Abstract
Background
Mesenchymal stem cell-based therapy is known to have the potential to induce angiogenesis. However, there are still some limitations regarding their clinical application. Photomodulation/photobiomodulation is non-invasive and non-toxic phototherapy able to stimulate cell viability, proliferation, differentiation, and migration, when the right irradiation parameters are applied. A review of the published articles on human conditioned-by-photobiomodulation mesenchymal cells in an in vitro set up was carried out. Our aim was to describe the studies' results and identify any possible tendency that might highlight the most suitable procedures.
Methods
A search in English of the PubMed database was carried out with the search criteria: photobiomodulation or photoactivation or photomodulation, and mesenchymal cells. All irradiations applied in vitro, on human mesenchymal cells, with wavelengths ranged from 600 to 1000 nm.
Results
The search yielded 42 original articles and five reviews. Finally, 37 articles were selected with a total of 43 procedures. Three procedures (7.0%) from 620 to 625 nm; 26 procedures (60.5%) from 625 to 740 nm; 13 procedures (30.2%) from 740 to 1000 nm; and one procedure (2.3%) with combinations of wavelengths. Of the 43 procedures, 14 assessed cell viability (n = 14/43, 32.6%); 34 cell proliferation (n = 34/43, 79.1%); 19 cell differentiation (n = 19/43, 44.2%); and three cell migration (n = 3/43, 7.0%).
Conclusions
Photobiomodulation is a promising technology that can impact on cell viability, differentiation, proliferation, or migration, leading to enhance its regenerative capacity.
No Level Assigned
This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cell-based therapy is known to have the potential to induce angiogenesis, primarily through the secretion of angiogenic growth factors. Furthermore, it has been shown that the paracrine properties of MSCs can improve collateral vessel growth in ischemic tissue, bone regeneration, cardiovascular repair after myocardial infarction, and wound healing [1,2,3]. Human mesenchymal stem cells can be retrieved from different sources, such as adipose tissue, cartilage, cord blood, dental pulp, gut, perichondrium, salivary glands or tendons [4]. In vitro, mesenchymal cells have lineage differentiation potential after induction (e.g., into adipocytes after induction with dexamethasone, indomethacin, insulin, methylbutylxanthine or thiazolidinedione; into chondrocytes after induction with ascorbate, bone morphogenic protein 6, dexamethasone or transforming growth factor β; and into osteoblasts after induction with ascorbate, bone morphogenetic protein, dexamethasone, or 1.25 dihydroxy vitamin D3) [4].
However, there are still some limitations regarding the clinical application of MSCs. To overcome them, some methods that improve cell viability (cell death and survival rates), proliferation, differentiation, and migration, have been explored, including MSC preconditioning, genetic modification, and optimization of culture conditions [5].
Generally, photomodulation/photobiomodulation (PBM) or low-power laser refers to non-invasive, non-toxic phototherapy with wavelengths ranging from 600 to 1000 nm, that is, light in the red-near-infra red region of the spectrum [6]. The definition also applies to the word photoactivation. It has been observed that, while red (660 nm) or near-infrared (810 nm) light stimulates proliferation, blue (415 nm) and green (540 nm) light inhibits it, for example, in stem cells derived from human adipose tissue [7]. PBM is biologically attributed to the absorption of light by internal photoreceptors of the respiratory chain located in the mitochondria, which induce mitochondrial activation within cells [6]. The photons absorbed by mitochondria cause an increase in adenosine triphosphate [8]. Another proposed mechanism for PBM action relies on ion channels' sensitivity, allowing calcium to enter the cell [9].
Scientific research on PBM started about 50 years ago [10]. To date, the beneficial effects of PBM have been verified in a variety of diseases and physiological processes, in which the reduction of inflammation or the stimulation of lesion repair has been observed in vivo and in vitro [11], as well as other effects, such as the reduction of hypoxic damage or brain degeneration [12]. However, PBM has not been widely accepted yet, mainly due to the uncertainty regarding its molecular, cellular, and tissue mechanisms of action [13].
