Abstract
Mussels provide important ecological functions in freshwater ecosystems but the associations between Amazonian mussels, macroinvertebrate assemblage and habitat quality remain poorly understood. We investigated whether changes in macroinvertebrate assemblage structure and ecological functioning were associated with mussel presence. We compared sites with and without mussels, with similar habitat conditions, in an eastern Amazonian river, using field measurements of macroinvertebrate structure, hydrological variables and sediment organic matter, and laboratory experiments of mussel clearance rate and biodeposition. Sites with mussels were associated with higher macroinvertebrate abundance and number of taxa, especially for trichopterans Marilia (shredder), Oecetis (predator) and Antarctoecia (collector). Decreased chlorophyll-a in the water column and increased sediment organic matter were positively associated with mussel presence. Laboratory experiments corroborated these patterns, which were stronger with higher mussel density. Mussel filtration and biodeposition may be associated with habitat quality for other invertebrates by lowering phytoplankton density in the water column and increasing inputs of sediment organic matter. This suggests a potential role of freshwater mussels in ecosystem function associated with high taxonomic and functional diversity in the macroinvertebrate assemblages of an eastern Amazon river, enhancing the already high mussel conservation priority.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater mussels of the order Unionida (hereafter mussels) constitute a large part of the invertebrate biomass in freshwater habitats (Vaughn, 2018). Aggregations of many or single species (Strayer, 2008) may introduce structural complexity and heterogeneity to the benthic habitat or increased resource availability (Ilarri et al., 2018; Vaughn, 2018; Simeone et al., 2021). Therefore, mussels may be considered foundational species since they provide important ecological functions in freshwater ecosystems that may influence both the aquatic and terrestrial food web (Howard & Cuffey, 2006; Allen & Vaughn, 2011). For example, feces and pseudofeces deposited by mussels (Vaughn & Hakenkamp, 2001) and benthic algae associated with mussel beds (Atkinson et al., 2021) are important food sources for associated macroinvertebrates, which are prey for fish and other insect predators, or as adults, for spiders and birds (Holomuzki et al., 2010). High mussel densities may also increase penetration of oxygen and water into the sediment (Boeker et al., 2016). Furthermore, mussels may influence primary production indirectly by releasing dissolved nutrients into the water column (Strayer, 2014; Zieritz et al., 2019) that influence periphyton growth (Spooner & Vaughn, 2006; Benelli et al., 2019), which serves as food for scrapers (Vaughn & Hakenkamp, 2001), or directly through filtering (Tuttle-Raycraft & Ackerman, 2018; Buelow & Waltham, 2020), which decreases phytoplankton density (Vaughn et al., 2004; Lummer et al., 2016).
Much of the knowledge quantifying mussel ecological function comes from North American and European habitats, and have focused on interactions between mussels and food webs (Vaughn et al., 2008; Atkinson et al., 2014, 2021), biodeposition and filtration (Atkinson et al., 2011; Lummer et al., 2016; Zieritz et al., 2020) and associated macroinvertebrates (Spooner & Vaughn, 2006; Vaughn & Spooner, 2006; Richter et al., 2016). On the other hand, little or nothing is known of the ecological functions provided by the South American mussel fauna; only the association between sponges and mussels was qualitatively described from the Xingu River, Brazil (Volkmer-Ribeiro et al., 2019). The South American mussel fauna has lower diversity, with approximately 168 species, mostly Hyriidae and Mycetopodidae (Pereira et al., 2014), compared to about 300 species in North America (Vaughn, 2018). However, many mussel species are declining worldwide (Lopes-Lima et al., 2018), resulting in losses of both biodiversity and function in aquatic ecosystems (Vaughn, 2010, 2018). Thus, measurements of mussel ecological functions are important for understanding, managing and conserving their benefits in aquatic ecosystems (Ferreira-Rodríguez et al., 2019).
Unionida freshwater mussels generally have a patchy distribution (Vaughn & Hakenkamp, 2001), occurring in areas that provide refuge during periods of high flow, i.e., depositional areas with low shear velocity and stable sediments, that are also important for macroinvertebrate diversity (Simeone et al., 2018). In the Amazon, large rivers have mussel beds that are discrete, relatively distant from one another, and distributed over a wide spatial scale (Pereira et al., 2014). Variation in patterns of mussel distribution and macroinvertebrate composition may be associated with different hydrodynamic conditions that create patches of suitable and unsuitable habitats (Simeone et al., 2018). For example, sites without mussels and low macroinvertebrate abundance and diversity were found in high hydrodynamic areas, but stable low hydrodynamic areas may either support intermediate levels of macroinvertebrates where mussel beds are absent, or where present, higher macroinvertebrate abundance and diversity (Simeone et al., 2018). The present study aimed to focus on these suitable stable habitats with low hydrodynamics to further explore the relationship between macroinvertebrates and mussels. For this purpose, we used sites with mussel beds (mussel sites) and sites where mussels were absent (no mussel sites) in a single river in the eastern Amazon, in order to better control for habitat conditions during fieldwork. We were interested in verifying whether the presence or absence of mussels would be associated with differences in macroinvertebrate structure, hydrological variables, primary production, and organic matter in the sediment (Vaughn & Hakenkamp, 2001; Spooner & Vaughn, 2012; Vaughn, 2018). Laboratory experiments measuring mussel clearance rates (the decrease in microalgal density in the water column due to mussel filtering; Tuttle-Raycraft & Ackerman, 2018) and biodeposition (organic matter deposited in the form of feces and pseudofeces) were used to check whether these variables corroborated with observations in the field. We hypothesized that the presence of mussels would be associated with increased macroinvertebrate abundance, number of taxa and changes in the river food web associated with an increased number of functional groups. We expected patterns in macroinvertebrate structure to be associated with mussel ecological functions such as filtration, which decreases algae and suspended solids in the water column, and biodeposition, which increases organic matter content in the sediment (Atkinson et al., 2011).
