Abstract
Purpose
Currently, regenerative endodontic treatments are gaining more and more attention, and stem cells play a significant role in these treatments. In order to enhance stem cell proliferation and differentiation, a variety of methods and materials have been used. The purpose of this study was to determine the effects of magnesium oxide nanoparticles and LED irradiation on the survival and differentiation of human stem cells from apical papilla.
Methods
The MTT test was used to measure the cell survival of SCAPs that had been exposed to different concentrations of magnesium oxide nanoparticles after 24 and 48 h, and the concentration with the highest cell survival rate was picked for further studies. The cells were classified into four distinct groups based on their treatment: (1) control, which received no exposure, (2) exposure to magnesium oxide nanoparticles, (3) exposure to light emitting diode (LED) irradiation (635 nm, 200 mW/cm2) for 30 s, (4) exposure simultaneously with magnesium oxide nanoparticles and LED irradiation. A green approach was employed to synthesize magnesium oxide nanoparticles. Quantitative real time PCR was used to measure the gene expression of osteo/odontogenic markers such as BSP, DSPP, ALP and DMP1 in all four groups after treatment, and Alizarin red S staining (ARS) was used to determine the osteogenic differentiation of SCAPs by demonstrating the Matrix mineralization.
Results
The highest viability of SCAPs was observed after 24 h in concentration 1 and 10 µg/mL and after 48 h in concentration 1 µg/mL, which were not significantly different from the control group. In both times, the survival of SCAPs decreased with increasing concentration of magnesium oxide nanoparticles (MgONPs). According to the results of Real-time PCR, after 24 and 48 h, the highest differentiation of BSP, DMP1, ALP and DSPP genes was observed in the LED + MgONPs group, followed by MgONPs and then LED, and in all 3 experimental groups, it was significantly higher than control group (P < 0.05). Also, after 24 and 48 h, the density of ARS increased in all groups compared to the control group, and the highest density was observed in the MgONPs + LED and MgONPs groups.
Conclusion
This research concluded that exposure to SCAPs, MgONPs, and LED irradiation has a significant effect on enhancing gene expression of odontogenic/osteogenic markers and increasing matrix mineralization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After trauma or dental carries, the pulp of the developing permanent tooth may become damaged, potentially causing inflammation and necrosis of the pulp. The death of odontoblasts and the interruption of root development is caused by pulp necrosis (Albuquerque et al. 2014). The classical technique for treating immature permanent teeth with pulp necrosis is Apexification, wherein calcium hydroxide paste is used to generate apical barrier or mineral trioxide aggregate (MTA) plug is utilized for apical seal (Lin et al. 2017). Despite its success, one of the major issues with Apexification is its inhibition of root development in both the longitudinal and transverse directions, resulting in shortened roots with thin walls that are particularly vulnerable to fracture (Llaquet et al. 2017). In contrast to the traditional treatments that do not provide tissue regeneration, regenerative treatments provide a solution which consists of replacing the pulp-dentin complex, enabling it to proliferate and operate in a physiological manner (Murray et al. 2007). Stem cells, characterized by their capacity to remain in an undifferentiated state and respond to both internal and external signals by undergoing proliferation and differentiation, play a crucial role in various biological processes. Pluripotent cells, a subset of adult (somatic) stem cells with remarkable adaptability, are particularly noteworthy. These pluripotent cells are commonly found in bone marrow and can take the form of hematopoietic, endothelial, and mesenchymal (stromal) stem cells (MSCs) (Karkehabadi et al. 2023a; Padial-Molina et al. 2015). Beyond bone marrow, research has identified alternative sources of MSCs in adults, such as adipose tissues known as adipose-derived stem cells (ASCs), as well as locations like the lung and specific dental structures, including the perivascular niche of dental pulp and the periodontal ligament within teeth. Recognizing this diverse array of MSC sources highlights the potential for exploring and utilizing their regenerative capabilities in various therapeutic applications (Karkehabadi et al. 2023d; Padial-Molina et al. 2015).
Various stem cell populations have been identified within dental structures, including dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), stem cells from apical papilla (SCAP), and gingiva-derived mesenchymal stem cells (GMSCs). These mesenchymal stem cells (MSCs) exhibit unique properties that make them integral to the regeneration of various compromised or absent periodontal tissues. In vitro studies have demonstrated that, when exposed to specific stimuli, mesenchymal stem cells derived from dental tissues possess the capability to generate dental tissues such as dentin, cementum, and bone (Kobolak et al. 2016; Liu et al. 2015; Rodríguez-Lozano et al. 2011).
The dental pulp of adult teeth, notably harboring dental pulp stem cells (DPSCs), stands as a pivotal source of oral mesenchymal stem cells with profound implications for regenerative dentistry. These cells have undergone meticulous scrutiny, revealing their extensive in-vitro multilineage differentiation potential spanning osteo/odontogenic, adipogenic, chondrogenic, neurogenic, angiogenic, and myogenic lineages. Complementing these in-vitro observations, in-vivo studies affirm their capacity to regenerate functional dentin/pulp complexes and various tissues, encompassing bone, cementum, blood vessels, and neural tissues(Bakopoulou et al. 2017).
In 1961, Dr. Nygaard Ostby (Nygaard 1961) was the first to propose tissue regeneration, a procedure through which a blood clot is formed in the pulp space to promote the generation of new tissue in the root canal. In addition to blood clot stability, fibrin plays a role as a scaffold for apical papilla stem cells (Llaquet et al. 2017). The scaffold is a necessity as well as certain factors to enable the differentiation of stem cells into odontoblasts (Wan et al. 2016). The primary components in the process of tissue engineering in order to generate a new tissue or organ are mesenchymal stem cells, morphogen growth factor, and scaffold (Gandhi et al. 2011). MSCs demonstrate the ability of self-renewal and multi-lineage differentiation, making them an essential part of tissue engineering (Fonticoli et al. 2022; Shaikh et al. 2023). These cells, which are in the apical papilla of immature permanent teeth, provide a source of primary odontoblasts, which continue the process of root development (Llaquet et al. 2017). The apical papilla is the soft tissue at the apices of developing permanent teeth. It assists in tooth formation and becomes the dental pulp tissue. Thus, Stem cell from the apical papilla can only be isolated at certain stages of tooth development. Sonoyama et al. (Sonoyama et al. 2006) were the first to report the isolation of such cells. Two reliable methods to obtain them exist. The first, consists of the tissue with collagenase type I followed by culture of the obtained cells in culture dishes. The second is based on the culture of small pieces of apical papilla samples on culture dishes, without digestion. Stem cell from the apical papilla are characterized by flow cytometry to identify specific stem cell surface markers. Therapeutic implications of Stem cell from the apical papilla are those related to the repair and the regeneration of different tissues such as pulp, dentin, root, periodontal tissue, bone, neurons, and blood vessels (Costela-Ruiz et al. 2022).
