different conservation strategies for aquatic systems compared with terrestrial? Aquat Conserv 19:1–3. https://doi.org/10.1002/aqc.1017
Muniz CM, García-Berthou E, Ganassin MJM, Agostinho AA, Gomes LC (2021) Alien fish in Neotropical reservoirs: assessing multiple hypotheses in invasion biology. Ecol Indic 121:107034
Naeem S, Duffy JE, Zavaleta E (2012) The functions of biological diversity in an age of extinction. Science 336:1401–1406. https://doi.org/10.1126/science.1215855
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the world. Wiley, Hoboken
Oksanen J et al (2022) vegan: Community Ecology Package. R package version 2.6-4. https://CRAN.R-project.org/package=vegan
Olden JD (2006) Biotic homogenization: a new research agenda for conservation biogeography. J Biogeogr 33:2027–2039. https://doi.org/10.1111/j.1365-2699.2006.01572.x
Padial AA et al (2017) The “Tilapia Law” encouraging non-native fish threatens Amazonian River basins. Biodivers Conserv 26:243–246. https://doi.org/10.1007/s10531-016-1229-0
Pavoine S, Bonsall MB (2010) Measuring biodiversity to explain community assembly: a unified approach. Biol Rev 86:792–812. https://doi.org/10.1111/j.1469-185X.2010.00171.x
Pavoine S, Vallet J, Dufour A-B, Gachet S, Hervé D (2009) On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos 118:391–402. https://doi.org/10.1111/j.1600-0706.2008.16668.x
Pelicice FM, Agostinho AA (2008) Fish fauna destruction after the introduction of a non-native predator (Cichla kelberi) in a Neotropical reservoir. Biol Invas 11(8):1789–1801. https://doi.org/10.1007/s10530-008-9358-3
Pelicice FM, Bialetzki A, Camelier P, Carvalho FR, García-Berthou E, Pompeu PS, Mello FT, Pavanelli CS (2021) Human impacts and the loss of Neotropical freshwater fish diversity. Neotrop Ichthyol 19(3):e210134. https://doi.org/10.1590/1982-0224-2021-0134
Pelicice FM et al (2022) Ecosystem services generated by neotropical freshwater fishes. Hydrobiologia. https://doi.org/10.1007/s10750-022-04986-7
Pereira SP et al (2017) Non-native species in reservoirs: How are we doing in Brazil? Hydrobiologia 87:71–84. https://doi.org/10.1007/s10750-017-3446-2
Peressin A, Gonçalves CS, Cetra M (2018) Ichthyofauna diet changes in response to urbanization: the case of upper Paranapanema River basin (Brazil). Urban Ecosyst 21:795–803. https://doi.org/10.1007/s11252-018-0755-9
Pinheiro J et al (2020) _nlme: Linear and Nonlinear Mixed Effects Models_R package version 3.1-150. https://CRAN.R-project.org/package=nlme
Poff NL (1997) Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J N Am Benthol Soc 16:391–409. https://doi.org/10.2307/1468026
Pool TK, Olden J (2012) Taxonomic and functional homogenization of an endemic desert fish fauna. Divers Distrib 18:366–376. https://doi.org/10.1111/j.1472-4642.2011.00836.x
R Development Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Reid AJ, Carlson AK, Creed IF et al (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Reis RE, Albert JS, Dario FD, Mincarone MM, Petry P, Rocha LA (2016) Fish biodiversity and conservation in South America. J Fish Biol 89:12–47. https://doi.org/10.1111/jfb.13016
Ribeiro MD, Teresa FB, Casatti L (2016) Use of functional traits to assess changes in stream fish assemblages across a habitat gradient. Neotrop Ichthyol 14(1):e140185. https://doi.org/10.1590/1982-0224-20140185
Rocha CFD, Bergallo HG, Mazzoni R (2011) Invasive Vertebrates in Brazil. In: Pimentel D (ed) Economic and environmental costs of alien plant, animal, and microbe species. Taylor & Francis, New York, pp 53–103
Rodrigues-Filho CAS et al (2017) What governs the functional diversity patterns of fishes in the headwater streams of the humid forest enclaves: environmental conditions, taxonomic diversity or biotic interactions? Environ Biol Fish 100:1023–1032. https://doi.org/10.1007/s10641-017-0603-4
Rodrigues-Filho CAS et al (2018) How are local fish communities structured in Brazilian semiarid headwater streams? Hydrobiologia 819:93–108. https://doi.org/10.1007/s10750-018-3650-8
Rosa DM, Sene AM, Moreira MZ, Pompeu PS (2021) Non-native prey species supporting fish assemblage biomass in a Neotropical reservoir. Biol Invas 23:2355–2370. https://doi.org/10.1007/s10530-021-02510-x
Santana MLC, Carvalho FR, Teresa FB (2021) Broad and fine-scale threats on threatened Brazilian freshwater fish: variability across hydrographic regions and taxonomic groups. Biota Neotrop 21(2):e20200980. https://doi.org/10.1590/1676-0611-BN-2020-0980
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52(3–4):591–611. https://doi.org/10.1093/biomet/52.3-4.591
Strahler AN (1957) Quantitative analysis of watershed geomorphology. Trans Am Geophys Union 38:913–920. https://doi.org/10.1029/TR038i006p00913
Su G, Logez M, Xu J, Tao S, Villéger S, Brosse S (2021) Human impacts on global freshwater fish biodiversity. Science 371(6531):835–838. https://doi.org/10.1126/science.abd3369