Several experiments have shown that administering PBM to MSC cultures accelerated the repair process of skin lesions in normal and ischemic simulations, improved the viability of MSCs, and promoted the release of cytokines in normal and ischemic organs [14, 15]. That is why PBM can be used as MSC preconditioning to improve their regenerative capacity [11, 16]. Furthermore, stem cells and progenitor cells appear to be particularly susceptible to PBM[13]: Its potential to promote MSC growth factor proliferation, differentiation and secretion has already been shown [12]. In several animal models, MSCs were conditioned with PBM to stimulate neoangiogenesis and showed improvements in tissue healing [12, 17].
The PBM devices used are diverse: Helium–Neon (He–Ne) gas lasers; gallium arsenide (GaAs), neodymium-doped yttrium aluminum garnet (Nd:YAG), gallium aluminum arsenide (GaAlAs) and indium-gallium-aluminum-phosphide (InGaAlP) lasers; non-thermal, non-ablative carbon dioxide (CO2) lasers; light-emitting diode (LED) arrays, and visible light [6]. It is well established that the biostimulatory effects of lasers are influenced by parameters such as wavelength, energy density, output power, frequency, or irradiation duration [18]. In addition to the different devices used in the studies carried out with MSCs, a great diversity of parameters had been set, resulting in multiple treatment protocols with different—and sometimes even contradictory—results [7].
Our aim was to conduct a review of published articles carried out in human MSCs with in vitro PBM preconditioning, and identify any possible tendency that might highlight the most suitable procedures.
Methods
A search in English of the PubMed database was carried out using the following search criteria: photobiomodulation or photoactivation or photomodulation, and mesenchymal cells (https://www.ncbi.nlm.nih.gov/pubmed/?term=(photobiomodulation+OR+photoactivation+OR+photobiomodulation)+AND+mesenchimal+cells).
All articles found (including reviews) were listed in an Excel file and arranged by author, year, title, and abstract. Title and abstract were checked for coherent inclusion in the starting list. After a first screening, study duplicates arriving from more than one review were identified and removed. Once the list of studies was available, main text, procedure, results, and conclusions were checked for minimum requirement meeting.
Study Inclusion Criteria
To be included in this review, three criteria had to be met: studies had to be carried out on human MSCs, cell PBM had to be performed in vitro, and its effects had to be analyzed in vivo or ex vivo. Light sources could differ in each study, but the used wavelengths had to range from 600 to 1000 nm. Data of studies that also used other wavelengths were collected as well.
The main registered parameter was wavelength (nm), and secondary parameters were irradiance (W/cm2), dose/fluence (J/cm2), power output (mW), duration of treatment (seconds), frequency of treatment (number of times the treatment was carried out and the period), and cumulative dose (sum of all individual doses). Studies had to assess at least one of the following variables: viability, proliferation, differentiation, or migration.
Other parameters, such as irradiation mode (continuous, fractioned, or punctual) or spot size (cm2), were collected, although they were not included in the analysis. Likewise, variables, such as apoptosis, adherence and secretion of growth factors, or blood vessel count, were included in this review when collected by the studies, though excluded from its conclusions, for that analysis would exceed the focus of this work.
Studies were grouped by wavelengths as follows: Group I (620–625 nm, yellow-orange-green); Group II (625–740 nm, red); Group III (740–1000 nm, near infra-red); and Group IV (studies in which wavelength combinations were applied). Procedures with different wavelengths within the same study were allocated to their corresponding group.
