Abstract
Background
This study assessed the effect of Biodentine coated with Emdogain (Biodentine/Emdogain) on proliferation and differentiation of human stem cells from the apical papilla (SCAPs).
Methods and results
In this in vitro, experimental study, SCAPs were isolated from two immature impacted third molars and cultured. After ensuring the stemness of the cells by assessing the cell surface markers, they were exposed to Biodentine, Emdogain, and Biodentine/Emdogain for 24 and 72 h. The control cells did not receive any intervention. Cell viability was evaluated by the methyl thiazolyl tetrazolium assay. Expression of odontogenic differentiation genes was analyzed by the quantitative reverse transcription polymerase chain reaction. Alkaline phosphatase (ALP) activity was quantified by the respective kit. Data were analyzed by one-way ANOVA, t-test, and Mann-Whitney test (α = 0.05). Cell viability did not change after 24 h of exposure to biomaterials. At 72 h, the viability of the cells exposed to Biodentine and Biodentine/Emdogain decreased compared with the control group. The expression of dentin sialophosphoprotein, dentin matrix protein 1, and bone sialoprotein genes, and ALP activity significantly increased in all three experimental groups, compared with the control group at both 24 and 72 h; this increase was significantly greater in Biodentine/Emdogain group. The number of mineralized nodules significantly increased in all groups after 72 h with a greater rate in Biodentine/Emdogain group.
Conclusions
All biomaterials increased the differentiation of SCAPs, expression of odontogenic genes, and ALP activity, but Biodentine/Emdogain was significantly more effective for this purpose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Management of immature necrotic teeth is clinically challenging. Such teeth may require endodontic treatment due to trauma, severe anomalies, or extensive caries. Regenerative endodontic procedures (REPs) are the treatment of choice for permanent immature necrotic teeth with open apices to allow completion of root development and reinstate the normal physiological responses [1]. Mesenchymal stem cells required for REPs are isolated from the apical tissue of mature and immature teeth [2]. The apical papilla is the main source of such cells [3]. Evidence shows that the apical papilla remains viable in dental infections in both animal models [4] and humans [3]. Thus, stem cells from the apical papilla (SCAPs) are commonly used for REPs. Bioceramic materials especially calcium silicate cements are commonly used for REPs due to favorable properties such as optimal biocompatibility and antimicrobial activity [5,6,7].
A viable pulp tissue is imperative for root development in immature permanent teeth. Pulp infection or trauma can cease the process of root development and leave an open apex. REPs are increasingly used to allow completion of root development and apex closure by differentiation of stem cells and continuation of mineralization [8,9,10]. REPs are superior to the old apexification technique since they are faster and reinforce the root structure by increasing the root length and thickness [10,11,12].
Mineral trioxide aggregate (MTA) is a root-end filling material with optimal biocompatibility, which has been successfully used for single-session apexification. However, MTA is costly, and has a long setting time, difficult handling, and tooth discoloration potential [8, 13]. Also, despite the high success rate of single-session apexification treatment, the root structure remains weak in this procedure [5, 14].
Biodentine is a tricalcium silicate-based cement, which was introduced as a replacement for dentin in 2009. It has shown superior properties to MTA with regard to faster cementum deposition, and higher mechanical resistance [15]. Biodentine is commonly used for apexification and perforation repair, and as a pulp capping agent and regenerative biomaterial [16]. Studies on the optimal concentration of Biodentine for regeneration of the dentin-pulp complex in the clinical setting are limited [17]. However, its optimal efficacy for proliferation, migration, and adhesion of human dental pulp stem cells (DPSCs) has been documented [18].
Emdogain is an enamel matrix derivative derived from the developing porcine enamel matrix. Amelogenin is its main constituent. It also contains matrix metalloproteinases and several growth factors. Emdogain can enhance the migration, attachment, and proliferation of periodontal ligament cells [19], and has also been suggested for induction of pulp regeneration. Evidence shows that amelogenin particles participate in proliferation and maturation of DPSCs [20].
Considering the positive role of Emdogain and Biodentine in REPs, this study sought to assess the effects of Biodentine, Emdogain and Biodentine coated with Emdogain (Biodentine/Emdogain) on proliferation and differentiation of SCAPs.