ter Braak CJF, Cormont A, Dray S (2012) Improved testing of species traits–environment relationships in the fourth-corner problem. Ecology 93:1525–1526. https://doi.org/10.1890/12-0126.1
Thioulouse J, Dray S, Dufour AB, Siberchicot A, Jombart T, Pavoine S (2018) Multivariate analysis of ecological data with ade4. Springer, Berlin
Toussaint A, Charpin N, Brosse S, Villéger S (2016) Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci Rep 6:1–9. https://doi.org/10.1038/srep22125
Toussaint A, Charpin N, Beauchard O et al (2018) Non-native species led to marked shifts in functional diversity of the world freshwater fish faunas. Ecol Lett 21:1649–1659. https://doi.org/10.1111/ele.13141
Villéger S, Grenouillet G, Brosse S (2014) Functional homogenization exceeds taxonomic homogenization among European fish assemblages. Glob Ecol Biogeogr 23:1450–1460. https://doi.org/10.1111/geb.12226
Villéger S, Brosse S, Mouchet M, Mouillot D, Vanni MJ (2017) Functional ecology of fish: current approaches and future challenges. Aquat Sci 79:783–801. https://doi.org/10.1007/s00027-017-0546-z
Vitule JRS (2009) Introduction of fishes in Brazilian continental ecosystems: review, comments, and suggestions for actions against the almost invisible enemy. Neotrop Biol Conserv 4(2):111–122. https://doi.org/10.4013/5123
Vitule JRS, Prodocimo V (2012) Introdução de espécies não nativas e invasões biológicas. Est Biol Amb Divers 34:225–237. https://doi.org/10.7213/estud.biol.7335
Vitule JRS, Freire CA, Simberloff D (2009) Introduction of non-native freshwater fish can certainly be bad. Fish Fish 10:98–108. https://doi.org/10.1111/j.1467-2979.2008.00312.x
Vitule JRS, Skóra F, Abilhoa V (2012) Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in Neotropics. Divers Distrib 18:111–120. https://doi.org/10.1111/j.1472-4642.2011.00821.x
Vitule JRS, Costa APL, Frehse FA, Bezerra LAV, Occhi TVT, Daga VS, Padial AA (2017) Comments on “Fish biodiversity and conservation in South America by Reis et al. (2016)”. J Fish Biol 9:1182–1190. https://doi.org/10.1111/jfb.13239
Warwick RM, Clarke KR (1998) Taxonomic distinctness and environmental assessment. J Appl Ecol 35:532–543. https://doi.org/10.1046/j.1365-2664.1998.3540532.x
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annu Rev Ecol Syst 33:475–505. https://doi.org/10.1146/annurev.ecolsys.33.010802.150448
Weyl OLF et al (2016) A review of Clarias gariepinus invasions in Brazil and South Africa. J Fish Biol 89:386–402. https://doi.org/10.1111/jfb.12958
Acknowledgements
We thank to the BioEcol lab team that contributed with field data collection; Maria Angélica Pérez Mayorga for her assistance in obtaining fish pictures; Carlos Filipe Camilo Cotrim and Thais Sampaio Silva for his help with ecomorphological measurements of species. We are also grateful for contributions given by the Editor and anonymous reviewers.
Funding
PPB was supported by a PhD scholarship provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (proc. 23038.000452/2017-16). FRC was supported by CNPq (process # 420620/2018-4) and FUNDECT (“Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul”, process # 59/300.093/2017, SIAFEM 27248). LC is granted by CNPq (304403/2021-0). FBT was supported by CNPq (431094/2016-0 and 306912/2018-0). Data provided by FLTG was collected with the financial support of the FAPEG. Data provided by LC was collected with the financial support of the FAPESP (proc. 2001/13340-7). Data provided by MC was collected with the financial support of the FAPESP (proc. 2013/24737-2). Data provided by GLB was collected with the financial support of the FAPESP (proc. 2006/04878-7). The study also had the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (proc. 431094/2016-0) and CNPq/MCT/CAPES/FAPEG (Programa de Pesquisa Ecológica de Longa Duração—PELD, sítio COFA (proc. 441278/2016-7).
Author information
Authors and Affiliations
Contributions
BRBS and RAC were responsible for the conception and design of the study, organization of data, statistical analyses, interpretation of data, and drafting the manuscript. FBT contributed with the conception and design of the study, acquisition of data and manuscript revision. TS contributed with statistical analyses and manuscript revision. PPB contributed with the acquisition of data and manuscript revision. LC, GB, FLTG, PSP, EB, FRC, MC, MSD, and YRS contributed with interpretation of data, and the revision of the final version of the manuscript. All authors approved the final version submitted for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest or competing interests.
Consent to participate
All persons entitled to authorship have been so named and agreed to participate.
Consent for publication
All authors agree with the final version submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, B.R.B., Teresa, F.B., Borges, P.P. et al. Tropical headwater streams and the role of non-native species on fish assemblage’s diversity. Biol Invasions 25, 3103–3118 (2023). https://doi.org/10.1007/s10530-023-03093-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03093-5