Results
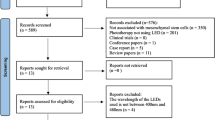
The search yielded a total of 47 articles: 42 single-study articles and five reviews [6, 19,20,21,22]. These reviews contained a total of 67 articles. Of them, 43 were discarded: nine (13.4%, n = 9/67) were excluded because PBM procedures were conducted in vivo; 20, because they were not conducted in human cells (29.9%, n = 20/67); five (7.5%, n = 5/67) were discarded for not including 600–1000 nm wavelengths; and 9 (13.4%, n = 9/67) for being duplicates. This resulted in a total of 24 articles selected, published between 2005 and 2019. Of the 42 single-study articles found, a total of 18 (42.6%, n = 18/42) were discarded because they did not meet the criteria of this review: five (27.8%, n = 5/18) performed PBM procedures in vivo; six studies were not conducted on human cells (33.3%, n = 6/18); in one study (5.6%, n = 1/18), wavelengths did not fit the range; and the last six (33.3%, n = 6/18) were narratives about PBM but did not describe any research study. This resulted in a total of 24 single-study articles selected for this review, published between 2013 and 2020 (Fig. 1).
All articles (n = 48) were put together and again, checked for duplicates. Eleven (22.9%, n = 11/48) were discarded, resulting in 37 articles selected for this review. For a general timeframe reference of the 37 articles included in this review: 18 articles (48.6%, n = 18/37) were published between 2016 and 2020; 13 (35.1%, n = 13/37) were published between 2010 and 2015; and six (19%, n = 6/37) were published between 2005 and 2009. In addition to this, six (16.2%, n = 6/37) articles contained several PBM protocols with different wavelengths, amounting to a total of 43 procedures (Fig. 1). These 43 procedures constituted the final n of this review, and were grouped as follows: (a) Group I (620–625 nm), three procedures (7.0%, n = 3/43) [7, 23,24,25] (Table 1); (b) Group II (625–740 nm), 26 procedures (60.5%, n = 26/43) [7, 8, 12, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] (Table 2); (c) Group III (740–1000 nm), 13 procedures (30.2%, n = 13/3) [7, 27, 31, 32, 48,49,50,51,52,53,54,55,56] (Table 3); and (d) Group IV (combinations of wavelengths), one procedure (2.3%, n = 1/43) [27] (Table 4).
Results on Viability
Fourteen procedures assessed cell viability (32.6%, n = 14/43) (Table 5). Wavelengths ranged from 630 nm to 808 nm and one protocol combined 630 + 810 nm. Ten procedures (71.4%, n = 10/14) showed an increase in viability; in four (28.6%, n = 4/14), there was no effect. No procedure showed a decrease on viability.
Results on Proliferation
Thirty four procedures assessed cell proliferation (79.1%, n = 34/43) (Table 6), with wavelengths ranging from 620 to 940 nm. In 27 procedures (79.4%, n = 27/34), there was an increase in proliferation; in three procedures (8.8%, n = 3/34), there was a decrease, and in three procedures (8.8%, n = 3/34), no effect was witnessed. In one study (2.9%, n = 1/34), the effect varied depending on the dose irradiated.
Results on Differentiation
Sixteen procedures assessed cell differentiation (37.2%, n = 16/43) (Table 7). Wavelengths ranged from 620 to 940 nm. In 13 procedures (81.25%, n = 13/16), there was an increase in differentiation; and in three (18.8%, n = 3/16), no effect was reported.
Results on Migration
Three procedures assessed cell migration (7.0%, n = 3/43) (Table 8). Wavelengths ranged from 625 to 660 nm. Two procedures (66.7%, n = 2/3) showed an increase in cell migration and one (33.3%, n= 1/3) reported no effect.
Discussion
The studies included in this review showed great diversity in protocol design, not only due to the type of device and wavelength applied, but also to important differences in energy parameters, such as dose, exposure time, or light source-sample distance, among others. Furthermore, retrieval location of MSCs varied (adipose tissue, dental pulp, umbilical cord, bone marrow). This diversity made it difficult to draw solid conclusions towards the identification of the best PBM protocols for cell viability, proliferation, differentiation, and migration. Our goal with this review was to confirm PBM´s action on MSCs and, if possible, identify trends in the way cells responded. Thus, further studies are mandatory and should tackle and compare procedure effectiveness.