Biomaterials play an important role in regenerative endodontic treatments. Metal ions and compounds can be part of biomaterials. Since in regenerative treatments, cells are exposed to scaffolds and biomaterials, Biocompatibility of materials in regenerative treatments is necessary to maintain the ability of cell proliferation and differentiation (Laurenti & Cauda 2017; Trevino et al. 2011). Biomaterials can be used in the nano scale (< 1–100 nm). Nanoparticles have unique properties due to their small size and high surface-to-volume ratio, but the use of nanoparticles in high concentration can cause cytotoxicity. Therefore, it is imperative to select the accurate concentration (Dolai et al. 2021).
Magnesium is the fourth most abundant cation in the body, with an average concentration of 1% (wt/wt) in dentin. Magnesium has a profound influence on various cellular processes, including the regulation of cell mobility, energy metabolism, cell proliferation, and cell differentiation.
Photobiostimulation therapy employs light sources such as light-emitting diodes (LEDs) and low-level lasers (LLL) to promote cell growth, tissue regeneration, and healing. These light sources emit wavelengths within the visible red to near-infrared (NIR) range and collectively constitute Photobiomodulation (PBM) (Fekrazad et al. 2016; Khorsandi et al. 2020).
In-depth studies, both in vitro and in vivo, have explored the diverse effects of PBM. These effects span a spectrum from inhibitory to stimulatory, serving purposes such as pain control, anti-inflammatory response, and modulation of metabolic and immunological processes. Notably, PBM actively influences endogenous enzyme photoacceptors, initiating intricate cell signaling pathways(Fekrazad et al. 2016; Pinto et al. 2021). Recent investigations have illuminated the potential of light emitted from specific-wavelength light-emitting diodes (LEDs) to intricately influence the behavior of various cells through physical stimulation. Notably, this form of physical stimulation at distinct wavelengths has been shown to induce apoptosis in cancer cells, exerting precise control over their growth. Moreover, this light-induced stimulation has the fascinating ability to guide stem cells towards specific differentiation pathways (Ahrabi et al. 2019; Cho et al. 2023; Hanna et al. 2021). Extracellular substances traditionally undergo active rather than passive transport for cellular uptake. However, an intriguing phenomenon emerges under light irradiation, where the cell membrane facilitates the translocation of extracellular substances without reliance on active transport mechanisms. This phenomenon is attributed to the heightened sensitivity of the phospholipid bilayer, constituting the primary boundary of the cell membrane, to LED light of specific wavelengths (Muley et al. 2020; Wan et al. 2020). Furthermore, the light-induced modulation of cell membrane behavior plays a pivotal role in expediting the delivery of various substances, including nanoparticles (NPs). The swift absorption and intracellular delivery of substance-loaded nanomaterials stand as critical factors in the precise manipulation of cell behavior(Cho et al. 2023).
Our prior study involved the investigation of effects of CuONPs and LED irradiation on proliferation and osteogenic/odontogenic differentiation of stem cells from the apical papilla (SCAPs) (Karkehabadi et al. 2023b). Following the noticed advantageous effects of the copper nanoparticle, we set out to examine the influence of Magnesium Oxide Nanoparticles (MgONPs) and Light-Emitting Diodes (LEDs) on the proliferation and osteogenic/odontogenic differentiation of Stem Cells from the Apical Papilla (SCAPs). This investigation aimed to fill a gap in existing literature, as this specific interaction had not been previously explored. We hypothesized that a positive effect would be observed, suggesting an enhancing influence of MgONPs and LEDs on the mentioned cellular processes.
Materials and methods
Isolation and culture of SCAPs
Following the Guidelines for Stem Cell Research and Clinical Translation of the International Society for Stem Cells Research (ISSCR), the experiment was performed. The Ethics Committee of Hamadan University of Medical Sciences granted permission for the protocol of this study (IR.UMSHA.REC.1402.660).
To attain the SCAPs, we followed the same method as our preceding study, using the intact upper left and right third permanent molars taken from a healthy 18 year old patient. Over two-thirds of their roots had matured (Karkehabadi et al. 2023c).
The patient provided a written informed consent prior to the incorporation of this research process. A dental tweezer was implemented to differentiate the apical papilla from the apical area of teeth that had not yet fully developed. The SCAPs were obtained by enzyme digestion and cultured in congruence with protocols written previously (Garrido et al. 2021; Karkehabadi et al. 2023c; Rahmati et al. 2022). Collection of the stem cells was accomplished through the use of a solution comprised of 3 mg/mL type I collagenase (Worthlington Biomedical, Lakewood, NJ, USA) and 1 mol/L phosphate-buffered saline (PBS) (Worthlington Biomedical, Lakewood, NJ). Subsequently, the cells were transferred to Dulbecco’s modified Eagle’s medium (Gibco, GrandIsland, NY, USA) at a temperature of 37 °C for a period of one hour. They were stored at a temperature of 37 °C and a relative humidity of 85%, and a carbon dioxide concentration of 5%. The sterile cell culture flasks (SPL Life Science, Gyeonggi-do, South Korea) were supplemented with a combination of 15% fresh bovine serum and 1% penicillin and streptomycin. Taking into account the design and size of past in vitro studies, three repetitions were carried out in both control and experimental groups (Bakopoulou et al. 2011; Karkehabadi et al. 2023b; Liang et al. 2017).
Assuring the stemness of cells
When the flask had 85% confluence, the medium was taken away and the cells were given a double rinse with phosphate buffered solution (PBS). The detachment of the cells using trypsin/EDTA was followed by the incorporation of the medium culture into the flask. Fifteen milliliters of culture medium and cells were put into a Falcon tube and centrifuged at 1200×g for six minutes. After two rinses with PBS, the cell sediment was put through flow cytometry to detect specific stem cell surface markers (CD105 and CD90) and hematopoietic cell surface markers (CD45 and CD34). The cells displayed a favorable outcome for mesenchymal cell surface markers, whereas the hematopoietic cell surface markers were absent.
Experimental groups
The SCAPs were divided into four distinct groups based on the type of treatment they received: (I) no-treatment control, (II) exposure to MgONPs, (III) LED irradiation (635 nm, 200 mW/cm2) for 30 s, and (IV) a combination of MgONPs and LED irradiation.