Materials and methods
This in vitro experimental study was conducted on SCAPs isolated from two sound immature impacted mandibular third molars scheduled for extraction. The patients signed informed consent forms and consented to the use of their extracted teeth for research purposes. The sample size was calculated according to previous studies, and three repetitions were considered for each test at each assessment time point [21, 22]. The study was approved by the Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.787).
Cell culture
The extracted teeth were immediately rinsed with sterile phosphate buffered saline (PBS; Gibco BRL, Grand Island, NY, USA) and stored in it. Stem cells were isolated from the apical papilla by enzymatic digestion using 2 mg/mL of type I collagenase (Worthlington Biomedical, Lakewood, NJ, USA) and placed in Dulbecco’s modified Eagle’s medium (Gibco, GrandIsland, NY, USA). The cells were then re-cultured in the culture medium supplemented with 15% fetal bovine serum (Gibco, Invitrogen, USA) and then in alpha-minimum essential medium supplemented with 10% fetal bovine serum in sterile cell culture flasks (SPL Life Science, Gyeonggi-do, South Korea). The culture medium was refreshed every 2–3 days, and the cells were passaged after 1 week. After 4 passages, the cells reached adequate confluence, and 4-µm insert plates (SPL Life Science, Gyeonggi-do, South Korea) were used for treatment of the cells with the respective biomaterials. Biodentine (Septodont, Saint-Maur-des-Fosses, France) was prepared according to the manufacturer’s instructions under sterile conditions. Biodentine was mixed and applied in paraffin wax molds with 10 mm diameter and 1 mm thickness, compressed, and incubated at 37 °C and 96% humidity for 10 min to set. Emdogain gel (30 mg/mL and 0.7 mL) (Biora AB, Malmo, Sweden) was diluted with sterile distilled water to obtain 100 µg/mL concentration. The cells in the control group did not receive any treatment. The cells in the three experimental groups were treated with Emdogain, Biodentine and a combination of Emdogain and Biodentine. Each test was repeated in triplicate for each group [22, 23].
Characterization of SCAPs
SCAP surface markers were analyzed using flow cytometry. After the cells reached 80% confluence, the culture medium was removed from the flask, and the cells were rinsed with PBS twice. The cells were detached from the bottom of the flask using trypsin/EDTA, and the culture medium was added to the flask. The cell suspension was then transferred into 15-mL Falcon tubes and centrifuged at 1200 × g for 6 min. The cell sediment was rinsed with PBS twice, and the cells were then evaluated for stem cell specific markers (CD105, and CD90) and hematopoietic cell markers (CD45 and CD34). Data were analyzed with the FACScalibur cytometer (Becton Dickinson) and CellQuest software. Of all, 97.9% of the cells expressed the CD-105 marker and 95.8% of them expressed the CD-90 marker. Also, 99.8% of the cells did not express the CD-34 or the CD-45 markers.
Assessment of cell viability
The methyl thiazolyl tetrazolium (MTT) assay was used for assessment of cell viability. For this purpose, the cells were cultured in a 96-well plate and treated with Emdogain, Biodentine, and a combination of the two. After 24 and 72 h, 10 λ of the MTT solution was added to all wells, and the plate was incubated at 37 °C. After 2 h, the overlaying medium was removed and replaced with 100 λ of dimethyl sulfoxide. After 20 min, the percentage of cell viability was determined by reading the optical density of the solution at 570 nm wavelength.
Assessment of the expression of odontogenic genes
After treatment of the cells with the biomaterials, RNA was extracted by Trizol, and the amount of extracted total RNA was quantified by NanoDrop at 260 and 280 nm wavelengths. The cDNA was then synthesized by superscript II first-strand cDNA synthesis kit (Invitrogen, CA, USA) according to the manufacturer’s instructions. The expression of dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), and bone sialoprotein (BSP) genes was quantified by using the beta-actin gene as the housekeeping gene. The cDNA was quantified by real-time polymerase chain reaction using specific primers. The gene expression of specific markers in different experimental groups was normalized against the control group.
Assessment of alkaline phosphatase (ALP) activity
The ALP activity was assessed by the ALP staining kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The cells were rinsed with PBS twice and incubated overnight with 0.2% TritonX-100 (Jiancheng, Nanjing, China) at 37 °C. The working solution was then added, and the absorbance was read by an automatic microplate reader (BioTek, Winooski, VT, USA) at 520 nm wavelength.
