Abstract
Self-purification is one of the most important ecosystem functions of rivers. Multiple human activities regularly impact this ecosystem service, consequently altering river morphology, hydrology, and the composition of biotic assemblages that contribute to self-purification. However, little quantitative information is available about the importance of such impacts. Hence, we tested how invasive mussel species contribute to self-purification under disturbed riverine conditions. In laboratory experiments, invasive mussel species equipped with magnetic sensors that recorded filtration activity were exposed to artificial waves of varying intensity that simulated the hydraulic effects of inland navigation. Our results suggest that invasive mussel species are more resistant to wave disturbance compared to native species, as estimated threshold values for initiating shell closure are very high (Dreissena rostriformis bugensis) or the duration (Corbicula fluminea) and degree of shell closing (D. rostriformis bugensis, C. fluminea) very low. Also we demonstrated that the invasive species D. rostriformis bugensis and C. fluminea continued filtering during wave impact, whereas Dreissena polymorpha did not behave significantly differently than previously studied native mussel species, based on the studied susceptibility parameters. Thus, D. rostriformis bugensis and C. fluminea appear to be pre-adapted to hydraulic or morphological disturbance, and may compensate against other losses regarding this important ecosystem function in rivers that are intensively used for inland navigation. However, as the dominance of invasive species in river systems may disrupt natural food webs, this compensation of filter-feeding activity may be accompanied by the loss of other ecosystem functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human civilization depends, to a certain extent, on ecosystem services provided by rivers; however, these services are often overused (Costanza et al. 1997; Everard and Powell 2002; Kareiva et al. 2007). Among other functions, many river systems worldwide are intensively used for inland navigation and boating activities (Food and Agriculture Organization of the United Nations 2008). Worldwide, 671,886 km of waterways exist for inland navigation (Central Intelligence Agency 2011), many of which use natural rivers and lakes. As many inland waterways are connected to other river systems by canals, inland navigation facilitates or accelerates the spread of invasive invertebrate species to new biogeographic regions. Thereby, species may be actively translocated as a result of being attached to the hull of boats or within the ballast water, or may spread independently along newly built waterways, as documented for the Danube-Main Canal (Leuven et al. 2009; Mills et al. 1993; Pusch et al. 2009). As a consequence, invasive species arriving from various biogeographical regions meet habitat conditions in other river systems that have been modified by humans in many aspects (Tockner et al. 2010, 2011). This situation often enables non-native species to successfully establish novel ecological niches, and build up large populations (Darrigran 2002). As rivers subjected to multiple human pressures only offer suboptimal habitat conditions to native species, invasive species often replace native species (Byers 2002). Thereby, some invasive species may also benefit from pre-adaptations acquired from their original natural habitats, which result in gaining a superior competitive position relative to native species (Correa and Gross 2008; Gabel et al. 2011a). Yet there is still a tremendous lack of knowledge on the mechanisms how non-native species become successful colonizers especially in waterways, and both theories of pre-adaptations as well as of rapid adaptive changes are supported in literature (Alford et al. 2009; Correa and Gross 2008; Gabel et al. 2011a; Henery et al. 2010).
Given the profound alterations in the physical and biotic structure of major inland waters described above, there is also a high probability of changes in key ecosystem services, such as self-purification capacity, which includes the removal of organic matter from the water column (Pusch and Hoffmann 2000; Tockner et al. 2011). Recent publications have demonstrated that waves induced by navigation and boating may cause significant hydraulic disturbances to benthic invertebrates and fish (Bishop and Chapman 2004; Gabel et al. 2008, 2011b), which may also affect the filtration activity of mussel populations (Lorenz et al. 2013; Payne et al. 1999; Widdows et al. 1979).