Preparation of MgONPs
By utilizing the extract from pomegranate peels, a green method was embraced to synthesize MgONPs in compliance with the prior protocol (Karkehabadi et al. 2023b; Sankar et al. 2014; Suresh et al. 2014). An analysis of the properties of MgONPs was conducted using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM).
The process of synthesizing magnesium oxide nanoparticles required the systematic washing of pomegranate peels using deionized water, followed by shadow drying for a period of 14 days. Following the washing of 100 mg of pomegranate peels with double distilled water, the peels were then cut and subjected to a hot air drying process in an oven. Subsequent to the drying process being completed, the materials were ground into a fine dust and mixed with distilled water (10 g dust/100 mL distilled water) in an Erlenmeyer flask, then boiled for 10 min at 60 °C. The filtration was carried out utilizing Whatman filter paper No.1. The filtrate was freeze-dried for preservation and kept at 4 degrees Celsius.
A magnetic stirrer was used at 60 °C to combine 90 mL of an analytical grade solution of cupric sulphate (5 mM) with 20 mL of filtrate obtained in a magnetic stirrer with deionized water. The mixture was kept at ambient temperature while stored. A brownish-black sediment was produced in the conical flask over time. Following that process, it was desiccated and preserved for the goal of utilizing it as a green synthesized MgONPs.
A Perkin 118 was employed to carry out Fourier transform infrared spectroscopy (FT-IR) to ascertain the surface chemistry of NPs. Chattopadhyay et al. (Chattopadhyay et al. 2015)conducted an analysis of the hydrodynamic sizes and zeta potential distribution of nanoparticles with the use of the Zetasizer-Nano ZS (Malvern, Malvern Hills, U.K.).
X-Ray diffraction (XRD) study of green synthesized MgONPs
An X-ray powder diffraction study of NPs was performed in a solid state. The diffractometer XPERT-PRO was used to figure out diffraction patterns (PANalytical Ltd., The Netherlands) according to the Das et al. method (Das et al. 2017).
The surface morphology and particle size of magnesium oxide nanoparticles were established with the help of high resolution scanning electron microscopy (Hitachi S-3400N).
Methyl thiazolyl tetrazolium (MTT) assay
To evaluate the impact of MgONPs on cell viability and decide on the appropriate concentration, 1*104 cells were incubated in each well of a 96-well plate (n = 12). The plates were placed in the incubator (Binder, NY, USA)at a temperature of 37 °C and humidity of 96% for a period of 24 h. The cells were randomly allocated to six groups and 100 µL of the culture medium with MgO concentrations of 1, 10, 100, 200 and 500 µg/mL was added to each well in a sterile condition.
The plates were placed in an incubator for 24 and 48 h. Following the removal of the plates from the incubator, 10 mL of MTT solution and 90 mL of alpha-MEM culture medium containing 10% fetal bovine serum were added to each well, and the plates were incubated again at an environment of 37 °C, 95% humidity and 5% CO2 for a period of 4 h. The top layer was then cautiously eliminated, and 100 mL of dimethyl sulfoxide (Gibco BRL, Grand Island, NY, USA) were added to each well. Following the breaking down of formazan crystals, the optical density values were studied using an ELISA Reader (BioTek, USA) in a wavelength scope of 540–690 nm (Saberi et al. 2016).
LED irradiation
The methyl thiazolyl tetrazolium (MTT) assay was used to first ascertain the most effective concentration of MgONPs. This concentration of MgONPs was added to the cultured cells before LED irradiation, 30 min in advance. Following this, the cells were irradiated with LED light in a semi-dark atmosphere with a single gap between the wells. The Fotosan 630 LED (Fotosan 630, MDD, CMS Dental Denmark) was used, with a 1 mm2 diameter end tip, emitting light of a wavelength range between 620–640 nm (85%) with a peak at 630 nm, having an intensity of 200 mW/cm2 and energy density (fluence) of 4 mJ/cm2, irradiated for a period of 30 s. The expected energy level, as well as the formula (energy density = power density × irradiation time), is detailed according to the research from prior studies (Beolchi et al. 2015). The initial irradiation was conducted 24 h after the primary cell culture had been established, while a second irradiation was administered 48 h later. The same operator managed all irradiations.
Assessment of osteogenic/odontogenic differentiation by real-time polymerase chain reaction (PCR)
An assessment of osteogenic/odontogenic differentiation was conducted in each group after 24 and 48 h, with and without LED irradiation. At each time interval, six wells were allocated for evaluation to each group (Bakopoulou et al. 2016).
Trizol reagent (Invitrogen, CA, USA) was employed to extract RNA from the stem cells found in the medium. After that, cDNA synthesis was done by using the Superscript II first-strand cDNA synthesis kit (Invitrogen, CA, USA) in line with the manufacturer’s instruction. Following that, a reverse transcription PCR experiment was conducted (7500 Fast Real-Time PCR System for dentin sialophosphoprotein (DSPP), bone sialoprotein (BSP), alkaline phosphatase (ALP), and dentin matrix acidic phosphoprotein 1 (DMP1).
Alizarin red staining (ARS)
In order to analyze the odontogenic/osteogenic differentiation of cells qualitatively, the cells were seeded in 96-well plates after being subject to MgONPs and LED irradiation (n = 24). Subsequently, they were cultivated in an osteogenic/odontogenic environment with 10 mM beta glycerophosphate (Sigma Aldrich, St. Louis, MO, USA), 10 nm dexamethasone (Sigma Aldrich, St. Louis, MO, USA), and 50 mg/mL L-ascorbic acid. The cells were stabilized with 4% paraformaldehyde. After a period of 21 days, they were rinsed with phosphate buffered saline (PBS) and subjected to 1% alizarin red stain (Sigma Aldrich, St. Louis, MO, USA) at 37 °C for 30 min to identify the formed mineral nodules. An assessment was carried out via the SpectraMax M2 spectrophotometer at the 562 nm wavelength (Pereira et al. 2012).
Statistical analysis
Analysis of data was carried out using SPSS version 18 (SPSS Inc., Chicago, IL). The Kruskal–Wallis test and Mann–Whitney U post hoc test were implemented to analyze the data. For alizarin red staining, the data were interpreted as present/absent, and the chi-square test was used for analysis. The statistical significance was accepted at 0.05.