Assessment of odontogenic–osteogenic differentiation
The cells were cultured in a 24-well plate and after exposure to biomaterials, osteogenic–odontogenic medium including regular medium containing 10 mM beta-glycerophosphate (Sigma-Aldrich, St. Louis, MO, USA), 10 nM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA) and 50 mg/mL ascorbic acid was added to the cells. The culture medium was refreshed every 72 h. After 21 days, the cells were fixed with 4% formaldehyde, rinsed with PBS, and incubated with Alizarin Red stain at room temperature for 15 min. The differentiated cells were stained red due to the presence of calcium deposits [24].
Statistical analysis
The four groups were compared regarding cell viability and expression of odontogenic genes by t-test in case of normal distribution of data and by the Mann-Whitney test for non-normally distributed data.
Results
Cell viability
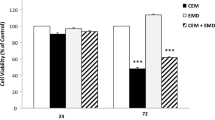
At 24 and 72 h, Emdogain had no significant difference with the control group with respect to cell viability; however, treatment with Biodentine and Biodentine/Emdogain significantly decreased the cell viability compared with the control group. At 24 and 72 h, Emdogain showed significantly higher cell viability than the Biodentine and Biodentine/Emdogain groups. Also, at both 24 and 72 h, the Biodentine/Emdogain group showed significantly higher cell viability than the Biodentine group.
One-way ANOVA revealed a significant difference among the groups in cell viability at both 24 and 72 h. Pairwise comparisons by the Tukey’s test revealed significant differences between the control and Emdogain/Biodentine groups at both 24 and 72 h, and Biodentine and control groups at both 24 and 72 h. At both 24 and 72 h, the biocompatibility of Emdogain was higher than Biodentine. Also, the biocompatibility of Biodentine/Emdogain was significantly lower than the control group at both time points. No other significant differences were noted (Fig. 1).
Expression of odontogenic/osteogenic differentiation genes
Greater expression of BSP, DMP1, and DSPP genes was noted in the experimental groups compared with the control group at both 24 and 72 h (Figs. 2, 3, and 4). The maximum expression of BSP, DMP1, and DSPP genes was noted in the Emdogain/Biodentine group followed by the Emdogain, Biodentine, and control groups. One-way ANOVA revealed a significant difference in gene expression among the groups at both 24 and 72 h. The Tukey’s test revealed significantly higher expression of BSP, DMP1 and DSPP genes in the Emdogain/Biodentine, Emdogain, and Biodentine groups compared with the control group. Moreover, gene expression in the Emdogain group was higher than that in the Biodentine group.
ALP activity
An increase in ALP activity was noted in the experimental groups at both 24 and 72 h, (Fig. 5). The maximum ALP activity was noted in the Emdogain/Biodentine group followed by the Emdogain, Biodentine, and control groups.
One-way ANOVA revealed a significant difference in ALP activity among the groups at both 24 and 72 h. The Tukey’s test revealed significantly higher ALP activity in Emdogain/Biodentine, Emdogain, and Biodentine groups compared with the control group at both time points. Also, ALP activity was significantly greater in the Emdogain than Biodentine group.
Odontogenic–osteogenic differentiation
Alizarin Red staining revealed an increase in the number of calcified nodules after 21 days in all experimental groups (24 and 72 h of exposure to the biomaterials). The number of calcified nodules in the Biodentine/Emdogain group was greater than that in other groups at both time points (Fig. 6).
Discussion
The discovery of stem cells and their prominent role in regenerative processes greatly contributed to the advances in REPs. The present study evaluated the effect of Biodentine coated with Emdogain on proliferation and differentiation of human SCAPs. Biodentine is a tricalcium silicate-based cement, with unique properties such as dentin-like biomechanical properties, high biocompatibility, and cementum deposition [15]. Biodentine is used for REPs. However, despite the excellent biomechanical properties of Biodentine, cytotoxicity is one drawback of this biomaterial [17, 18] which decreases when coated with Emdogain; although this reduction may not be significant. Assessment of cell viability by the MTT assay evealed that after the control group, Emdogain had the highest biocompatibility followed by Emdogain/Biodentine. The Emdogain group had no significant difference with the control group in this regard. However, Biodentine group had significantly lower biocompatibility than the control group. Also, Biodentine had significantly lower biocompatibility than Emdogain. Thus, it appears that Biodentine is cytotoxic early after use. As shown in Fig. 1, this effect appears to be alleviated by addition of Emdogain (Emdogain/Biodentine) due to its proliferative effect. However, this change was not significant. The biocompatibility of Emdogain at 24 h was lower than that at 72 h, which indicates that Emdogain has some cytotoxic effects at first, which are neutralized after 72 h due to chemical stabilization of the cement.