As freshwater mussels are primary consumers of phytoplankton and seston in aquatic habitats, this group supplies significant food resources to the benthic food web (Howard and Cuffey 2006), in addition to significantly contributing to the self-purification of running waters. Thus, mussels may significantly improve water quality, particularly in eutrophicated surface waters (Welker and Walz 1998). Consequently, anthropogenic impacts on the filtration activity of freshwater mussels are likely to affect the productivity of the benthic food web and decrease ecosystem resilience, in addition to increasing the eutrophication of aquatic ecosystems. However, in the case river when systems have been colonized by invasive mussel species, the filtration rate of the benthic community may be even increased, as their filtration capacities and rates are typically higher compared to native species (Atkinson et al. 2011; Leff et al. 1990; Weitere et al. 2008). Aside from the greater capacity to compete for food (Strayer and Smith 1996), invasive species may exhibit other biological characteristics that better fit the habitat conditions of altered river systems, such as substrate preference (as for Dreissena spp.), temperature preference, or mechanical resistance (as for Corbicula spp.) (Tockner et al. 2011). However, there is limited information clarifying which of these multiple modes of anthropogenic disturbance is the most decisive for a given species, or how these modes favor specific invasive species (e.g. Gabel et al. 2011a).
We conducted a laboratory study to test whether wave disturbance, which represents an anthropogenic disturbance typical to large rivers, affects the filtration activity of three invasive mussel species. The results were discussed with similar data obtained from a previous study on native mussel species (Lorenz et al. 2013). We hypothesized that invasive mussels exhibit pre-adaptations to hydraulic disturbance, and are more likely to perform better under wave disturbance than native mussel species. This hypothesis would be supported by obtaining a consistent difference for both wave sensitivity and shell closing behavior between native and invasive species. We predicted that invasive mussel species are less susceptible to ship-induced waves, and that filtration activity is higher under disturbed conditions.
Material and methods
Experimental settings
We obtained 15 individuals of three invasive mussel species in Germany; specifically (1) the Asian clam Corbicula fluminea MÜLLER 1774 from Rhine River, (2) the quagga mussel Dreissena rostriformis bugensis ANDRUSOV 1897 from Main River, and (3) the zebra mussel Dreissena polymorpha PALLAS 1771 from the Spree River. The mussels were acclimatized in separate aerated laboratory aquaria to a water temperature of 18 °C. After acclimatization, five individuals of the two Dreissena species were placed on ceramic tiles inside the respective aquaria, and were kept for another 2 weeks at 18 °C in a climate chamber. After all individuals used byssus threads to attach to the tiles, each mussel was equipped with a magnetic sensor (radiometric linear Hall-effect sensor A1321, Allegro Microsystems Inc., Worcester, MA, USA) on one shell, and a disc magnet (magnet grade N48, diameter 2 mm, thickness 2 mm) on the other shell. This equipment was used to record shell gape as a parameter of filtration activity (Hopkins 1933). Subsequently, one tile with five individuals was placed on the sediment inside an experimental wave tank that had three replicate flumes (Fig. 1). For C. fluminea, five magnet and sensor equipped individuals were transferred to each replicate section, and individuals were allowed to burrow into the sediment before the experiment. The wave tank was filled with aerated unchlorinated tap water, with a similar temperature of 18 °C. The sediment bed consisted of a 10 cm layer of silica sand, with a grain size of 0.2–0.63 mm. All three species were kept inside this wave tank for an additional 24 h. During all time in laboratory aquaria, individuals were fed with dried Spirulina sp. algae (food concentration 10 mg L−1).
After all individuals exhibited filtration activity, waves of different intensity (5, 8, 11, 14, 17, 21, and 24 cm s−1) were produced with a wave paddle driven by a car windshield wiper motor in random order to avoid individual mussels becoming acclimated to the waves. Each type of wave intensity was repeated three times. Data were recorded and processed using our own software written in LabVIEW (National Instruments, Germany). Shell gape was calibrated against voltage (mV), and the measured voltage data (x) was then converted into gape opening (in mm) by using the following linear inverse (x) polynomial equation (Lorenz et al. 2013):
Afterwards, data were converted into relative values (percentage of maximum gape opening).