In the procedures where viability was assessed, 630 nm [26, 27], 635 nm [31, 32], 636 nm [31, 33, 57], 660 nm [36, 43, 44], 668 nm [47], and 808 nm [49]; the most significant increase was found in procedures with 630 nm [27] and 635 nm[31] wavelengths. One study [27] compared different wavelengths (630 nm, 810nm), and again, the most significant increase in viability was seen at 630 nm. On the other hand, the study of Tani et al. [32] did not show viability changes at 635 nm, and the study of Andrade et al. [36] found no differences between compared groups. These findings could be explained by: (i) different cell line, (ii) different exposure time, and, most probable, (iii) different energy density. As a matter of fact, the procedures that reported an increased viability applied lower energy densities: less than 5 J/cm2. In general, studies that reported no effect applied higher energy densities (Table 3).
Proliferation was the most tested variable in these procedures. Most of the procedures that ranged from 620 to 660 nm wavelengths (Tables 2 and 4) showed cell proliferation increase [7, 12, 24, 26, 28,29,30,31,32,33,34,35,36,37,38,39, 43, 44, 46, 49, 57, 58], while a decrease was observed in one procedure [58]. In spite of this positive trend, some results are still difficult to understand: the roles that other variables may play towards cell proliferation remain hidden and a challenge for future studies. The procedures performed with wavelengths ranging from 808 to 980 nm showed the following: in four of them[7, 51, 54, 56], there was an increase; in three [31, 32, 51], no effect was observed; and in one [55], proliferation decreased. In a study carried out by Soleimani et al. [51] at 810 nm, proliferation increased or was not affected, depending on the energy density applied to cells: 2.4 J/cm2 and 3J/cm2 increased proliferation, while a density of 6 J/cm2 had no effect [51].
When wavelengths ranged from 620 to 940 nm (Tables 2, 3 and 4), most authors found an increase in differentiation [24, 25, 28, 31, 35, 48, 50, 51, 53, 56, 59],
In studies assessing migration, Yin K et al. found an increase [46], while Ong et al. did not find any effect at 625 nm [23].
Discussion would be enriched with the presentation of trends, regarding the most effective and popular protocols used, for the reported outcomes to be achieved, however, it has been challenging to find standard protocols of generalized use in humans. For example, in a review about photobiomodulation in bone repair [60], authors found only one article in humans about a clinical case [61]. They included in this review this comment “A lack of persistence in the standardization of methodology employed by authors was observed, with instances of absence of important data, such as output power, energy density and application time, a pattern also observed in reviews relating PBMT to other types of lesions”. The same concern is observed in other reviews, such as review on nerve regeneration, where the authors mentioned regarding the lack of standardization in relation to the application protocols [62]. Another study about photobiomodulation of human osteoblast-like cells in vitro by low-intensity-pulsed LED light cannot compare their results with a previous report because the experimental setups were not identical [63]. With these examples, we want to remark PBM's utility and the importance of focused the research in finding the best variable values to develop standard protocols for each objective that will be of great value to analyze and compare different studies data.
The limitations of the study may include: (1) the fact that it is not a systematic review, since only the PubMed database has been consulted; (2) the great variability of the protocols described in the articles reviewed has made it difficult to trace clear and contrasted trends, although it has been possible to pinpoint a few; and (3) although all the cells studied were human mesenchymal cells, they were grafted from several locations, such as orbital fat, umbilical cord, dental pulp, or bone marrow, among others.
Despite the methodological difficulties already stated, some relationships/trends could be witnessed: (i) blue and green light tended to inhibit the proliferation of hADSCs, (ii) red and NIR light tended to favor cell proliferation and differentiation, (iii) red light tended to favor cell viability, (iv) yellow-orange-green and red light seemed to increase migration, and (v) so far, the combination of any two wavelengths was usually less effective than the most effective of them alone [64,65,66].