Emdogain has properties similar to those of the extracellular matrix. It regulates the proliferation, migration and differentiation of osteoblasts [25, 26]. Wang et al. [27] demonstrated that Emdogain enhanced the mineralization of pulp cells [27]. The effects of Emdogain in combination with Biodentine are probably due to the possible molecular mechanisms and release of growth factors [28]. Karkehabadi et al. [29] indicated that addition of Emdogain to different biomaterials did not affect the cell viability after 24 and 48 h; however, it significantly enhanced the cell viability at 7 days. Their results were different from the present findings since a reduction in cell viability occurred at 72 h in the Emdogain/Biodentine group in the present study. It appears that cell proliferation neutralized the cytotoxic effects at 7 days. Differences in the results of the two studies may be due to the fact that Karkehabadi et al. [29] evaluated DPSCs; while, SCAPs were assessed in the present study. The present results regarding the cytotoxicity of biomaterials (Boidentine, Emdogain/Biodentine) at 24 and 72 h were inconsistent with the findings of Saberi et al. [30] who found no significant difference in cytotoxicity of different biomaterials and the control group at 168 h. However, in the present study, Biodentine and Biodentine/Emdogain showed cytotoxicity at 72 h, which was different from their results at 168 h, and may be due to the fact that cell proliferation and release of calcium ions are considerably lower at 72 h compared with 168 h. The present results were in accordance with the findings of Mohamed and Fayyad [31] who reported a reduction in cell viability at 24 h, which was compensated by cell proliferation on the next day.
The rate of release of calcium ions at different time points may explain the variations in cell viability in presence of different cements. Calcium silicate-based cements (such as Biodentine) continuously release calcium ions [32]. Calcium silicate hydrate is then formed, and calcium carbonate phosphate deposits. Release of calcium ions can induce inflammatory toxic reactions [33]; however, it is also critical for the viability of mesenchymal stem cells [34]. Calcium ions play a fundamental role in signaling pathways and regulation of cellular activities such as cell migration [35].
ALP activity was also evaluated in this study since ALP is the primary marker of osteogenic differentiation [36]. The present results indicated a significant increase in ALP activity in all experimental groups at 24 and 72 h. The maximum ALP activity was noted in the Emdogain/Biodentine group followed by Emdogain, Biodentine and finally the control group. These findings highlight the role of Emdogain and Biodentine (alone or in combination) in enhancement of pulp and dentin regeneration. Similar to the present study, Li et al. [37] indicated a significant increase in ALP activity of DPSCs after 7 days of incubation with Emdogain compared with the control group, and Miller et al. [38] reported higher ALP activity of SCAPs induced by some bioceramic materials. Moreover, Wu et al. [36] reported significant enhancement of ALP activity for 3 h caused by Emdogain. Min et al. [39] demonstrated that Emdogain + MTA significantly increased the ALP activity, which was in line with the present findings regarding the use of Biodentine/Emdogain.
Osteogenic–odontogenic differentiation of SCAPs was also evaluated in this study by assessment of the expression of DSPP, BSP, and DMP genes after 14 days, which play a fundamental role in odontoblastic differentiation and dentin mineralization [40]. The results indicated a significant increase in BSP expression after both 24 and 72 h of exposure to the biomaterials in all experimental groups, compared with the control group. This upregulation was significantly greater in the Biodentine/Emdogain group followed by the Emdogain group. Min et al. [39] reported upregulation of BSP gene in the MTA and MTA/Emdogain groups, which reached its maximum level after 3 days. Wang et al. [27] reported the upregulation of odontoblast-like and osteoblast-like cell markers by Emdogain. Also, Jue et al. [41] demonstrated over-expression of BSP by human mesenchymal stem cells exposed to Emdogain. The abovementioned findings all support the present results.