Results
Characterization of MgONPs
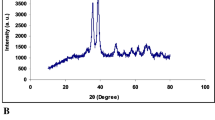
Figure 1 shows the XRD pattern of MgO nanoparticles synthesized from magnesium nitrate (II) trihydrate and pomegranate extract. The formation of synthesized MgO nanoparticle was confirmed by X-ray diffraction measurement. The diffraction peaks at 31.22°, 35.87°, 38.92°, 49.42°, 53.87°, 58.42°, 62.02°, 66.820°, 68.37° and 73.07° in Fig. 1 correspond to (100), (002), (200), (202), (020), (202), (113), (022), (020), (311), and (004), respectively (30–32).
Characterization of nanoparticles. A: XRD pattern of MgO nanoparticles, the XRD pattern of MgONPs synthesized from magnesium nitrate (II) trihydrate and pomegranate peel extract. (The formation of synthesized MgO nanoparticle was confirmed by X-ray diffraction measurement. The diffraction peaks at 31.22°, 35.87°, 38.92°, 49.42°, 53.87°, 58.42°, 62.02°, 66.820°, 68.37° and 73.07° in Fig. 1 correspond to (100), (002), (200), (202), (020), (202), (113), (022), (020), (311), and (004), respectively)
Figure 2 shows the FTIR spectrum of synthesized magnesium oxide nanoparticles. Based on the FTIR spectrum of nanoparticles, the most significant absorption peak in the region at 524 cm−1 is related to the stretching vibration of the Mg-O bond (20). Strong absorptions at 1041 cm−1 can be related to stretching vibrations related to C-O in the structure of the carboxylic group and flavonoids in the structure of the plant extract. The characteristic peak observed at 1382 cm−1 is due to C-N stretching vibration in the amine group [34]. The strong absorption band in the region of 1623 cm−1 is due to the bending vibration of the C = C bond. Absorption at 2917 cm−1 is also related to asymmetric and symmetric C-H stretching caused by phenolic compounds. Also, the broad band observed at 3402 cm−1 is due to the stretching vibrations of the hydroxyl group O–H (21).
The surface morphology of the synthesized MgO nanoparticles was studied by SEM and the results are presented in Fig. 3. The SEM image reveals that the synthesized nanoparticles exhibit quasi-spherical shapes with some degree of aggregation. The isolated cells had fibroblast-like morphology (spindle-shaped) with homogeneity (Fig. 4). Flow cytometric analysis using antibodies against CD45, CD34, CD90, and CD105 showed stromal surface markers on the cytoplasmic membrane of these cells. The results of flow cytometry showed the expression of a high proportion of the mentioned cell markers (Fig. 5).
In vitro assessment of cell viability
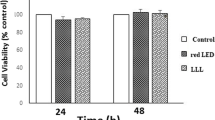
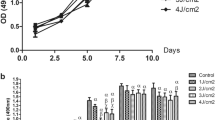
Results from Fig. 6 indicated that an elevated concentration of magnesium oxide nanoparticles led to a notable decrease in the survival rate of apical papilla stem cells, displaying a significant difference compared to the control group at both the 24 and 48-h intervals (p < 0.05). Tukey’s test and a two-by-two comparison revealed that, after 24 h, concentrations of 1 and 10 μg/mL, and after 48 h at a concentration of 1 μg/mL, stem cell survival was comparable to the control. However, at higher concentrations, the survival of stem cells was significantly lower than that of the control group (p < 0.05).Therefore, in the continuation of the study, we used a concentration of 1 μg/mL of MgONPs to investigate the osteo/odontogenic differentiation.
Cell viability of the groups at 24 and 48 h. ***and ###: P < 0.001.** and ##: P < 0.01. *and #: P < 0.05. The higher concentrations of magnesium oxide nanoparticles resulted in a significant reduction in the survival rate of apical papilla stem cells compared to the control group at both 24 and 48 h (p < 0.05). Tukey’s test and a two-by-two comparison indicated that at concentrations of 1 and 10 μg/mL after 24 h, and at 1 μg/mL after 48 h, stem cell survival was comparable to the control. However, at higher concentrations, stem cell survival markedly decreased, significantly differing from the control group (p < 0.05)
Quantitative real-time PCR
According to the results of Fig. 7, after 24 and 48 h, the highest expression of all genes was observed in the LED + MgONPs group, then in the MgONPs group, and finally in the LED group (P < 0.05). The control group had the lowest expression of genes.
The expression profile of SCAPs treated with LED, MgONPs, and LED + MgONPs. The relative mRNA expressions of genes were compared among the three groups and also with the control group (undifferentiated SCAPs). A: DSPP, B: DMP1, C: BSP, D: ALP, after 24 and 48 h, the highest expression of all genes was observed in the LED + MgONPs group, then in the MgONPs group, and finally in the LED group (P < 0.05). The control group had the lowest expression of genes
Alizarin red S staining
ARS indicates calcium deposition in the extracellular matrix. According to Fig. 8, after 24 and 48 h, the density of ARS increased in all experimental groups compared to the control group, and LED + MgONPs and MgONPs groups showed the highest density. In other words, exposure to MgONPs and LED irradiation increased bone mineralization.
Discussion
In this study, we investigated the effect of magnesium oxide nanoparticles and LED irradiation on survival and odontogenic/osteogenic differentiation of SCAPs. Due to their anatomical placement and critical function in regenerative endodontic treatments, SCAPs were the suitable choice for this research. (Theocharidou et al. 2017). Furthermore, SCAPs demonstrated a dramatically increased rate of multiplication, tissue regeneration ability, and a larger amount of STRO-1 positive cells compared to dental pulp stem cells (DPSCs) (Karkehabadi et al. 2022; Karkehabadi et al. 2023b; Radio et al. 2006).
Materials that are measured to contain at least one dimension in the range of 1–100 nm are classified as nanomaterials. Apart from their distinctive properties, these materials may also cause a certain degree of cytotoxicity. Numerous approaches have been proposed concerning the cytotoxicity linked to nanomaterials. It is theorized that exposure to nanoparticles can cause a rise in oxidative stress and upset calcium homeostasis intracellularly, ultimately leading to cellular destruction, apoptosis, and cell cycle disruption (Huang et al. 2017). To minimize the level of cytotoxicity caused by exposure to nanoparticles in this study, we initially exposed SCAPs to different concentrations of nanoparticles with the MTT assay to select the maximum cell survival after 24 and 48 h. MTT is a widely used technique to measure the survival of cells, and to assess cell toxicity (Chang et al. 2014a, b; Karkehabadi et al. 2018). According to the results of the MTT assay in Fig. 4, with the increase in the concentration of magnesium oxide nanoparticles, after 24 and 48 h, the survival of SCAPs decreased, and the highest survival of cells was observed at a concentration of 1 μg/mL. Consequently, a concentration of 1 μg/mL was utilized in the following study to evaluate the result of nanoparticles and LED irradiation on odontogenic/osteogenic differentiation.