Conclusions
As a general conclusion, it can be stated that PBM is an extremely promising way to trigger and stimulate cell metabolic paths that may impact on viability, differentiation, proliferation, or migration, and that might ultimately lead to an enhancement in the cellular regenerative capacity. To determine accurately the clinical potential of PBM and develop efficient and appropriate treatment protocols, future controlled in vivo studies should be performed.
References
Chen L, Tredget EE, Wu PYG, Wu Y, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3(4):e1886
Linero I, Chaparro O (2014) Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 9(9):1–12
Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S et al (2004) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109(12):1543–1549
Lindner U, Kramer J, Rohwedel J, Schlenke P (2010) Mesenchymal stem or stromal cells: toward a better understanding of their biology? Transfus Med Hemother 37(2):75–83
Baldari S, Di Rocco G, Piccoli M, Pozzobon M, Muraca M, Toietta G (2017) Challenges and strategies for improving the regenerative effects of mesenchymal stromal cell-based therapies. Int J Mol Sci 18(10):2087
Ahrabi B, Tavirani MR, Khoramgah MS, Noroozian M, Darabi S, Khoshsirat S et al (2019) The effect of photobiomodulation therapy on the differentiation, proliferation, and migration of the mesenchymal stem cell: a review. J Lasers Med Sci 10(4):S96-103
Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR (2017) Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci Rep 7(1):1–10
Han B, Fan J, Liu L, Tian J, Gan C, Yang Z et al (2019) Adipose-derived mesenchymal stem cells treatments for fibroblasts of fibrotic scar via downregulating TGF-β1 and Notch-1 expression enhanced by photobiomodulation therapy. Lasers Med Sci 34(1):1–10
Fallahnezhad S, Jajarmi V, Shahnavaz S, Amini A, Ghoreishi SK, Kazemi M et al (2019) Improvement in viability and mineralization of osteoporotic bone marrow mesenchymal stem cell through combined application of photobiomodulation therapy and oxytocin. Lasers Med Sci 35(3):557–566
Heiskanen V, Hamblin MR (2018) Photobiomodulation: lasers: vs. light emitting diodes? Photochem Photobiol Sci 17(8):1003–17
Kouhkheil R, Fridoni M, Abdollhifar MA, Amini A, Bayat S, Ghoreishi SK et al (2019) Impact of photobiomodulation and condition medium on mast cell counts, degranulation, and wound strength in infected skin wound healing of diabetic rats. Photobiomodul, Photomed, Laser Surg 37(11):706–714
Kim K, Lee J, Jang H, Park S, Na J, Myung JK et al (2019) Photobiomodulation enhances the angiogenic effect of mesenchymal stem cells to mitigate radiation-induced enteropathy. Int J Mol Sci 20(5):1–19
De Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3):1–37
Park IS, Chung PSAJ (2014) Enhanced angiogenic effect of adipose-derived stromal cell spheroid with low-level light therapy in hind limb ischemia mice. Biomaterials 35(34):9280–9289
Kim H, Choi K, Kweon OKKW (2012) Enhanced wound healing effect of canine adipose-derived mesenchymal stem cells with low-level laser therapy in athymic mice. J Dermatol Sci 68(3):149–156
Zare F, Bayat M, Aliaghaei A, Piryaei A (2020) Photobiomodulation therapy compensate the impairments of diabetic bone marrow mesenchymal stem cells. Lasers Med Sci 35(3):547–556
Park IS, Mondal A, Chung PS, Ahn JC (2015) Vascular regeneration effect of adipose-derived stem cells with light-emitting diode phototherapy in ischemic tissue. Lasers Med Sci 30(2):533–541