The current results also revealed upregulation of DMP1 at both time points in all experimental groups compared with the control group. The expression of DMP1 was maximum in the Emdogain/Biodentine group followed by the Emdogain group. DMP1 has a regulatory role in the mineralization process of reparative dentin, and is an odontoblastic marker [42]. The present results regarding the over-expression of DMP1 was in agreement with the findings of Asgary et al. [43] although they evaluated DPSCs.
Expression of DSPP indicates the presence of mature osteoblasts, and is correlated with dentinogenesis [44, 45]. Upregulation of DSPP was also noted in the experimental groups, compared with the control group at both time points, which was maximum in the Biodentine/Emdogain group followed by the Emdogain group. This finding was in accordance with the results of Miller et al. [38] who reported the over-expression of DSPP by SCAPs in presence of Biodentine and EndoSequence, Hajizadeh et al. [46] who reported the over-expression of DSPP by SCAPs after 3 weeks of using different biomaterials, and Saberi et al. [47] who showed the upregulation of osteogenic markers by SCAPs due to exposure to Biodentine and MTA.
The mechanism of action of Emdogain in odontoblastic–osteoblastic differentiation has not been well elucidated. It may directly stimulate the odontoblasts or pulp cells to produce collagen matrix [48]. Alternatively, presence of transforming growth factor B1 or amelogenin peptides in Emdogain may induce cell signaling and matrix formation, and lead to subsequent mineralization [49].
Alizarin Red staining of odontoblast-like cells was also performed in this study. This test reveals calcium deposits in the extracellular matrix [50]. The results indicated staining of all experimental groups.
Overall, Biodentine has some toxic effects on the stem cells, which subside over time. In the long-term, use of Emdogain can help alleviate this effect by induction of cell proliferation. On the other hand, combined use of Emdogain and Biodentine can have a synergistic effect on expression of odontoblastic markers and formation of calcified nodules, which is probably due to the release of calcium ions from Biodentine and growth factors from Emdogain.
This study evaluated cell viability, gene expression, and mineralization after 24 and 72 h of exposure of SCAPs to biomaterials. Future studies are required to assess the effects of biomaterials over longer periods of time. Also, the possible synergistic effects of other biomaterials should be investigated in future studies on different types of stem cells.
Conclusions
All biomaterials increased the differentiation of SCAPs, expression of odontogenic genes, and ALP activity but Biodentine/Emdogain was significantly more effective than each biomaterial alone in the latter two parameters.
References
Diogenes A, Ruparel NB, Shiloah Y, Hargreaves KM (2016) Regenerative endodontics: a way forward. J Am Dent Assoc 147:372–380. https://doi.org/10.1016/j.adaj.2016.01.009
Chrepa V, Henry MA, Daniel BJ, Diogenes A (2015) Delivery of apical mesenchymal stem cells into root canals of mature teeth. J Dent Res 94:1653–1659. https://doi.org/10.1177/0022034515596527
Chrepa V, Pitcher B, Henry MA, Diogenes A (2017) Survival of the apical papilla and its resident stem cells in a case of advanced pulpal necrosis and apical periodontitis. J Endod 43:561–567. https://doi.org/10.1016/j.joen.2016.09.024
Tobias Duarte PC, Gomes-Filho JE, Ervolino E, Marçal Mazza Sundefeld ML, Tadahirowayama M, Lodi CS, Dezan-Júnior E, Angelo Cintra LT (2014) Histopathological condition of the remaining tissues after endodontic infection of rat immature teeth. J Endod 40:538–542. https://doi.org/10.1016/j.joen.2013.09.015
Parirokh M, Torabinejad M (2010) Mineral trioxide aggregate: a comprehensive literature review—part III: clinical applications, drawbacks, and mechanism of action. J Endod 36:400–413. https://doi.org/10.1016/j.joen.2009.09.009