Results similar to these were seen in a separate study that evaluated different concentrations of MgONPs in the form of a cell scaffold; it was observed that the proliferation rate of bone marrow mesenchymal stem cells at a concentration of under 1 μg/mL was similar to the control sample (Wetteland et al. 2016). Due to discrepancies in particle size and cell type, the safe concentration may differ between studies (Gurunathan et al. 2019).
The Real-Time Quantitative Reverse Transcription PCR results in Fig. 5 showed that the MgONPs + LED group had the highest gene expression of odontogenic/osteogenic markers, followed by the MgONPs group. These findings are comparable to experiments in which the odontogenic differentiation of DPSCs was notably increased when magnesium phosphate (MgP) was incorporated into the cell scaffold structure. (Ahmed et al. 2023; Farag et al. 2022; Qu et al. 2014). The Mg ions were thought to play a role in the activation of ALP (alkaline phosphatase) and the progression of the crystallization and pattern formation processes of the inorganic mineral phase, which were the basis for these results(Salem et al. 2021). In accordance with the current results, a study which looked into the impact of MgONPS on the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs), noted that MgONPs enhance the expression of genes and proteins associated with osteogenesis differentiation. These results were found to be caused by the activation of canonical Wnt/β-catenin and BMP signaling pathways that were activated by MgONPs(Babuska et al. 2022; Bozorgi et al. 2021; Raghav et al. 2022; Wetteland et al. 2016).
Studies have revealed that magnesium deficiency causes a rise in osteoclast formation, causing a decrease in bone mass, abnormal bone growth and a weakened skeletal structure. (Belluci et al. 2013; Martiniakova et al. 2022; Xing et al. 2022). Supplementation of magnesium to phosphate glasses and ceramics boosts the osteogenesis associated bioactivity of scaffolds (Gu et al. 2022; Martiniakova et al. 2022).
According to the study of Yu et al.(Yu et al. 2017), The addition of Mg and Zn ions on the titanium surfaces of dental implants promotes the adhesion and proliferation of rat bone marrow mesenchymal stem cells. In addition, these ions promoted bone formation, angiogenesis and osseointegration by augmenting the amounts of OCN (osteocalcin) and ALP (alkaline phosphatase) (Yu et al. 2017). The results of the current study are in agreement with the positive effects of magnesium ion on bone formation as demonstrated in aformentioned studies. As opposed to the present study, a low concentration of magnesium nanoparticles was incorporated to reduce the cytotoxicity and to benefit from the nano structure.
As Fig. 5 demonstrated, the effect of MgONPs on gene expression was enhanced by LED irradiation, with maximal success in the combination of MgONPs and LED irradiation. LLLT has been determined to be of assistance in the treatment of many diseases. It has been ascertained that following treatment with low level laser therapy, a rise in mitochondrial activity, ATP production, DNA and RNA synthesis has been observed. In addition, the synthesis of reactive oxygen species (ROS) and the release of nitric oxide (NO) elevates, hence bringing about an upsurge in cell proliferation and survival. Low-Level Laser Therapy has been proven to be successful in diminishing pain, inflammation and promoting wound healing. It increases the migration, proliferation and survival of stem cells and the expression of proteins and stimulates cell differentiation of progenitor cells (Abrahamse 2012).
In accordance with the current findings, LLLT has been seen to induce an upturn of bone formation, angiogenesis and fibroblasts proliferation (Khadra et al. 2004). Furthermore, low level laser irradiation has been seen to improve bone regeneration and reduce inflammation levels (Pretel et al. 2007).
Research that included the utilization of both LLLT and mesenchymal stem cells (MSCs) for bone repair demonstrated that combining these two resulted in a heightened level of new bone formation in comparison to MSCs utilized by themselves (Fekrazad et al. 2015). Research has demonstrated that the application of LLLT in cell culture results in an increase in proliferation and differentiation of stem cells, greater secretion of growth factors, and heightened levels of calcium and alkaline phosphatase enzyme activity (Eduardo et al. 2008; Kim et al. 2009).
An additional study revealed that LED irradiation on Mg-based and Zn-doped bioceramic scaffolds could enhance the odontogenic differentiation and biomineralization of hDPSCs (Theocharidou et al. 2017).
The results of the present study concerning the positive effect of LED irradiation in regard to odontogenic/osteogenic differentiation was not far from what was expected, yet the observation of better results in the MgONPs + LED combination group compared to LED irradiation alone, could point to a synergistic effect of the two in regenerative endodontics.
According to Fig. 6, the density of ARS has increased in all experimental groups within 24 and 48 h, compared to the results of the control group. The greatest density was seen in the MgONPs and MgONPs + LED groups. In summary, MgONPs had a positive effect on bone mineralization. In this situation, MgONPs have been used to enhance calcium deposition in BMSCs (Wetteland et al. 2016).
Limitations and avenues for future studies
Time Constraints: The present study faced limitations in terms of time constraints, impacting the depth of analysis and the exploration of more extended time points. Future studies should allocate adequate time to ensure a comprehensive understanding of the long-term effects.
Financial Constraints: Financial limitations hindered the inclusion of certain analyses, such as Western blot tests for assessing synthesized proteins and signaling pathways. Future research with expanded resources could delve into these molecular aspects for a more thorough investigation.
Short Interval Irradiation: Due to financial considerations, the study utilized shorter intervals (24, 48 h) for stem cell irradiation. Future investigations should explore diverse time intervals, aligning with the broader spectrum suggested in systematic reviews (6–48 h), to discern nuanced effects (Borzabadi-Farahani 2016; Marques et al. 2016).
Cell Expansion Challenges: The study emphasized the necessity of cell expansion to obtain sufficient quantities for clinical applications, particularly given the limited number of stem cells in the apical zone of the tooth. Future research should address strategies for efficient and rapid cell expansion.
Mechanistic Understanding: While the study examined the impact of nanoparticles and LED irradiation on SCAP proliferation and differentiation, the exact underlying mechanisms remain unclear. Future studies should delve into the molecular mechanisms involved, contributing to a more comprehensive understanding of these regenerative processes.
Functional Analysis: The study did not conduct functional analyses, such as assessing cell migration, homing, and apoptosis. Future research should incorporate these functional aspects to provide a holistic evaluation of the effects of LED and nanoparticles on SCAPs.