Methods
Study area and mussel beds
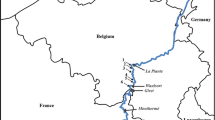
Field sampling took place in the middle course of the Caeté River, an alluvial lowland river approximately 150 km long, located in northeastern Pará state, in the eastern Brazilian Amazon (Fig. 1). The climate is tropical and humid, with annual rainfall between 2,500 and 3,000 mm, of which 70% falls between January and April, and a dry season from July to December, with an average monthly rainfall of 60 mm (Moraes et al., 2005). There is marked seasonality in river hydrology, which is a feature of Amazonian rivers (Junk, 1997), with an average discharge (± SD) of 48.3 ± 11.5 m3/s (range 33–67 m3/s) in the rainy season and 8.4 ± 2.9 m3/s (range 5–15 m3/s) in the dry season. The Caeté has a predominantly meandering morphology, with a sinuosity index of 61.1 and a low channel slope. The landscape consists of secondary forest floodplain, with small scattered human settlements, subsisting mainly by fishing and family based farming (Simeone et al., 2018). Since large human settlements are scarce along the Caeté River, fluvial habitats are in relatively natural conditions and there have been no artificial modifications to the channel along the course of the river. In the Caeté River, Castalia ambigua Lamarck, 1819 (Hyriidae) predominates in mussel beds, whereas Anodontites elongates (Swainson, 1823) (Mycetopodidae) is found at lower densities (Table 1). In a stretch of approximately 20 km (Fig. 1b), we selected five mussel beds (Fig. 1c) that occur as discrete aggregations. In these sites (mussel sites) the sediment, depth, water flow and near-bed (1 cm) shear velocity were similar at a variety of discharge levels (Table 1). To test our hypothesis about the association of mussels with the macroinvertebrate assemblage structure, ecosystem function and habitat quality, we selected five sites where mussels were absent (no mussel sites) for comparison with mussel sites. These sites were similar to the mussel sites in terms of area, river canopy cover, substrate composition and hydrodynamic conditions at low and high flows (Table 1), in order to minimize any effects of habitat heterogeneity.
Study area in the eastern Amazon region of northern Brazil (a) in the middle course of the Caeté River, approximately 30 km upstream of the City of Bragança, in Pará state (b), with location of the five sites with mussels (mussel site), the five sites without mussels (no mussel site), and the single site for mussel sampling used in the laboratory experiments (c)
Field sampling design
We carried out sampling for quantifying the macroinvertebrate assemblage structure and measuring environmental variables between November and December 2017, when water levels were sufficiently low to allow access and robust quantitative sampling. At each site, we established one 20-m transect and randomly placed 20 replicate plots of 1 m2 located 0.5 m from each other, to avoid spatial autocorrelation (Braun et al., 2012) and cover all microhabitats, resulting in a total sample size of 200 (10 sites × 20 replicates). Sampling was carried out in the upstream direction, where environmental variables were sampled first to avoid potential interference from downstream disturbance of the water column (Richter et al., 2016).
Measurement of the environmental variables
For each replicate plot, we measured a set of hydrological variables, including pH, dissolved oxygen (mg/L), and electrical conductivity (μS/cm). The measurements were taken at around 9 a.m. using a digital probe (Hanna Instruments, Woonsocket, USA), at the bottom of the water column, to avoid differences caused by daily variation. We measured concentrations of nitrate (NO3, mg/L) and phosphate (PO4, mg/L) in the water column using the Prodac Test kit (Prodac International, Cittadella, Italy) following the manufacturer's usage information. To determine primary production at each site, we collected five replicates of water at each site to quantify chlorophyll-a (µg/L), which were kept in containers with ice. In the laboratory, 100 mL of each water replicate was filtered through a GF/F filter and frozen for 24 h. Chlorophyll-a was extracted with acetone and analyzed spectrophotometrically (American Public Health Association, APHA, 1995). To quantify the sediment organic matter (g/0.1 m2), we randomly sampled five replicates of sediment at each site using a 0.1 m2 quadrat. Twenty grams of the dry sediment (40 °C for 48 h) was ignited in a muffle furnace at 550 °C for 4 h, and reweighed after ignition, the difference being the weight of organic matter (0.001 g precision) in the sediment.
Sampling of macroinvertebrates
To determine the abundance and composition of macroinvertebrates at each replicate plot, we manually disturbed the substrate for 1 min using a reinforced rectangular (30 by 15 cm) hand net with 300 µm mesh to a depth of approximately 15 cm. The sediment sampled for macroinvertebrates was immediately placed in individual plastic bags, tagged and preserved in 70% alcohol. In the laboratory, each replicate of sediment was washed through a 300 µm mesh to remove fine particles and coarse debris. Under the stereomicroscope, macroinvertebrates were counted and identified. Chironomidae and Naididae were identified to subfamily, but other macroinvertebrates were identified to genus and their functional feeding groups using a recent trophic classification of Amazonian macroinvertebrates (Hamada et al., 2014). As macroinvertebrates were identified to different taxonomic levels, we used taxa to describe these operational taxonomic units (subfamily/genus).