El Gammal ZH, Zaher AM, El-Badri N (2017) Effect of low-level laser-treated mesenchymal stem cells on myocardial infarction. Lasers Med Sci 32(7):1637–1646
Fekrazad R, Eslaminejad MB, Shayan AM, Kalhori KAM, Abbas FM, Taghiyar L et al (2016) Effects of photobiomodulation and mesenchymal stem cells on articular cartilage defects in a rabbit model. Photomed Laser Surg 34(11):543–549
Vicenti G, Bizzoca D, Caruso I, Nappi VS, Giancaspro G, Carrozzo M et al (2018) New insights into the treatment of non-healing diabetic foot ulcers. J Biol Regul Homeost Agents 32(6):15–21
Marques MM, Diniz IMA, De Cara SPHM, Pedroni ACF, Abe GL, D’Almeida-Couto RS et al (2016) Photobiomodulation of dental derived mesenchymal stem cells: a systematic review. Photomed Laser Surg 34(11):500–508
Odinokov D, Hamblin MR (2018) Aging of lymphoid organs: Can photobiomodulation reverse age-associated thymic involution via stimulation of extrapineal melatonin synthesis and bone marrow stem cells? J Biophotonics 11(8):1–13
Ong WK, Chen HF, Tsai CT, Fu YJ, Wong YS, Yen DJ et al (2013) The activation of directional stem cell motility by green light-emitting diode irradiation. Biomaterials 34(8):1911–1920
Yang D, Yi W, Wang E, Wang M (2016) Effects of light-emitting diode irradiation on the osteogenesis of human umbilical cord mesenchymal stem cells in vitro. Sci Rep 6:1–7
Babaee A, Nematollahi-Mahani SN, Dehghani-Soltani S, Shojaei M, Ezzatabadipour M (2019) Photobiomodulation and gametogenic potential of human Wharton’s jelly-derived mesenchymal cells. Biochem Biophys Res Commun 514(1):239–245
Li W-T, Chen H-L, Wang C (2006) Effect of light emitting diode irradiation on proliferation of human bone marrow mesenchymal stem cells. J Med Biol Eng 26(1):35–42
Zare F, Moradi A, Fallahnezhad S, Ghoreishi SK, Amini A, Chien S et al (2019) Photobiomodulation with 630 plus 810 nm wavelengths induce more in vitro cell viability of human adipose stem cells than human bone marrow-derived stem cells. J Photochem Photobiol B Biol 201:111658
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23(2):161–166
Mvula B, Mathope T, Moore T, Abrahamse H (2008) The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med Sci 23(3):277–282
Wang J, Huang W, Wu Y, Hou J, Nie Y, Gu H et al (2012) MicroRNA-193 pro-proliferation effects for bone mesenchymal stem cells after low-level laser irradiation treatment through inhibitor of growth family, member 5. Stem Cells Dev 21(13):2508–2519
Chen H, Wu H, Yin H, Wang J, Dong H, Chen Q et al (2019) Effect of photobiomodulation on neural differentiation of human umbilical cord mesenchymal stem cells. Lasers Med Sci 34(4):667–675
Tani A, Chellini F, Giannelli M, Nosi D, Zecchi-Orlandini S, Sassoli C (2018) Red (635 nm), near-infrared (808 nm) and violet-blue (405 nm) photobiomodulation potentiality on human osteoblasts and mesenchymal stromal cells: a morphological and molecular in vitro study. Int J Mol Sci 19(7):1–23
Mvula B, Moore TJ, Abrahamse H (2009) Effect of low-level laser irradiation and epidermal growth factor on adult human adipose-derived stem cells. Lasers Med Sci 25(1):33–39
de Villiers JA, Houreld NN, Abrahamse H (2011) Influence of low intensity laser irradiation on isolated human adipose derived stem cells over 72 hours and their differentiation potential into smooth muscle cells using retinoic acid. Stem Cell Rev Rep 7(4):869–882