Clinical Translation: The study highlighted the challenge of providing cells for injured tissue promptly. Future research should focus on bridging the gap between laboratory findings and clinical applications, exploring the translational potential of SCAP-based regenerative therapies.
Diversity in Nanoparticle Effects: Future studies could investigate the diverse effects of different nanoparticles, considering variations in size, composition, and surface properties. This would contribute to a nuanced understanding of the role of nanoparticles in stem cell responses(Patil et al. 2022; Tizu et al. 2022).
In summary, addressing these limitations and exploring the outlined avenues for future studies will contribute to advancing the field, fostering a more robust understanding of the therapeutic applications of Stem Cells from the Apical Papilla (SCAPs) in conjunction with LED irradiation and nanoparticles.
Conclusion
The current results suggest that the combination of magnesium oxide nanoparticles and LED irradiation in the culture medium of stem cells from the apical papilla plays a crucial role in promoting the survival and osteogenic/odontogenic differentiation of these cells.
Data availability
The complete documentation of participant enrolled in this study belongs to the corresponding author, Roshanak Abbasi, and is available only upon reasonable request.
Abbreviations
- SCAPs:
-
Stem cells from the apical papilla
- MTT :
-
Methyl thiazolyl tetrazolium
- ISSCR:
-
International Society for Stem Cells Research
- qrt-PCR:
-
Quantitative reverse-transcription polymerase chain reaction
- ARS:
-
Alizarin red staining
- LLL:
-
Low level laser
- LLLI:
-
Low level laser irradiation
- LLLT:
-
Low level laser therapy
- ALP :
-
Alkaline phosphatase
- DSPP:
-
Dentin sialophosphoprotein
- DMP1:
-
Dentin matrix protein 1
- BSP:
-
Bone sialoprotein
- DPSCs:
-
Dental pulp stem cells
- hPDL:
-
Human periodontal ligament
- hPDLSCs:
-
Human periodontal ligament stem cells
- h:
-
Hour
- LED:
-
Light-emitting diode
- PBS:
-
Phosphate-buffered saline
- SPSS:
-
Statistical Package of the Social Sciences
- Fig.:
-
Figure
- CuONPs :
-
Copper oxide nanoparticles
- MgONPs:
-
Magnesium oxide nanoparticle
- CuO:
-
Copper oxide
- MgO:
-
Magnesium oxide
- XRD:
-
X-ray diffraction
- FT-IR:
-
Fourier-transform infrared spectroscopy
- SEM:
-
Scanning electron microscopy
References
Abrahamse H (2012) Regenerative medicine, stem cells, and low-level laser therapy: future directives. Photomed Laser Surg 30(12):681–682
Ahmed B, Ragab MH, Galhom RA, Hassan HY (2023) Evaluation of dental pulp stem cells behavior after odontogenic differentiation induction by three different bioactive materials on two different scaffolds. BMC Oral Health 23(1):1–13
Ahrabi B, Tavirani MR, Khoramgah MS, Noroozian M, Darabi S, Khoshsirat S, Abbaszadeh HA (2019) The effect of photobiomodulation therapy on the differentiation, proliferation, and migration of the mesenchymal stem cell: a review. J Lasers Med Sci 10(Suppl 1):S96
Albuquerque MT, Valera MC, Nakashima M, Nor JE, Bottino MC (2014) Tissue-engineering-based strategies for regenerative endodontics. J Dent Res 93(12):1222–1231. https://doi.org/10.1177/0022034514549809
Babuska V, Kasi PB, Chocholata P, Wiesnerova L, Dvorakova J, Vrzakova R, Nekleionova A, Landsmann L, Kulda V (2022) Nanomaterials in bone regeneration. Appl Sci 12(13):6793
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W (2011) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56(7):709–721
Bakopoulou A, Papachristou E, Bousnaki M, Hadjichristou C, Kontonasaki E, Theocharidou A, Papadopoulou L, Kantiranis N, Zachariadis G, Leyhausen G (2016) Human treated dentin matrices combined with Zn-doped, Mg-based bioceramic scaffolds and human dental pulp stem cells towards targeted dentin regeneration. Dent Mater 32(8):e159–e175
Bakopoulou A, Apatzidou D, Aggelidou E, Gousopoulou E, Leyhausen G, Volk J, Kritis A, Koidis P, Geurtsen W (2017) Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res Ther 8(1):1–21
Belluci MM, Schoenmaker T, Rossa-Junior C, Orrico SR, de Vries TJ, Everts V (2013) Magnesium deficiency results in an increased formation of osteoclasts. J Nutr Biochem 24(8):1488–1498
Beolchi RS, Moura-Netto C, Palo RM, Torres CRG, Pelissier B (2015) Changes in irradiance and energy density in relation to different curing distances. Braz Oral Res 29:1–7
Borzabadi-Farahani A (2016) Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J Photochem Photobiol, B 162:577–582
Bozorgi A, Khazaei M, Soleimani M, Jamalpoor Z (2021) Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomater Sci 9(13):4541–4567
Chang S-W, Lee S-Y, Ann H-J, Kum K-Y, Kim E-C (2014a) Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J Endodont 40(8):1194–1200
Chang S-W, Lee S-Y, Kum K-Y, Kim E-C (2014b) Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. J Endodont 40(1):113–118
Chattopadhyay S, Dash SK, Tripathy S, Das B, Mandal D, Pramanik P, Roy S (2015) Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chem Biol Interact 226:58–71
Cho HB, Kim HJ, Lee S, Kim H-R, Lee S, Park J-I, Park K-H (2023) Reduction of nanoparticle size and promotion of cell membrane permeability by LED irradiation. Mater Today Nano 24:100397
Costela-Ruiz VJ, Melguizo-Rodríguez L, Bellotti C, Illescas-Montes R, Stanco D, Arciola CR, Lucarelli E (2022) Different sources of mesenchymal stem cells for tissue regeneration: a guide to identifying the most favorable one in orthopedics and dentistry applications. Int J Mol Sci 23(11):6356
Das B, Tripathy S, Adhikary J, Chattopadhyay S, Mandal D, Dash SK, Das S, Dey A, Dey SK, Das D (2017) Surface modification minimizes the toxicity of silver nanoparticles: an in vitro and in vivo study. J Biol Inorg Chem 22:893–918
Dolai J, Mandal K, Jana NR (2021) Nanoparticle size effects in biomedical applications. ACS Appl Nano Mater 4(7):6471–6496