Clearance rate and biodeposition laboratory experiments
We carried out experiments to measure the clearance rates (L/mussel/h) and biodeposition (g/mussel/h) by mussels in December 2018. Prior to the experiments, we cultivated two genera of microalgae, Chlorella sp. and Scenedesmus sp., in the laboratory as food for the mussels, using a liquid NPK (nitrogen, phosphorus and potassium) gardening supplement, which resulted in excellent microalgal growth in our study.
Only specimens of C. ambigua were used in the experiments, since this is the prevailing species in all Caeté River mussel beds (Simeone et al., 2018, 2021). Mussels were collected from a single site (Fig. 1c) by excavating the sediment by hand to a depth of approximately 15 cm, where 40 mussels with similar shell lengths were obtained to avoid bias in size (mean length of 27.5 ± 0.77 mm, range 26–28.5 mm). We carefully transported the mussels to the laboratory, where they acclimatized for 4 days in a constantly aerated aquarium containing river water and a 20 cm sediment bed. For the clearance rate and biodeposition experiments, we used two different density treatments (1 and 3 mussels, equivalent to 4 and 10 mussels per m2; Vaughn et al., 2004) with five replicates for each treatment and five control replicates without mussels. After all experiments ended, the mussels were returned to their original habitat, with no record of mortality during or after the experiments.
Clearance rate
Prior to the experiment, we gently scrubbed each mussel shell to remove any attached periphyton. For both treatments, mussels were placed in beakers with a 10 cm sediment bed and filled with 0.5 L of the cultivated microalgae (estimated biomass of 5.7 mg/L of microalgae). The experiment began when mussels were observed filtering with their filtration apertures open (Supplementary Fig. 1). We carefully took 1 mL of water from each beaker, which was fixed in 5% formalin to measure the initial microalgal density at the beginning of the experiment and again, after 1 h, to measure the final microalgal density. The initial and final microalgal densities were estimated using a Neubauer chamber under an optical microscope. To calculate the mussel clearance rate (CR) we used the following equation modified from Coughlan (1969):
where Vol is the beaker volume (0.5 L), n is the number of mussels (1 and 3), t is the duration of the experiment (1 h), ln is the natural logarithm, and Di/Df and D′i/D′f are the initial (i) and final (f) microalgal densities in replicates with either 1 or 3 mussels per beaker (D), and the control (D′), respectively.
Biodeposition
We carried out a separate experiment to obtain a baseline estimate of mussel biodeposition. We conducted the experiment under no-flow conditions so as not to confound biodeposition with potential transport of organic matter in the current (Vaughn et al., 2004). For both treatments, mussels were placed in beakers with a 10 cm deep clean sediment bed. Prior to the experiment, the sediment was incinerated in a muffle furnace, at 550 °C for 4 h, to sterilize and completely remove any organic material not associated with mussel deposition. Mussels were fed with 0.5 L of the cultivated microalgae and allowed to filter for 1 h (Howard & Cuffey, 2006). After the filtering period ended, mussels were removed from the beakers. We filtered the water from each beaker using a 300 µm mesh and carefully pipetted the biodeposits; afterwards, we removed 5 cm of the surficial sediment to obtain feces and pseudofeces suspended in the water column and deposited onto the sediment, respectively. The sediment together with the biodeposits (see Supplementary Fig. 1) were dried at 40 °C for 48 h, ignited in a muffle furnace at 550 °C for 4 h and reweighed after ignition. The difference was the weight of the biodeposits (0.001 g precision). We determined biodeposition, controlled for microalgae sedimentation, as the difference between organic matter mass from mussel treatments and controls.
Statistical analysis
For each macroinvertebrate replicate, we obtained the total abundance, the number of taxa and the number of functional feeding groups. We used classification trees, which perform unbiased partitioning based on regression that has a strong predictive performance in selecting important associated variables (Hothorn et al., 2006), to perform a forward selection of macroinvertebrate assemblage and functional group metrics that best explained differences between mussel and no mussel sites. The mincriterion function, which defines the significance value for tree splitting, was used as the stopping criterion with a significance of P < 0.05. Prior to analysis, the data distributions for all macroinvertebrate metrics and environmental variables were examined using box-plots and histograms. The most appropriate model to test the hypothesis was selected according to the data distribution. A negative binomial distribution best described all macroinvertebrate metrics. For the other variables, the data followed a normal distribution. Assumptions of homogeneity of variances, normality and uniformity of residual distributions were checked using residual plots after modeling (Zuur et al., 2009). All normal data were analyzed without the need for transformation.
To test whether the presence of mussels was associated with an increase in macroinvertebrate abundance and number of taxa, we used a generalized linear mixed-effects model (GLMM) for repeated measures (family = nbinom2, the negative binomial distribution) to take into account possible non-independence of the 20 randomly selected replicates (plots) within each site, and potential pseudoreplication (Pinheiro & Bates, 2000). Mixed-effects models provide a flexible and powerful tool for modeling the within-group correlation often present in repeated measures data (Pinheiro & Bates, 2000; Zuur et al., 2009). We corrected the model using the functions ziformula and dispformula to minimize any effects of zero-inflation and overdispersion of the data, respectively (Zuur et al., 2009). With this model, we tested for differences in the total abundance, number of taxa and the macroinvertebrate metrics selected with the classification trees. We used site (mussel and no mussel) as the fixed effect and the repeated measures (replicates) at the 20 plots in each site as the random effect. We used the coefficient of determination (R2) to quantify the contributions to the GLMM model fit.