Calculation of shear stress
The bottom flow velocity that was associated with experimental waves was recorded using an Acoustic Doppler Velocimeter (ADV, Micro ADV 16 MHz, Sontek, San Diego, CA, USA), at a rate of 50 Hz. The ADV was placed in the middle section of each wave tank flume, using the same technical set-up as in previous experiments (Gabel et al. 2011a). The three flumes of the wave tank showed no significant differences in orbital velocities (Gabel et al. 2011a). The sampling volume of the ADV probe head was adjusted to 1 cm above the sediment bed. As ADV measurements in clear tap water tend to be subject to high backscatter, one drop of Lycopodium clavatum spore suspension was added directly over the probe head before creating each wave to enhance particle concentration, and hence reduce backscatter. Using the bottom orbital velocity U w and the wave friction factor f w , wave friction shear stress τ w was calculated for each wave that was produced, as:
(see Soulsby (1997) for detailed description).
Data analysis
The recorded shear stress values were double-square-root transformed to obtain a normal distribution, while the other data were left untransformed. Sigmoid regression models of the type:
were calculated for the relationship between shear stress (x) and the duration of shell closure and the degree of shell closure (percent reduction of maximum shell gape) as functions of x. This type of sigmoid regression model explained the highest proportion of mussel response pattern with rising shear stress levels. In the relationships of shear stress versus shell closure duration, the inflection point of the curves (x0) was determined and re-transformed to an unpotentiated scale. The inflection point x0 was defined as a threshold value separating tolerable and severe impacts on filtration activity, representing a predicted moderate value for the effect of shear stress, PMES (Lorenz et al. 2013). Accordingly, shear stress levels above this threshold produced less shell gape in all experimental specimens, whereas some specimens were not affected below this threshold. The starting point of any shell closing behavior was defined as the value where 10 % of the maximum closing intensity is reached (Lorenz et al. 2013). The value was re-transformed to an unpotentiated scale, and considered as the predicted no-effect shear stress level (PNES).
We tested for differences in the parameter ‘a’ (asymptotic maximum), PMES, and PNES between the three invasive species using unpaired t tests as described in Motulsky (1998). As the biotic and abiotic conditions (water temperature, turbidity, dissolved oxygen, food concentration) of this laboratory experiment were highly comparable to the experimental conditions in a previous field experiment (Lorenz et al. 2013), we statistically compared sigmoid regression models for D. polymorpha from both studies. Thereby, we tested differences in the regression parameters ‘a’ (asymptotic maximum), ‘b’ (slope), and ‘x0’ (PMES) between field versus laboratory approaches using unpaired t tests as described in Motulsky (1998). All statistical regressions and plots were performed using PASW (version 17.0, SPSS Inc., Chicago, IL, USA) and SigmaPlot (version 11.0, Systat Software Inc., Chicago, IL, USA).
Results
Degree and duration of shell closing
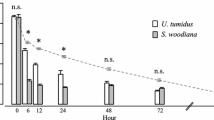
According to the sigmoid regression analysis (Table 1), the longest average closing duration was shown by D. polymorpha (a = 92 s), followed by D. rostriformis bugensis (87 s) and C. fluminea (20 s) (Fig. 2). Duration of shell closing did not differ significantly between both Dreissena species (df = 21, p = 0.87) but was significantly lower in C. fluminea than in D. rostriformis bugensis and D. polymorpha (df = 21, p < 0.0001 and p < 0.0001, respectively). The average degree of closing after wave disturbance was greatest in D. polymorpha (68 %), followed by C. fluminea (40 %) and D. rostriformis bugensis (39 %) (Fig. 2). Degree of shell closing did not differ significantly between D. rostriformis bugensis and C. fluminea (df = 21, p = 0.77) but was significantly higher in D. polymorpha than in D. rostriformis bugensis and C. fluminea (df = 21, p < 0.0001 and p < 0.0001, respectively).