Bloise N, Ceccarelli G, Minzioni P, Vercellino M, Benedetti L, De AMGC et al (2013) Investigation of low-level laser therapy potentiality on proliferation and differentiation of human osteoblast-like cells in the absence/presence of osteogenic factors. J Biomed Opt 18(12):128006
de Andrade ALM, Luna GF, Brassolatti P, Leite MN, Parisi JR, de Oliveira Leal ÂM et al (2019) Photobiomodulation effect on the proliferation of adipose tissue mesenchymal stem cells. Lasers Med Sci 34(4):677–683
de Oliveira TS, Serra AJ, Manchini MT, Bassaneze V, Krieger JE, de Carvalho PDTC, Antunes DE, Bocalini DS, Tucci PJF, Silva JA (2015) Effects of low level laser therapy on attachment, proliferation, and gene expression of VEGF and VEGF receptor 2 of adipocyte-derived mesenchymal stem cells cultivated under nutritional deficiency. Lasers n Med Sci 30(1):217–223
Diniz IMA, Carreira ACO, Sipert CR, Uehara CM, Moreira MSN, Freire L et al (2018) Photobiomodulation of mesenchymal stem cells encapsulated in an injectable rhBMP4-loaded hydrogel directs hard tissue bioengineering. J Cell Physiol 233(6):4907–4918
Eduardo FDP, Bueno DF, De Freitas PM, Marques MM, Passos-Bueno MR, Eduarde CDP et al (2008) Stem cell proliferation under low intensity laser irradiation: a preliminary study. Lasers Surg Med 40(6):433–438
Ferreira LS, Diniz IMA, Maranduba CMS, Miyagi SPH, Rodrigues MFSD, Moura-Netto C et al (2019) Short-term evaluation of photobiomodulation therapy on the proliferation and undifferentiated status of dental pulp stem cells. Lasers Med Sci 34(4):659–666
Garrido PR, Pedroni ACF, Cury DP, Moreira MS, Rosin F, Sarra G et al (2018) Effects of photobiomodulation therapy on the extracellular matrix of human dental pulp cell sheets. J Photochem Photobiol B Biol 2019(194):149–157
Pereira LO, Longo JPF, Azevedo RB (2012) Laser irradiation did not increase the proliferation or the differentiation of stem cells from normal and inflamed dental pulp. Arch Oral Biol 57(8):1079–1085
Soares DM, Ginani F, Henriques ÁG, Barboza CAG (2015) Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med Sci 30(3):1171–1174
Zaccara IM, Ginani F, Mota-Filho HG, Henriques ÁCG, Barboza CAG (2015) Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med Sci 30(9):2259–2264
Park IS, Chung PS, Ahn JC, Leproux A (2017) Human adipose-derived stem cell spheroid treated with photobiomodulation irradiation accelerates tissue regeneration in mouse model of skin flap ischemia. Lasers Med Sci 32(8):1737–1746
Yin K, Zhu R, Wang S, Zhao RC (2017) Low-level laser effect on proliferation, migration, and antiapoptosis of mesenchymal stem cells. Stem Cells Dev 26(10):762–775
Lenna S, Bellotti C, Duchi S, Martella E, Columbaro M, Dozza B et al (2020) Mesenchymal stromal cells mediated delivery of photoactive nanoparticles inhibits osteosarcoma growth in vitro and in a murine in vivo ectopic model. J Exp Clin Cancer Res 39(1):1–15
Diniz IMA, Matos AB, Marques MM (2015) Laser phototherapy enhances mesenchymal stem cells survival in response to the dental adhesives. Sci World J 2015:1–6
Nurković J, Zaletel I, Nurković S, Hajrović Š, Mustafić F, Isma J et al (2017) Combined effects of electromagnetic field and low-level laser increase proliferation and alter the morphology of human adipose tissue-derived mesenchymal stem cells. Lasers Med Sci 32(1):151–160
Arany PR, Huang GX, Gadish O, Feliz J, Weaver JC, Kim J et al (2014) Multi-lineage MSC differentiation via engineered morphogen fields. J Dent Res 93(12):1250–1257
Soleimani M, Abbasnia E, Fathi M, Sahraei H, Fathi Y, Kaka G (2012) The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts-an in vitro study. Lasers Med Sci 27(2):423–430