We performed a redundancy analysis (RDA) using the complete macroinvertebrate assemblage dataset as response variables, to describe the association of environmental variables with the macroinvertebrate assemblage and functional groups between mussel and no mussel sites. RDA is a constrained analysis which combines regression and principal component analysis to explore the relationships between a response and an explanatory matrix (Bocard et al., 2011). Only the macroinvertebrate metrics selected by the classification trees that were most associated with differences between mussel and no mussel sites were labeled on the RDA ordination. We tested for high collinearity of environmental variables using the criterion of variance inflation factors (VIF). As recommended by Bocard et al. (2011), a VIF ≥ 10 indicates high collinearity and we found that no variables were highly correlated, therefore, all variables were maintained in our RDA model. Afterwards, we tested the significance of the RDA model to verify whether the effect of the environmental variables on macroinvertebrates was associated with the presence of mussels by subjecting the RDA eigenvalues to a bootstrap permutation test (999 runs) with a significance of P < 0.05. We calculated the coefficient of determination (R2) for each environmental variable to quantify their contributions to the RDA model fit. We formally tested the environmental variables with an R2 > 0.45 using the mixed-effects model for repeated measures (family = gaussian, the normal distribution). Finally, to verify for correspondence with environmental variables measured in the field, we used an analysis of variance (ANOVA) model to test for differences in the experimental values of clearance rate and biodeposition between the controls and mussel density treatments. Where significant, a post hoc Tukey HSD test for pairwise comparisons was carried out between the control and each treatment and between both treatments (three comparisons in total).
All analyses were carried out in GNU R 4.0.1 (R Core Team, 2020) using the packages vegan (Oksanen et al., 2019) for RDA and VIF, glmmTMB (Brooks et al., 2017) for the mixed-effects model with repeated measures, party (Hothorn et al., 2006) for the classification trees, and bootstrap (Tibshirani & Leisch, 2019) for the bootstrap permutation test.
Results
Field observations of macroinvertebrate assemblage and environmental variables
A total of 2,786 individuals were distributed among 26 taxa and 5 functional groups of macroinvertebrates (Supplementary Table 1). The classification trees selected the abundance of three trichopteran taxa (Marilia, Oecetis and Antarctoecia) and of two macroinvertebrate functional groups (shredders and predators) that best explained the variation between mussel and no mussel sites (Supplementary Fig. 2). Higher abundances of shredders and predators were associated with mussel sites in most of the replicates (Supplementary Fig. 2). Although not selected by the classification tree, abundance of scrapers was also associated with mussel sites (Supplementary Table 1). All macroinvertebrate metrics were consistently and significantly higher at mussel sites. The number of taxa, abundance of Marilia (shredder), and abundance of predators and shredders had the greatest effects in the comparisons between mussel and no mussel sites (Fig. 2a–d). In addition, the genus Oecetis (predator) occurred exclusively at mussel sites (Supplementary Table 1; Fig. 2e). Higher total macroinvertebrate abundance, and abundances of Antarctoecia and scrapers were also significantly associated with mussel sites (Fig. 2f–h). GLMM R2 values for macroinvertebrate metrics varied from 0.11 to 0.66 (Fig. 2).
Median (± median absolute deviation) of the total abundance, number of taxa, and the macroinvertebrate taxa and functional groups selected by the classification tree, sampled from the five sites with mussels (mussel site; n = 100) and the five sites without mussels (no mussel site; n = 100) in the middle course of the Caeté River, Pará, Brazil. Macroinvertebrate metrics were placed in order of decreasing effect size of the GLMM analysis (with P and R2 values). Degrees of freedom: Site (1), Error (195)
On the RDA ordination plot (Fig. 3a), there was a clear division between mussel and no mussel sites, with higher abundance of trichopteran genera Marilia, Oecetis and Antarctoecia, and predators, shredders and scrapers associated with mussel sites (bootstrap significance; P = 0.017), where the first two axes together explained 84% of the total variation. Mussel sites were associated with low chlorophyll-a (RDA R2 = 0.52; GLMM F1,179 = 1,034, P < 0.0001, R2 = 0.84; Fig. 3b) in the water column and high organic matter in the sediment (RDA R2 = 0.47; GLMM F1,179 = 168, P < 0.0001, R2 = 0.46; Fig. 3c) in comparison with no mussel sites. The other environmental variables were less associated with differences between mussel and no mussel sites, with R2 values < 0.45 (Supplementary Table 2).
Redundancy analysis ordination plot (a) showing the association among environmental variables, mussels and the macroinvertebrate taxa. Environmental variable abbreviations: Chl-a (chlorophyll-a), OM (sediment organic matter), DO (dissolved oxygen), PO4 (phosphate), EC (electrical conductivity) and NO3 (nitrate). Mean (± standard deviation) of (b) chlorophyll-a (µg/L) and (c) sediment organic matter (g/0.1 m2), sampled from the five sites with mussels (mussel site; n = 100) and the five sites without mussels (no mussel site; n = 100) in the middle course of the Caeté River, Pará, Brazil. All environmental vectors are shown. Only macroinvertebrate metrics selected by the classification trees are labeled on the RDA ordination
Clearance rate and biodeposition experiments
Laboratory experiments demonstrated that clearance rate (ANOVA F2,9 = 13, P = 0.002; Fig. 4a) and biodeposition (ANOVA F2,9 = 19, P = 0.0006; Fig. 4b) were consistently higher in treatments with mussels than in controls, significantly increasing with mussel density.