Threshold values for the inhibition of filtration activity
Predicted moderate effect shear stress (PMES) values were higher in D. rostriformis bugensis (0.47 N m−2) and D. polymorpha (0.38 N m−2) compared to C. fluminea (0.14 N m−2) (Fig. 3). PMES values did not differ significantly between both Dreissena species (df = 21, p = 0.36) and between D. polymorpha and C. fluminea (df = 21, p = 0.46), but was significantly higher in D. rostriformis bugensis than in C. fluminea (df = 21, p < 0.001). Similarly, the predicted no-effect shear stress (PNES) value was higher in D. rostriformis bugensis (0.24 N m−2) compared to D. polymorpha (0.13 N m−2) and C. fluminea (0.04 N m−2) (Fig. 3). PNES values did not differ significantly between D. polymorpha and C. fluminea (df = 21, p < 0.01), but was significantly higher in D. rostriformis bugensis than in C. fluminea and D. polymorpha (df = 21, p < 0.0001 and p < 0.001, respectively).
a Duration (in s) and b degree (in %) of shell closing as functions of shear stress (N m−2), and respective c PMES and d PNES threshold values for the native (three left species) and invasive (three right species) mussel species that were studied. The results for the native species were obtained from Lorenz et al. (2013)
Comparison of field and laboratory studies
The shell closing behavior of D. polymorpha followed similar sigmoidal patterns under both field and laboratory conditions (Fig. 4). Respective regressions did not significantly differ between field or laboratory data for the relationship of shear stress and shell closing duration [unpaired t test, df = 114, p = 0.99 (a), p = 0.85 (b), p = 0.92 (x0)], or the relationship of shear stress and shell closing degree [unpaired t test, df = 112, p = 0.45 (a), p = 0.94 (b), p = 0.81 (x0)].
Discussion
To date, few studies have attempted to link the success of invasive species in newly colonized river systems to the specific habitat conditions that are available, which might reflect the respective biological or ecological traits of particular invasive species (Bij de Vaate et al. 2002; Devin and Beisel 2007; Ricciardi and Rasmussen 1998). In river systems subjected to strong or multiple human pressures, habitat conditions become suboptimal for native species, consequently favoring the establishment of invasive species. Thereby, the successful establishment of invasive species is not only governed by physical habitat conditions, but also by interspecific interactions. Invasive species have been shown to cause a decline in the abundance of native species, such as through competition for food sources (Strayer and Smith 1996). This exploitative competition may even occur among invasive species, as exemplified by the spread of the quagga mussel D. rostriformis bugensis, which caused a decline in the abundance of D. polymorpha (Zhulidov et al. 2010).
Our results indicate that, besides alterations to river hydrology, temperature, water quality, and channel morphology (Rahel and Olden 2008), higher filtration activity under wave exposure provides an additional mechanism to explain the success of invasive species in fresh water habitats used for inland navigation. In our studies, experimentally created waves consisted the primary variable influencing filtration activity (but see e.g. Moore 1977; Riisgard et al. 2011; or Salanki et al. 1974 for further variables influencing filtration). As invasive invertebrates, such as mussels, in freshwater habitats are mainly spread by ships via navigation channels (Leuven et al. 2009), the benefits of transportation to novel habitats might be twofold. First, their spread is accelerated by the anthropogenic disturbances (Byers 2002), such as ship traffic, and second, they are less susceptible to strong exposure from disturbance by ship-induced waves.