Eduardo FDP, Bueno DF, de Freitas PM, Marques MM, Passos-Bueno MR, Eduardo CDP, Zatz M (2008) Stem cell proliferation under low intensity laser irradiation: a preliminary study. Lasers Surg Med 40(6):433–438
Farag MM, Beherei H, Al-Rashidy ZM, Farag DB, Salem ZA (2022) Dental pulp stem cell viability and osteogenic potential assessment of new Mg-phosphate magnetic bioceramic nanoparticles. J Mater Res 1–13
Fekrazad R, Ghuchani MS, Eslaminejad M, Taghiyar L, Kalhori K, Pedram M, Shayan A, Aghdami N, Abrahamse H (2015) The effects of combined low level laser therapy and mesenchymal stem cells on bone regeneration in rabbit calvarial defects. J Photochem Photobiol, B 151:180–185
Fekrazad R, Asefi S, Allahdadi M, Kalhori KA (2016) Effect of photobiomodulation on mesenchymal stem cells. Photomed Laser Surg 34(11):533–542
Fonticoli L, Della Rocca Y, Rajan TS, Murmura G, Trubiani O, Oliva S, Pizzicannella J, Marconi GD, Diomede F (2022) A narrative review: gingival stem cells as a limitless reservoir for regenerative medicine. Int J Mol Sci 23(8):4135
Gandhi A, Gandhi T, Madan N (2011) Dental pulp stem cells in endodontic research: a promising tool for tooth tissue engineering. RSBO Revista Sul-Brasileira De Odontologia 8(3):335–340
Garrido M, Morales D, Saldías MP, Fernández C, Villalobos V, Cerda O, Cáceres M (2021) Cellular response of human apical papilla cells to calcium hydroxide and tricalcium silicate-based cements. BMC Oral Health 21:1–8
Gu X, Li Y, Qi C, Cai K (2022) Biodegradable magnesium phosphates in biomedical applications. J Mater Chem B
Gurunathan S, Kang M-H, Jeyaraj M, Kim J-H (2019) Differential cytotoxicity of different sizes of graphene oxide nanoparticles in leydig (TM3) and sertoli (TM4) cells. Nanomaterials 9(2):139
Hanna R, Dalvi S, Amaroli A, De Angelis N, Benedicenti S (2021) Effects of photobiomodulation on bone defects grafted with bone substitutes: a systematic review of in vivo animal studies. J Biophotonics 14(1):e202000267
Huang Y-W, Cambre M, Lee H-J (2017) The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int J Mol Sci 18(12):2702
Karkehabadi H, Yousefifakhr H, Zadsirjan S (2018) Cytotoxicity of endodontic irrigants on human periodontal ligament cells. Iran Endodontic J 13(3):390
Karkehabadi H, Ahmadyani E, Najafi R, Khoshbin E (2022) Effect of biodentine coated with emdogain on proliferation and differentiation of human stem cells from the apical papilla. Mol Biol Rep 49(5):3685–3692
Karkehabadi H, Abbasi R, Najafi R, Khoshbin E (2023a) The effects of melatonin on the viability and osteogenic/odontogenic differentiation of human stem cells from the apical papilla. Mol Biol Rep 50(11):8959–8969
Karkehabadi H, Rahmati A, Abbasi R, Farmany A, Najafi R, Behroozi R, Rezaei-Soufi L, Abbaspourrokni H (2023b) Effect of copper oxide nanoparticles and light-emitting diode irradiation on the cell viability and osteogenic/odontogenic differentiation of human stem cells from the apical papilla. BMC Oral Health 23(1):1–12
Karkehabadi H, Rahmati A, Abbasi R, Farmany A, Najafi R, Behroozi R, Rezaei-Soufi L, Abbaspourrokni H (2023c) Effect of copper oxide nanoparticles and light-emitting diode irradiation on the cell viability and osteogenic/odontogenic differentiation of human stem cells from the apical papilla. BMC Oral Health 23(1):249
Karkehabadi H, Zafari J, Khoshbin E, Abbasi R, Esmailnasab S, Doosti-Irani A (2023d) Effect of Low-level laser therapy on differentiation and proliferation of human dental pulp stem cells: a systematic review. J Lasers Med Sci 5:14
Khadra M, Kasem N, Haanæs HR, Ellingsen JE, Lyngstadaas SP (2004) Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 97(6):693–700
Khorsandi K, Hosseinzadeh R, Abrahamse H, Fekrazad R (2020) Biological responses of stem cells to photobiomodulation therapy. Curr Stem Cell Res Ther 15(5):400–413
Kim HK, Kim JH, Abbas AA, Kim D-O, Park S-J, Chung JY, Song EK, Yoon TR (2009) Red light of 647 nm enhances osteogenic differentiation in mesenchymal stem cells. Lasers Med Sci 24(2):214–222
Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A (2016) Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 99:62–68
Laurenti M, Cauda V (2017) ZnO nanostructures for tissue engineering applications. Nanomaterials 7(11):374
Liang Y, Leng R-X, Pan H-F, Ye D-Q (2017) Associated variables of myositis in systemic lupus erythematosus: a cross-sectional study. Med Sci Mon: Int Med J Exp Clin Res 23:2543
Lin J, Zeng Q, Wei X, Zhao W, Cui M, Gu J, Lu J, Yang M, Ling J (2017) Regenerative endodontics versus apexification in immature permanent teeth with apical periodontitis: a prospective randomized controlled study. J Endodont 43(11):1821–1827
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu G-T, Liang A, Liu S (2015) Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 33(3):627–638
Llaquet M, Mercadé M, Plotino G (2017) Regenerative endodontic procedures: a review of the literature and a case report of an immature central incisor. G Ital Endod 31(2):65–72
Marques MM, Diniz IMA, de Cara SPHM, Pedroni ACF, Abe GL, D’Almeida-Couto RS, Lima PLV, Tedesco TK, Moreira MS (2016) Photobiomodulation of dental derived mesenchymal stem cells: a systematic review. Photomed Laser Surg 34(11):500–508
Martiniakova M, Babikova M, Mondockova V, Blahova J, Kovacova V, Omelka R (2022) The role of macronutrients, micronutrients and flavonoid polyphenols in the prevention and treatment of osteoporosis. Nutrients 14(3):523
Muley H, Fado R, Rodriguez-Rodriguez R, Casals N (2020) Drug uptake-based chemoresistance in breast cancer treatment. Biochem Pharmacol 177:113959
Murray PE, Garcia-Godoy F, Hargreaves KM (2007) Regenerative endodontics: a review of current status and a call for action. J Endodont 33(4):377–390
Nygaard OB (1961) The role of the blood clot in endodontic therapy. Acta Odont Scand 19:323–342
Padial-Molina M, O’Valle F, Lanis A, Mesa F, Dohan Ehrenfest DM, Wang HL, Galindo-Moreno P (2015) Clinical application of mesenchymal stem cells and novel supportive therapies for oral bone regeneration. BioMed Res Int 2015