Mean (± standard deviation) of clearance rate (L/mussel/h) and organic matter deposition (g/mussel/h) from laboratory experiments with Castalia ambigua. Treatments were 1 Mussel (one mussel in each of the five replicates), 3 Mussels (three mussels in each of the five replicates), and five control replicates representing the no mussel site. Letters indicate post hoc Tukey HSD comparisons where different letters indicate significant differences between the control and each treatment and between both treatments
Discussion
This is the first use of both field and experimental observations to assess the association between freshwater mussels, macroinvertebrate assemblage structure and ecological functioning in a Neotropical river. As predicted, the presence of mussels was positively associated with higher macroinvertebrate abundance and number of taxa. Even in areas with suitable habitats for mussels, as found at no mussel sites, the establishment of mussel populations may depend on factors such as dispersal by host fish, or flow conditions around these habitats (Morales et al., 2006). In the Caeté River, substrate stability, a higher proportion of silt and low hydrodynamic conditions near the riverbed are associated with greater macroinvertebrate diversity (Simeone et al., 2018). In our study, macroinvertebrate diversity at no mussel sites increased when compared with areas of high hydrodynamics in the Caeté River (Simeone et al., 2018). However, diversity is even greater at mussel sites, which may support our hypothesis.
In Europe, studies comparing plots with and without mussels show that there were no differences in the number of macroinvertebrate taxa (Richter et al., 2016). However, in North America, patterns similar to those found in our study were observed, especially for Chironomidae, which had the highest abundance at sites with mussels (Spooner & Vaughn, 2006; Vaughn & Spooner, 2006). In contrast, our findings show that the abundance of Chironomidae, especially the subfamily Chironominae, was not associated with differences between mussel and no mussel sites. Organic matter deposited by mussels in the sediment may enhance the abundance of detritivorus invertebrates (Vaughn & Spooner, 2006). Most species of Chironominae in the Amazon region are infaunal (Hamada et al., 2014) and generally feed by collecting small organic debris in the sediment. Furthermore, this group is tolerant of habitat disturbance and is equally capable of colonizing habitats with high and low water quality (Hamada et al., 2014). Although sediment organic matter and water quality differed between mussel and no mussel sites, abundance of Chironominae was similar (Supplementary Table 1). In the Caeté River, Chironominae is associated with low pH and high electrical conductivity in meanders with strong habitat heterogeneity (Simeone et al., 2018). However, under relatively equal habitat conditions in the present study, these variables were not correlated with mussel presence and absence nor macroinvertebrate assemblage structure. Associations between mussels and Chironominae may occur at the species rather than at the level of the subfamily, but this was not assessed in our study.
Mussels were associated with higher trichopteran faunal and functional diversity, especially the genera Oecetis (predator), Marilia (shredder) and Antarctoecia (collector). Trichopterans have high species and functional diversity in Amazonian rivers and streams, preferring well-conserved habitats with high resource availability and benthic structural complexity (Hamada et al., 2014), which may be introduced by mussels (Vaughn, 2018) and are important for a more diverse macroinvertebrate fauna (Bódis et al., 2014; Ilarri et al., 2018; Simeone et al., 2021). Studies in temperate rivers have shown that macroinvertebrates, especially collectors and predators, are more abundant in mussel beds (Howard & Cuffey, 2006). Our results, in a Neotropical river highlighted a novel pattern, involving shredders as one of the most important functional groups associated with Amazonian mussels. From the four taxa identified as shredders, the trichopterans Marilia and Nectopsyche had the greatest abundance associated with mussel sites. These taxa feed by fragmenting organic matter (Hamada et al., 2014), and our results suggest that they may be using feces and pseudofeces, deposited by mussels, as food. Mussel sites on the Caeté were associated with higher sediment organic matter (Simeone et al., 2018; present study), which along with higher organic matter content in mussel treatments in our laboratory experiment, provide some evidence to support this hypothesis and corroborates similar studies in North American rivers (Vaughn & Hakenkamp, 2001; Vaughn, 2018). In addition, higher benthic algal biomass associated with mussels (Atkinson et al., 2021) may also be an important source of organic matter for shredders. River canopy cover is relatively low at our sites, with no significant accumulation of leaves on the riverbed (Simeone et al., 2018) that could serve as food for shredders (Hamada et al., 2014). Thus, high abundance of shredders in mussel sites is likely associated with organic matter originating from the presence of mussels. However, our experiment may have relatively high sedimentation of algae and seston due to the absence of flow, different to biodeposition under flow conditions in the field, and explains the presence of organic matter in our controls. In addition, removal of mussels after 1 h may not be sufficient to allow their guts to completely clear, which may lead to less biodeposition in mussel treatments. Nevertheless, organic matter observed in controls without mussels were much lower than in mussel treatments, which supports our hypothesis of greater biodeposition of organic matter associated with the presence of mussels.
Also associated with greater macroinvertebrate abundance and number of taxa, predators were consistently more abundant at mussel sites. In particular, the genus Oecetis was abundant in the presence of mussels and was absent from no mussel sites. An increase in predators due to greater prey availability is a well known feature of aquatic ecology (Holomuzki et al., 2010). Howard & Cuffey (2006) also found greater abundance of predators in mussel beds in temperate rivers, mainly due to increased abundance of their collector prey; however, in our study, predators were mostly associated with shredders.