A comparison of our results with variables representing the susceptibility to wave stress from former field experiments of native mussel species (Lorenz et al. 2013) revealed some differences in the shell closing behavior of native versus invasive mussel species. The invasive species D. polymorpha studied both under laboratory and field conditions showed no significant difference in its shell closing behavior, suggesting that available data of native and invasive species were comparable as well between both setups. In summary, the native species investigated in the previous study showed relatively low PNES values in combination with either the highest duration of shell closing (A. anatina) or the highest degree of shell closing (U. tumidus and U. pictorum) (Fig. 3). Thus, we conclude that native mussel species seem to be more susceptible to wave disturbance than invasive species. To demonstrate how this varying susceptibility to wave action could influence filtration, we calculated the remaining filtration activity for each mussel species according to Eq. 2 from Lorenz et al. (2013) (general assumption: single motorboat passages with a speed of 18 km h−1, mussels located at a water depth of 1.5 m). Thereby, the native species would show a remaining filtration activity of 80 % (U. tumidus), 71 % (U. pictorum), and 56 % (A. anatina), while the remaining filtration activities of invasive species would be 72 % (D. polymorpha), 88 % (C. fluminea), and 98 % (D. rostriformis bugensis) compared to undisturbed conditions. In comparison, this means an average reduction in filtration activity of 31 % for the studied native species, but just 14 % for invasive species. In contrast to D. rostriformis bugensis and C. fluminea, D. polymorpha differs not seriously from native species in any of the studied susceptibility parameters. Thus, the colonization success of this species (Aldridge et al. 2004; Johnson and Carlton 1996; Johnson and Padilla 1996) cannot be explained (at least for Europe) by invasive species exhibiting lower susceptibility to wave disturbance compared to native species.
We demonstrated that D. rostriformis bugensis and C. fluminea continue to filter under wave impacts, which would provide a crucial competitive advantage in fresh waters used intensively for navigation. Although both species exhibited similar degrees of shell closing at higher shear stress levels, they exhibited different sensitivity toward this stressor. For instance, D. rostriformis bugensis was the most stress–resistant species, with a minimal degree of shell closing, and maximal PNES and PMES values. Hence, D. rostriformis bugensis seems to be adapted to perform significant filtration activity even in habitats with high hydraulic disturbance. In comparison, while C. fluminea also showed a minimal degree of shell closing, which was even accompanied by a minimal duration of shell closing, this species exhibited minimal PMES and second minimal PNES values among the studied species. This ‘opportunistic’ pattern to exhibit a minimally sensitive response to hydraulic disturbance may represent an adaptation to life in hydraulically sheltered interstitial spaces within the sediments of fast-flowing rivers (McMahon 1999). In such habitats, disturbance may be mainly produced by relatively coarse particles transported along the river bottom, which pass by quickly. The ingestion of such particles may therefore be avoided by mussels, even by the short and incomplete closure of shells. During the experiments, we observed that C. fluminea individuals exhibited burrowing behavior in response to wave stress, which is a behavioral pattern that has not been previously observed in burrowing unionid species (Lorenz et al. 2013). Thereby, individuals escaped wave disturbances by burrowing into the sediment immediately after the passage of the first wave, leaving just their inhalant and exhalant siphons protruding from the sediment. In other cases, the mussels burrowed completely, without the siphon protruding but the shells remained open. This behavior supports the assumption that filter feeding may not consist the only foraging strategy for C. fluminea, as additional pedal feeding has been described for this species (Reid et al. 1992).
The underlying mechanism that causes low susceptibility may be based on a pre-adaptation that some invasive species may have acquired through evolution in their original biogeographical region (Correa and Gross 2008), or may reflect adaptive changes in their behavior in newly colonized habitats (Alford et al. 2009), or a combination of the two (Henery et al. 2010). In the case of C. fluminea, the minimal sensitive response to wave disturbance may be the result of a pre-adaptation acquired in the hydraulic disturbed environments of its native range. Its rapid burrowing response to wave stress may be seen as an adaptive behavior that has developed under even more disturbed conditions. Thus, this species reflects a high degree of behavioral flexibility, which represents a recently suggested explanation for invasion success in novel environments (Sol 2007). Therefore, our results support the hypothesis that the high invasion success of non-native species might be based on the interaction of pre-adaptations and rapid adaptive changes (Henery et al. 2010).