Patil RM, Deshpande PP, Aalhate M, Gananadhamu S, Singh PK (2022) An update on sophisticated and advanced analytical tools for surface characterization of nanoparticles. Surf Interf 102165
Pereira LO, Longo JPF, Azevedo RB (2012) Laser irradiation did not increase the proliferation or the differentiation of stem cells from normal and inflamed dental pulp. Arch Oral Biol 57(8):1079–1085
Pinto H, Goñi Oliver P, Sánchez-Vizcaíno Mengual E (2021) The effect of photobiomodulation on human mesenchymal cells: a literature review. Aesthetic Plast Surg 45:1826–1842
Pretel H, Lizarelli RF, Ramalho LT (2007) Effect of low-level laser therapy on bone repair: histological study in rats. Lasers Surg Med 39(10):788–796
Qu T, Jing J, Jiang Y, Taylor RJ, Feng JQ, Geiger B, Liu X (2014) Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration. Tissue Eng Part A 20(17–18):2422–2433
Radio NM, Doctor JS, Witt-Enderby PA (2006) Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J Pineal Res 40(4):332–342
Raghav PK, Mann Z, Ahlawat S, Mohanty S (2022) Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine. Eur J Pharmacol 918:174657
Rahmati A, Abbasi R, Najafi R, Rezaei-Soufi L, Karkehabadi H (2022) Effect of diode low level laser and red light emitting diode irradiation on cell proliferation and osteogenic/odontogenic differentiation of stem cells from the apical papilla. BMC Oral Health 22(1):1–9
Rodríguez-Lozano FJ, Bueno C, Insausti CL, Meseguer L, Ramírez MDC, Blanquer M, Marín N, Martínez S, Moraleda JM (2011) Mesenchymal stem cells derived from dental tissues. Int Endod J 44(9):800–806
Saberi EA, Karkehabadi H, Mollashahi NF (2016) Cytotoxicity of various endodontic materials on stem cells of human apical papilla. Iran Endodont J 11(1):17
Salem RM, Zhang C, Chou L (2021) Effect of magnesium on dentinogenesis of human dental pulp cells. Int J Biomat 2021
Sankar R, Manikandan P, Malarvizhi V, Fathima T, Shivashangari KS, Ravikumar V (2014) Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim Acta Part A Mol Biomol Spectrosc 121:746–750
Shaikh F, Langade N, Khan M, Muglikar S, Ansari NZ (2023) Mesenchymal stem cells and tissue engineering in dentistry
Sonoyama W, Liu Y, Fang D, Yamaza T, Seo B-M, Zhang C, Liu H, Gronthos S, Wang C-Y, Shi S (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1(1):e79
Suresh J, Yuvakkumar R, Sundrarajan M, Hong SI (2014) Green synthesis of magnesium oxide nanoparticles. Adv Mat Res 952:141
Theocharidou A, Bakopoulou A, Kontonasaki E, Papachristou E, Hadjichristou C, Bousnaki M, Theodorou G, Papadopoulou L, Kantiranis N, Paraskevopoulos K (2017) Odontogenic differentiation and biomineralization potential of dental pulp stem cells inside Mg-based bioceramic scaffolds under low-level laser treatment. Lasers Med Sci 32(1):201–210
Tizu M, Mărunțelu I, Cristea BM, Nistor C, Ishkitiev N, Mihaylova Z, Tsikandelova R, Miteva M, Caruntu A, Sabliov C (2022) PLGA nanoparticles uptake in stem cells from human exfoliated deciduous teeth and oral keratinocyte stem cells. J Funct Biomater 13(3):109
Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, Diogenes A (2011) Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod 37(8):1109–1115. https://doi.org/10.1016/j.joen.2011.05.013
Wan F, Gao L, Lu Y, Ma H, Wang H, Liang X, Wang Y, Ma C (2016) Proliferation and osteo/odontogenic differentiation of stem cells from apical papilla regulated by Zinc fingers and homeoboxes 2: an in vitro study. Biochem Biophys Res Commun 469(3):599–605
Wan Z, Zhang P, Lv L, Zhou Y (2020) NIR light-assisted phototherapies for bone-related diseases and bone tissue regeneration: a systematic review. Theranostics 10(25):11837
Wetteland CL, Nguyen N-YT, Liu H (2016) Concentration-dependent behaviors of bone marrow derived mesenchymal stem cells and infectious bacteria toward magnesium oxide nanoparticles. Acta Biomater 35:341–356
Xing F, Li S, Yin D, Xie J, Rommens PM, Xiang Z, Liu M, Ritz U (2022) Recent progress in Mg-based alloys as a novel bioabsorbable biomaterials for orthopedic applications. J Mag Alloys 10:1428
Yu Y, Jin G, Xue Y, Wang D, Liu X, Sun J (2017) Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater 49:590–603
Acknowledgements
The Vice-Chancellor of Research and Technology of the Hamadan University of Medical Sciences provided funding for this study (140211039634).
Funding
The Vice-Chancellor of Research and Technology of the Hamadan University of Medical Sciences provided funding for this study (140211039634).
Author information
Authors and Affiliations
Contributions
Conceptualization: HK; Methodology: RN, AF; Formal analysis and investigation: AR; Writing—original draft preparation: RA; Writing—review and editing: RA; Funding acquisition: HK; Resources: HA; Supervision: RB, LR.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no financial or proprietary interests in any material discussed in this article.
Ethics approval and consent to participate
The study was performed according to the ISSCR Guidelines for Stem Cell Research and Clinical Translation, and was approved by the ethics committee of the Hamadan University of Medical Sciences (IR.UMSHA.REC.1402.660). The procedure of this study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All procedures were explained to the patient that participated in this study. Participant consented to the use of the extracted teeth for research purposes prior to extraction at the department of oral and maxillofacial surgery, Hamadan University of Medical Sciences, and signed an informed consent form for this purpose.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karkehabadi, H., Rahmati, A., Abbaspourrokni, H. et al. Effect of magnesium oxide nanoparticles and LED irradiation on the viability and differentiation of human stem cells of the apical papilla. Biotechnol Lett 46, 263–278 (2024). https://doi.org/10.1007/s10529-024-03471-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-024-03471-6