Mussels are efficient filter-feeders, removing a large amount of suspended material and microalgae from the water column (Vaughn & Hakenkamp, 2001; Tuttle-Raycraft & Ackerman, 2018; Zieritz et al., 2020), with an estimated filtration rate of ~ 0.5 to 1 L of water per individual per hour (Vaughn et al., 2008). In our study, we did not measure filtration rates (the volume of water filtered by mussels; Vaughn & Hakenkamp, 2001), however, we observed that low values of chlorophyll-a were associated with mussel sites, supporting our hypothesis that mussel filtering would reduce suspended solids. Powerful effects of clearance rates were also demonstrated in laboratory experiments involving the North American (Vaughn et al., 2004; Atkinson et al., 2011; Tuttle-Raycraft & Ackerman, 2018) and European (Douda & Čadková, 2018) mussel faunas. Our work quantified laboratory clearance rates for the first time in an Amazonian mussel, C. ambigua, which is widely distributed in South America (Pereira et al., 2014) and is the prevailing species in Caeté River mussel beds (Simeone et al., 2018, 2021). Our experimental findings demonstrated that mussel treatments were associated with low values of microalgae, with the clearance rate increasing with mussel density. Mussel filtration has the greatest effect on water quality in dense mussel beds (Buelow & Waltham, 2020; Simeone et al., 2021), with individual mussel capacities associated with their physiology or food availability (Vaughn, 2018). In our study, mussels cleared more algae as a group than a single mussel. The increased clearance rate per mussel, in the high mussel density treatment, may be associated with adjustments in mussel feeding rates based on resource concentrations (decreasing algal concentrations over time in our experiment) to optimize food acquisition (Bril et al., 2014).
In our clearance control, there was no significant decrease in microalgae concentrations linked with the absence of mussel filtering activity, similarly observed in other studies (Vaughn et al., 2004; Douda & Čadková, 2018). High concentrations of phytoplankton or suspended solids in the water column may decrease the penetration of light to the riverbed (Junk, 1997) at no mussel sites. In contrast, at mussel sites, higher light penetration due to lower water turbidity associated with mussel filtering, may stimulate periphyton growth on mussel shells and substrate (Spooner & Vaughn, 2006, 2012), serving as food for macroinvertebrates (Atkinson & Vaughn, 2015; Benelli et al., 2019). When precipitation and flow are low, water takes longer to move downstream, allowing mussels to have significant local effects on habitat functioning (Vaughn et al., 2004). Caeté mussel beds are located in areas with low flow and shear velocity (Simeone et al., 2018), which may facilitate decreases in chlorophyll-a by mussel filtering. Although we did not quantify periphyton on mussel shells and sediment, scrapers, especially the trichopterans Protoptila (Glossosomatidae) and Helicopsyche (Helicopsychidae), and probably shredders, may respond more positively to benthic periphyton associated with mussels (Vaughn & Hakenkamp, 2001). The classification of macroinvertebrates into functional groups is a work in progress since a single species may have different feeding adaptations or belong to more than one functional group (Ramírez & Gutiérrez-Fonseca, 2014). Indeed, this may lead to misclassifications in macroinvertebrate functional groups, which may explain the non-inclusion of scrapers in the classification tree, despite the association of periphyton with mussels.
In the Caeté River, macroinvertebrate assemblages may be driven by different factors at different spatial scales. For example, at a broader scale, hydrodynamic conditions may create patches of suitable and unsuitable habitats for macroinvertebrates and mussels (Simeone et al., 2018). At a smaller scale, within these suitable habitats (present study), mussels are positively associated with increased macroinvertebrate abundance and number of taxa, especially with the trichopterans which are shredders, scrapers and predators, contrasting from patterns found in North American and European rivers. On the other hand, suitable habitats without mussels are associated with a different, less diverse and abundant macroinvertebrate assemblage. Mussel filtration and biodeposition, associated with decreased chlorophyll-a in the water column and increased organic matter in the sediment may suggest a mode of causality between mussel presence and enhanced macroinvertebrate assemblage structure. Though our study is restricted in geographical scope and mussel species, an important association is highlighted and more investigations into potential links among mussels, habitat function and aquatic biodiversity are needed in the Neotropical region (Ferreira-Rodríguez et al., 2019). In summary, filtration and biodeposition by freshwater mussels provide important ecological functions that appear to be associated with enhanced aquatic biodiversity in mussel beds in an eastern Amazonian river. This association highlighted that combined effects of potentially biotic (our study) and abiotic (Simeone et al., 2018) factors may be associated with the macroinvertebrate assemblage structure in a Neotropical river, increasing the already high conservation priority for freshwater mussels and riverine habitat.
Data availability
Data associated with this manuscript are available on Figshare (https://doi.org/10.6084/m9.figshare.14787813.v1)
References
Allen, D. C. & C. C. Vaughn, 2011. Density-dependent biodiversity effects on physical habitat modification by freshwater bivalves. Ecology 92: 1013–1019.
American Public Health Association, 1995. Standard Methods for the Examination of Eater and Wastewater. American Public Health Association, Washington, DC.
Atkinson, C. L. & C. C. Vaughn, 2015. Biogeochemical hotspots: temporal and spatial scaling of the impact of freshwater mussels on ecosystem function. Freshwater Biology 60: 563–574.
Atkinson, C. L., H. M. Halvorson, K. A. Kuehn, M. Winebarger, A. Hamid & M. N. Waters, 2021. Filter-feeders have differential bottom-up impacts on green and brown food webs. Oecologia 195: 187–198.
Atkinson, C. L., M. R. First, A. P. Covich, S. P. Opsahl & S. W. Golladay, 2011. Suspended material availability and filtration–biodeposition processes performed by a native and invasive bivalve species in streams. Hydrobiologia 667: 191–204.
Atkinson, C. L., J. F. Kelly & C. C. Vaughn, 2014. Tracing consumer-derived nitrogen in riverine food webs. Ecosystems 17: 485–496.
Benelli, S., M. Bartoli, M. Zilius, I. Vybernaite-Lubiene, T. Ruginis, D. Vaiciute, J. Petkuviene & E. A. Fano, 2019. Stoichiometry of regenerated nutrients differs between native and invasive freshwater mussels with implications for algal growth. Freshwater Biology 64: 619–631.