Human impacts on aquatic ecosystems, including wave disturbance by navigation, often result in dramatic and well-known reductions in population size or species richness of autochthonous biota (Tockner et al. 2010), whereas the detailed effects on key ecosystem services remain poorly documented (Chapin et al. 2000) despite their common acceptance (Binimelis et al. 2007). Threats to the supply of clean water by D. polymorpha consists one of the few well documented impacts causing risks on regulating ecosystem services (Charles and Dukes 2007). The estimated damage of this invasion has been estimated at 5 billion US$ solely in the United States (Pimentel 2005). Even though this economic loss is dramatic, the ecological assessment of fresh water habitats currently is mostly based on the composition of biotic assemblages, while the status of ecosystem functioning is rarely assessed. The important self-purification mechanism in rivers that is provided by the transport of organic matter from the water column to the benthic food web by the filtration activity of freshwater mussels (Howard and Cuffey 2006; Pusch et al. 2001) is a major process contributing to high water quality (Tockner et al. 2011). Consequently, a reduction in the filtration activity of freshwater mussels by ship waves will result in the loss of the capacity for the self-purification of surface waters and, thus, directly affect the provision of important ecosystem services. The lower susceptibility of invasive mussel species to wave disturbance indicates that the detrimental effects of ship waves may be compensated by the presence of less susceptible invasive mussel species within this community. The impact of those species might thus be valued both positively (as for water filtration, e.g. pointed out for D. polymorpha by Minchin et al. (2002)) and negatively (as on species composition), adding another controversy to the ongoing debate on the perception of invasive species impact on contrasting levels of examination (Binimelis et al. 2007).
As climate change will result in lower minimum water levels of many surface waters, the impact of boat generated waves on mussels is likely to increase (Lorenz et al. 2013). In parallel, the invasion of non-native mussel species, which are also favored by climate change (Rahel and Olden 2008) may ensure that an important component of self-purification in freshwater ecosystems is retained, despite the negative effects of climate change and high boating activities. Accordingly, the ecological carrying capacity of inland waters for boating, as calculated by Lorenz and Pusch (2012), might also benefit from the introduction of mussel species that are less susceptible to the impacts of ship-induced waves. However, as the dominance of invasive species in river systems may disrupt natural food webs, such compensation of filter-feeding activity may be accompanied by important losses in other ecosystem functions, which require identification.
References
Aldridge DC, Elliott P, Moggridge GD (2004) The recent and rapid spread of the zebra mussel (Dreissena polymorpha) in Great Britain. Biol Conserv 119:253–261
Alford RA, Brown GP, Schwarzkopf L, Phillips BL, Shine R (2009) Comparisons through time and space suggest rapid evolution of dispersal behaviour in an invasive species. Wildl Res 36:23–28
Atkinson CL, First MR, Covich AP, Opsahl SP, Golladay SW (2011) Suspended material availability and filtration-biodeposition processes performed by a native and invasive bivalve species in streams. Hydrobiologia 667:191–204
Bij de Vaate A, Jazdzewski K, Ketelaars HAM, Gollasch S, van der Velde G (2002) Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can J Fish Aquat Sci 59:1159–1174
Binimelis R, Born W, Monterroso I, Rodríguez-Labajos B (2007) Socio-economic impact and assessment of biological invasions. In: Nentwig W (ed) Biological invasions. Springer, Berlin, pp 331–347
Bishop MJ, Chapman MG (2004) Managerial decisions as experiments: an opportunity to determine the ecological impact of boat-generated waves on macrobenthic infauna. Estuar Coast Shelf Sci 61:613–622
Byers JE (2002) Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 97:449–458