Renno AC, McDonnell PA, Parizotto NA, Laakso EL (2007) The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg 25(4):275–280
Turrioni APS, Basso FG, Montoro LA, De Almeida LDFD, Costa CADS, Hebling J (2014) Phototherapy up-regulates dentin matrix proteins expression and synthesis by stem cells from human-exfoliated deciduous teeth. J Dent 42(10):1292–1299
Amini A, Pouriran R, Abdollahifar MA, Abbaszadeh HA, Ghoreishi SK, Chien S et al (2018) Stereological and molecular studies on the combined effects of photobiomodulation and human bone marrow mesenchymal stem cell conditioned medium on wound healing in diabetic rats. J Photochem Photobiol B Biol 182:42–51
Bagheri M, Amini A, Abdollahifar MA, Ghoreishi SK, Piryaei A, Pouriran R et al (2018) Effects of photobiomodulation on degranulation and number of mast cells and wound strength in skin wound healing of streptozotocin-induced diabetic rats. Photomed Laser Surg 36(8):415–423
Jawad MM, Husein A, Azlina A, Alam MK, Hassan R, Shaari R (2013) Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J Biomed Opt 18(12):128001
Mvula B, Abrahamse H (2016) Differentiation potential of adipose-derived stem cells when cocultured with smooth muscle cells, and the role of low-intensity laser irradiation. Photomed Laser Surg 34(11):509–515
Horvát-Karajz K, Balogh Z, Kovács V, Hámori A, Sréter L, Uher F (2009) In vitro effect of carboplatin, cytarabine, paclitaxel, vincristine, and low-power laser irradiation on murine mesenchymal stem cells. Lasers Surg Med 41(6):463–469
Kim HK, Kim JH, Abbas AA, Kim DO, Park SJ, Chung JY et al (2009) Red light of 647 nm enhances osteogenic differentiation in mesenchymal stem cells. Lasers Med Sci 24(2):214–222
Rosso MPDO, Buchaim DV, Pomini KT, Coletta BBD, Reis CHB, Pilon JPG, Duarte Júnior G, Buchaim RL (2019) Photobiomodulation therapy (PBMT) applied in bone reconstructive surgery using bovine bone grafts: a systematic review. Materials 12(24):4051
Bhardwaj S (2016) Low level laser therapy in the treatment of intra-osseous defect—a case report. J Clin Diagnostic Res. 10(3):10–12
Rosso MPDO, Buchaim DV, Kawano N, Furlanette G, Pomini KT, Buchaim RL (2018) Photobiomodulation therapy (PBMT) in peripheral nerve regeneration: a systematic review. Bioengineering 5(2):44
Rosenberg N, Gendelman R, Noofi N (2020) Photobiomodulation of human osteoblast-like cells in vitro by low-intensity-pulsed LED light. FEBS Open Bio. 10(7):1276–1287
Fekrazad R, Asefi S, Eslaminejad MB, Taghiyar L, Bordbar S, Hamblin MR (2019) Correction to: Photobiomodulation with single and combination laser wavelengths on bone marrow mesenchymal stem cells: proliferation and differentiation to bone or cartilage. Lasers Med Sci 34(1):115–126. https://doi.org/10.1007/s10103-018-2620-8
Hou JF, Zhang H, Yuan X, Li J, Wei YJ, Hu SS (2008) In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers Surg Med 40(10):726–733
Bouvet-Gerbettaz S, Merigo E, Rocca JP, Carle GF, Rochet N (2009) Effects of low-level laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoelasts. Lasers Surg Med 41(4):291–297
Acknowledgements
The authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pinto, H., Goñi Oliver, P. & Sánchez-Vizcaíno Mengual, E. The Effect of Photobiomodulation on Human Mesenchymal Cells: A Literature Review. Aesth Plast Surg 45, 1826–1842 (2021). https://doi.org/10.1007/s00266-021-02173-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-021-02173-y