Bocard, D., F. Gillet & P. Legendre, 2011. Numerical Ecology with R. Springer, New York.
Boeker, C., T. Lueders, M. Mueller, J. Pander & J. Geist, 2016. Alteration of physico-chemical and microbial properties in freshwater substrates by burrowing invertebrates. Limnologica 59: 131–139.
Bódis, E., B. Tóth, J. Szekeres, P. Borza & R. Sousa, 2014. Empty native and invasive bivalve shells as benthic habitat modifiers in a large river. Limnologica 49: 1–9.
Braun, A., K. Auerswald & J. Geist, 2012. Drivers and spatiotemporal extent of hyporheic patch variation: implications for sampling. PLoS ONE 7: 1–10.
Bril, J. S., J. J. Durst, B. M. Hurley, C. L. Just & T. J. Newton, 2014. Sensor data as a measure of native freshwater mussel impact on nitrate formation and food digestion in continuous-flow mesocosms. Freshwater Science 33: 417–424.
Brooks, M. E., K. Kristensen, K. J. van Benthem, A. Magnusson, C. W. Berg, A. Nielsen, H. J. Skaug, M. Maechler & B. M. Bolker, 2017. glmmTMB: balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9: 378–400.
Buelow, C. A. & N. J. Waltham, 2020. Restoring tropical coastal wetland water quality: ecosystem service provisioning by a native freshwater bivalve. Aquatic Sciences. https://doi.org/https://doi.org/10.1007/s00027-020-00747-7.
Coughlan, J., 1969. The estimation of filtering rate from the clearance of suspensions. Marine Biology 2: 356–358.
Douda, K. & Z. Čadková, 2018. Water clearance efficiency indicates potential filter-feeding interactions between invasive Sinanodonta woodiana and native freshwater mussels. Biological Invasions 20: 1093–1098.
Ferreira-Rodríguez, N., Y. B. Akiyama, O. V. Aksenova, R. Araujo, M. C. Barnhart, Y. V. Bespalaya, A. E. Bogan, I. N. Bolotov, P. B. Budha, C. Clavijo, S. J. Clearwater, G. Darrigran, V. T. Do, K. Douda, E. Froufe, C. Gumpinger, L. Henrikson, C. L. Humphrey, N. A. Johnson, O. Klishko, M. W. Klunzinger, S. Kovitvadhi, U. Kovitvadhi, J. Lajtner, M. Lopes-Lima, E. A. Moorkens, S. Nagayama, K.-O. Nagel, M. Nakano, J. N. Negishi, P. Ondina, P. Oulasvirta, V. Prié, N. Riccardi, M. Rudzite, F. Sheldon, R. Sousa, D. L. Strayer, M. Takeuchi, J. Taskinen, A. Teixeira, J. S. Tiemann, M. Urbanska, S. Varandas, M. V. Vinarski, B. J. Wicklow, T. Zajac & C. C. Vaughn, 2019. Research priorities for freshwater mussel conservation assessment. Biological Conservation 231: 77–87.
Gordon, N. D., T. M. McMahon, B. L. Finlayson, C. J. Gippel & R. J. Nathan, 2004. Stream Hydrology: An Introduction for Ecologists. Wiley, Chichester.
Hamada, N., J. L. Nessimian & R. B. Querino, 2014. Insetos aquáticos na Amazônia Brasileira: Taxonomia, biologia e ecologia. Editora INPA, Manaus.
Holomuzki, J. R., J. W. Feminella & M. E. Power, 2010. Biotic interactions in freshwater benthic habitats. Journal of the North American Benthological Society 29: 220–244.
Hothorn, T., K. Hornik & A. Zeileis, 2006. Unbiased recursive partitioning: a conditional inference framework. Journal of Computational and Graphical Statistics 15: 651–674.
Howard, J. K. & K. M. Cuffey, 2006. The functional role of native freshwater mussels in the fluvial benthic environment. Freshwater Biology 51: 460–474.
Ilarri, M. I., L. Amorim, A. T. Souza & R. Sousa, 2018. Physical legacy of freshwater bivalves: effects of habitat complexity on the taxonomical and functional diversity of invertebrates. Science of the Total Environment 634: 1398–1405.
Junk, W. J., 1997. The Central Amazon floodplain. Springer, New York.
Lopes-Lima, M., L. E. Burlakova, A. Y. Karatayev, K. Mehler, M. Seddon & R. Sousa, 2018. Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia 810: 1–14.
Lummer, E.-M., K. Auerswald & J. Geist, 2016. Fine sediment as environmental stressor affecting freshwater mussel behavior and ecosystem services. Science of the Total Environment 571: 1340–1348.
Moraes, B. C., J. M. N. Costa, A. C. L. Costa & M. Costa, 2005. Variação espacial e temporal da precipitação no Estado do Pará. Acta Amazônica 35: 207–214.
Morales, Y., L. J. Weber, A. E. Mynett & T. J. Newton, 2006. Effects of substrate and hydrodynamic conditions on the formation of mussel beds in a large river. Journal of the North American Benthological Society 25: 664–676.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Wagner, 2019. vegan: Community Ecology Package [available on internet at: http://CRAN.R-project.org/package=vegan].
Pereira, D., M. C. D. Mansur, L. D. S. Duarte, A. S. de Oliveira, D. M. Pimpão, C. T. Callil, C. Ituarte, E. Parada, S. Peredo, G. Darrigran, F. Scarabino, C. Clavijo, G. Lara, I. C. Miyahira, M. T. R. Rodriguez & C. Lasso, 2014. Bivalve distribution in hydrographic regions in South America: historical overview and conservation. Hydrobiologia 735: 15–44.