Central Intelligence Agency (2011) The World Factbook—country comparison: waterways. Central Intelligence Agency, Washington
Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Diaz S (2000) Consequences of changing biodiversity. Nature 405:234–242
Charles H, Dukes JS (2007) Impacts of invasive species on ecosystem services. In: Nentwig W (ed) Biological invasions. Springer, Berlin, pp 217–239
Correa C, Gross MR (2008) Chinook salmon invade southern South America. Biol Invasions 10:615–639
Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Darrigran G (2002) Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biol Invasions 4:145–156
Devin S, Beisel J-N (2007) Biological and ecological characteristics of invasive species: a gammarid study. Biol Invasions 9:13–24
Everard M, Powell A (2002) Rivers as living systems. Aquat Conserv Mar Freshw Ecosyst 12:329–337
Food and Agriculture Organization of the United Nations (2008) Inland aquatic biodiversity. FAO Fisheries and Aquaculture Department, Rome
Gabel F, Garcia XF, Brauns M, Sukhodolov A, Leszinski M, Pusch MT (2008) Resistance to ship-induced waves of benthic invertebrates in various littoral habitats. Freshw Biol 53:1567–1578
Gabel F, Pusch MT, Breyer P, Burmester V, Walz N, Garcia XF (2011a) Differential effect of wave stress on the physiology and behaviour of native versus non-native benthic invertebrates. Biol Invasions 13:1843–1853
Gabel F, Stoll S, Fischer P, Pusch MT, Garcia XF (2011b) Waves affect predator-prey interactions between fish and benthic invertebrates. Oecologia 165:101–109
Henery ML, Bowman G, Mráz P, Treier UA, Gex-Fabry E, Schaffner U, Müller-Schärer H (2010) Evidence for a combination of pre-adapted traits and rapid adaptive change in the invasive plant Centaurea stoebe. J Ecol 98:800–813
Hopkins AE (1933) Experiments on the feeding behavior of the oyster, Ostrea gigas. J Exp Zool 64:469–494
Howard JK, Cuffey KM (2006) The functional role of native freshwater mussels in the fluvial benthic environment. Freshw Biol 51:460–474
Johnson LE, Carlton JT (1996) Post-establishment spread in large-scale invasions: dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology 77:1686–1690
Johnson LE, Padilla DK (1996) Geographic spread of exotic species: ecological lessons and opportunities from the invasion of the zebra mussel Dreissena polymorpha. Biol Conserv 78:23–33
Kareiva P, Watts S, McDonald R, Boucher T (2007) Domesticated nature: shaping landscapes and ecosystems for human welfare. Science 316:1866–1869
Leff LG, Burch JL, McArthur JV (1990) Spatial-distribution, seston removal, and potential competitive interactions of the bivalves Corbicula fluminea and Elliptio complanata, in a coastal-plain stream. Freshw Biol 24:409–416
Leuven R, van der Velde G, Baijens I, Snijders J, van der Zwart C, Lenders HJR, de Vaate AB (2009) The river Rhine: a global highway for dispersal of aquatic invasive species. Biol Invasions 11:1989–2008
Lorenz S, Pusch MT (2012) Estimating the recreational carrying capacity of a lowland river section. Water Sci Technol 66:2033–2039
Lorenz S, Gabel F, Dobra N, Pusch MT (2013) Modelling the impacts of recreational boating on self-purification activity provided by bivalve mollusks in a lowland river. Freshw Sci 32:82–92
McMahon RF (1999) Invasive characteristics of the freshwater bivalve Corbicula fluminea. In: Claudi R, Leach JH (eds) Nonindigenous freshwater organisms: vectors, biology, and impacts. CRC Press, Boca Raton, pp 315–343
Mills EL, Leach JH, Carlton JT, Secor CL (1993) Exotic species in the Great Lakes—a history of biotic crises and anthropogenic introductions. J Great Lakes Res 19:1–54
Minchin D, Lucy F, Sullivan M (2002) Zebra mussel: impacts and spread. In: Olenin S (ed) Invasive aquatic species of Europe—distribution, impact and management. Kluwer, Dordrecht, pp 135–146
Moore PG (1977) Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanogr Mar Biol Annu Rev 15:225–363
Motulsky H (1998) Comparing dose-response or kinetic curves with GraphPad Prism. HMS Beagle: The BioMedNet Mag 34:1–13
Payne BS, Miller AC, Shaffer LR (1999) Physiological resilience of freshwater mussels to turbulence and suspended solids. J Freshw Ecol 14:265–276
Pimentel D (2005) Aquatic nuisance species in the New York State Canal and Hudson River system and the Great Lakes Basin: an economic and environmental assessment. Environ Manag 35:692–701
Pusch M, Hoffmann A (2000) Conservation concept for a river ecosystem (River Spree, Germany) impacted by flow abstraction in a large post-mining area. Landsc Urban Plan 51:165–176
Pusch M, Siefert J, Walz N (2001) Filtration and respiration rates of two unionid species and their impact on the water quality of a lowland river. In: Bauer G, Wächtler K (eds) Ecology and evolutionary biology of the freshwater mussels Unionoida. Springer, Heidelberg, pp 317–326
Pusch M, Andersen HE, Bäthe J et al (2009) Rivers of the central highlands and plains. In: Tockner K, Uehlinger U, Robinson CT (eds) Rivers of Europe. Elsevier, London, pp 525–576
Rahel FJ, Olden JD (2008) Assessing the effects of climate change on aquatic invasive species. Conserv Biol 22:521–533
Reid RGB, McMahon RF, Foighil DO, Finnigan R (1992) Anterior inhalant currents and pedal feeding in bivalves. Veliger 35:93–104
Ricciardi A, Rasmussen JB (1998) Predicting the identity and impact of future biological invaders: a priority for aquatic resource management. National Research Council of Canada, Ottawa
Riisgard HU, Egede PP, Barreiro Saavedra I (2011) Feeding behaviour of the mussel, Mytilus edulis: new observations, with a minireview of current knowledge. J Mar Biol. Article ID 312459
Salanki J, Lukacsovics F, Hiripi L (1974) The effect of temperature variations on the rhythmic and periodic activity of the freshwater mussel (Anodonta cygnea L.). Annal Inst Biol Tihany 41:69–79
Sol D (2007) Do successful invaders exist? Pre-adaptations to novel environments in terrestrial vertebrates. In: Nentwig W (ed) Biological invasions. Springer, Berlin, pp 127–141
Soulsby R (1997) Dynamics of marine sands. Thomas Telford Publications, London
Strayer DL, Smith LC (1996) Relationships between zebra mussels (Dreissena polymorpha) and unionid clams during the early stages of the zebra mussel invasion of the Hudson River. Freshw Biol 36:771–779
Tockner K, Pusch M, Borchardt D, Lorang MS (2010) Multiple stressors in coupled river-floodplain ecosystems. Freshw Biol 55:135–151
Tockner K, Gessner J, Pusch MT, Wolter C (2011) Domesticated ecosystems and novel biotic communities: challenges for water management. Ecohydrol Hydrobiol 11:167–174
Weitere M, Dahlmann J, Viergutz C, Arndt H (2008) Differential grazer-mediated effects of high summer temperatures on pico- and nanoplankton communities. Limnol Oceanogr 53:477–486
Welker M, Walz N (1998) Can mussels control the plankton in rivers?—A planktological approach applying a Lagrangian sampling strategy. Limnol Oceanogr 43:753–762
Widdows J, Fieth P, Worrall CM (1979) Relationships between seston, available food and feeding-activity in the common mussel Mytilus edulis. Mar Biol 50:195–207
Zhulidov A, Kozhara A, Scherbina G, Nalepa T, Protasov A, Afanasiev S, Pryanichnikova E, Zhulidov D, Gurtovaya T, Pavlov D (2010) Invasion history, distribution, and relative abundances of Dreissena bugensis in the old world: a synthesis of data. Biol Invasions 12:1923–1940
Acknowledgments
We thank Helge Norf and Katharina Heiler for providing specimens of C. fluminea and D. rostriformis bugensis. We thank Reinhard Hölzel, Thomas Hintze, and Nora Dobra for technical support with the experiments, and Angela Hayes for data processing. We thank Thomas Mehner and the participants of the workshop ‘Scientific Writing’ at the Leibniz-Institute of Freshwater Ecology and Inland Fisheries for helpful discussions on an earlier stage of this manuscript. Financial support was provided by the German Federal Ministry of Education and Research (BMBF, FKZ 01LR0803G) through the project INKA BB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lorenz, S., Pusch, M.T. Filtration activity of invasive mussel species under wave disturbance conditions. Biol Invasions 15, 2681–2690 (2013). https://doi.org/10.1007/s10530-013-0483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0483-2