Pinheiro, J. C. & D. M. Bates, 2000. Mixed-Effects Models in S and S-PLUS. Springer, New York.
R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna [available on internet at: http://www.R-project.org/].
Ramírez, A. & P. E. Gutiérrez-Fonseca, 2014. Functional feeding groups of aquatic insect families in Latin America: a critical analysis and review of existing literature. Revista de Biología Tropical 62: 155-167.
Richter, A., K. Stoeckl, M. Denic & J. Geist, 2016. Association between the occurrence of the thick-shelled river mussel (Unio crassus) and macroinvertebrate, microbial, and diatom communities. Freshwater Science 35: 922–933.
Simeone, D., C. Santos, F. Gisane, C. H. Tagliaro & C. R. Beasley, 2018. Greater macroinvertebrate diversity and freshwater mussel density in meander margins of an Amazon river. Freshwater Biology 63: 1118–1129.
Simeone, D., C. H. Tagliaro & C. R. Beasley, 2021. Amazonian freshwater mussel density: a useful indicator of macroinvertebrate assemblage and habitat quality. Ecological Indicators. https://doi.org/https://doi.org/10.1016/j.ecolind.2020.107300.
Spooner, D. E. & C. C. Vaughn, 2006. Context-dependent effects of freshwater mussels on stream benthic communities. Freshwater Biology 51: 1016–1024.
Spooner, D. E. & C. C. Vaughn, 2012. Species’ traits and environmental gradients interact to govern primary production in freshwater mussel communities. Oikos 121: 403–416.
Strayer, D. L., 2008. Freshwater Mussel Ecology: A Multifactor Approach to Distribution and Abundance. University of California Press, Los Angeles.
Strayer, D. L., 2014. Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia 735: 277–292.
Tibshirani, R. & F. Leisch, 2019. bootstrap: Functions for the Book “An Introduction to the Bootstrap” [available on internet at: https://CRAN.R-project.org/package=bootstrap].
Tichý, L., 2016. Field test of canopy cover estimation by hemispherical photographs taken with a smartphone. Journal of Vegetation Science 27: 427–435.
Tuttle-Raycraft, S. & J. D. Ackerman, 2018. Does size matter? Particle size vs. quality in bivalve suspension feeding. Freshwater Biology 63: 1560–1568.
Vaughn, C. C., 2010. Biodiversity losses and ecosystem function in freshwaters: emerging conclusions and research directions. BioScience 60: 25–35.
Vaughn, C. C., 2018. Ecosystem services provided by freshwater mussels. Hydrobiologia 810: 15–27.
Vaughn, C. C. & C. C. Hakenkamp, 2001. The functional role of burrowing bivalves in freshwater ecosystems. Freshwater Biology 46: 1431–1446.
Vaughn, C. C. & D. E. Spooner, 2006. Unionid mussels influence macroinvertebrate assemblage structure in streams. Journal of the North American Benthological Society 25: 691–700.
Vaughn, C. C., K. B. Gido & D. E. Spooner, 2004. Ecosystem processes performed by unionid mussels in stream mesocosms: species roles and effects of abundance. Hydrobiologia 35: 35–47.
Vaughn, C. C., S. J. Nichols & D. E. Spooner, 2008. Community and foodweb ecology of freshwater mussels. Journal of the North American Benthological Society 27: 409–423.
Volkmer-Ribeiro, C., M. C. D. Mansur, D. Pereira, J. S. Tiemann, K. S. Cummings & M. H. Sabaj, 2019. Sponge and mollusk associations in a benthic filter-feeding assemblage in the middle and lower Xingu River, Brazil. Proceedings of the Academy of Natural Sciences of Philadelphia 166: 1–24.
Zieritz, A., F. N. Mahadzir, W. N. Chan & S. McGowan, 2019. Effects of mussels on nutrient cycling and bioseston in two contrasting tropical freshwater habitats. Hydrobiologia 835: 179–191.
Zieritz, A., W. N. Chan, S. McGowan & C. Gibbins, 2020. High rates of biodeposition and N-excretion indicate strong functional effects of mussels (Bivalvia: Unionida) in certain anthropogenic tropical freshwater habitats. Hydrobiologia. https://doi.org/https://doi.org/10.1007/s10750-020-04464-y.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Savaliev & G. M. Smith, 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York.
Acknowledgements
This work was carried out as a part of DS’s Ph.D. degree in Environmental Biology, Universidade Federal do Pará (UFPA). DS thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a postgraduate scholarship and the Programa de Pós-Graduação em Biologia Ambiental at UFPA for logistic support. We are grateful to Ádila Kelly Rodrigues da Costa (UFPA) for help with the chlorophyll-a analysis. We also thank Jaqueline Feitosa, Jeovana Lima and Lilian Amorim (UFPA) for help with the laboratory experiments and the people of the Arimbu and Mocajuba settlements on the Caeté River for their kind assistance. Fieldwork was carried out under License 21187-1 from the Instituto Chico Mendes de Conservação da Biodiversidade – ICMBio.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: María del Mar Sánchez-Montoya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simeone, D., Tagliaro, C.H. & Beasley, C.R. Filter and deposit: a potential role of freshwater mussels in ecosystem functioning associated with enhanced macroinvertebrate assemblage structure in a Neotropical river. Hydrobiologia 848, 4211–4223 (2021). https://doi.org/10.1007/s10750-021-04633-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04633-7