Kim Y, Lee D, Kim HM, Kye M, Kim SY (2021) Biological characteristics and odontogenic differentiation effects of calcium silicate-based pulp capping materials. Materials (Basel) 14:4661
Jitaru S, Hodisan I, Timis L, Lucian A, Bud M (2016) The use of bioceramics in endodontics—literature review. Clujul Med 89:470–473. https://doi.org/10.15386/cjmed-612
Haglund R, He J, Jarvis J, Safavi KE, Spångberg LS, Zhu Q (2003) Effects of root-end filling materials on fibroblasts and macrophages in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95:739–745. https://doi.org/10.1067/moe.2003.231
Edrees HY, Abu Zeid STH, Atta HM, AlQriqri MA (2019) Induction of osteogenic differentiation of mesenchymal stem cells by bioceramic root repair material. Materials (Basel) 12:2311
Lee BN, Moon JW, Chang HS, Hwang IN, Oh WM, Hwang YC (2015) A review of the regenerative endodontic treatment procedure. Restor Dent Endod 40:179–187. https://doi.org/10.5395/rde.2015.40.3.179
Goyal L (2014) Clinical effectiveness of combining platelet rich fibrin with alloplastic bone substitute for the management of combined endodontic periodontal lesion. Restor Dent Endod 39:51–55. https://doi.org/10.5395/rde.2014.39.1.51
Hotwani K, Sharma K (2014) Platelet rich fibrin—a novel acumen into regenerative endodontic therapy. Restor Dent Endod 39:1–6. https://doi.org/10.5395/rde.2014.39.1.1
Bonson S, Jeansonne BG, Lallier TE (2004) Root-end filling materials alter fibroblast differentiation. J Dent Res 83:408–413. https://doi.org/10.1177/154405910408300511
Witherspoon DE, Small JC, Regan JD, Nunn M (2008) Retrospective analysis of open apex teeth obturated with mineral trioxide aggregate. J Endod 34:1171–1176. https://doi.org/10.1016/j.joen.2008.07.005
Camilleri J (2008) Characterization of hydration products of mineral trioxide aggregate. Int Endod J 41:408–417. https://doi.org/10.1111/j.1365-2591.2007.01370.x
Camilleri J, Kralj P, Veber M, Sinagra E (2012) Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int Endod J 45:737–743. https://doi.org/10.1111/j.1365-2591.2012.02027.x
Han J, Menicanin D, Gronthos S, Bartold PM (2014) Stem cells, tissue engineering and periodontal regeneration. Aust Dent J 59:117–130. https://doi.org/10.1111/adj.12100
Escobar-García DM, Aguirre-López E, Méndez-González V, Pozos-Guillén A (2016) Cytotoxicity and initial biocompatibility of endodontic biomaterials (MTA and Biodentine™) used as root-end filling materials. Biomed Res Int 2016:7926961. https://doi.org/10.1155/2016/7926961
Miron RJ, Caluseru OM, Guillemette V, Zhang Y, Gemperli AC, Chandad F, Sculean A (2013) Influence of enamel matrix derivative on cells at different maturation stages of differentiation. PLoS ONE 8:e71008. https://doi.org/10.1371/journal.pone.0071008
Frasheri I, Ern C, Diegritz C, Hickel R, Hristov M, Folwaczny M (2016) Full-length amelogenin influences the differentiation of human dental pulp stem cells. Stem Cell Res Ther 7:10. https://doi.org/10.1186/s13287-015-0269-9
Gambarini G, Plotino G, Grande NM, Nocca G, Lupi A, Giardina B, De Luca M, Testarelli L (2011) In vitro evaluation of the cytotoxicity of FotoSan™ light-activated disinfection on human fibroblasts. Med Sci Monit 17:MT21–MT25. https://doi.org/10.12659/msm.881435
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W (2011) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56:709–721. https://doi.org/10.1016/j.archoralbio.2010.12.008
Wu J, Jia Q, He W, Liu J, Hou L, Zhang J, Niu Z, Ni L (2013) Conditioned medium from periapical follicle cells induces the odontogenic differentiation of stem cells from the apical papilla in vitro. J Endod 39:1015–1022. https://doi.org/10.1016/j.joen.2013.04.011
Pereira LO, Longo JP, Azevedo RB (2012) Laser irradiation did not increase the proliferation or the differentiation of stem cells from normal and inflamed dental pulp. Arch Oral Biol 57:1079–1085. https://doi.org/10.1016/j.archoralbio.2012.02.012
Paranjpe A, Zhang H, Johnson JD (2010) Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J Endod 36:1042–1047. https://doi.org/10.1016/j.joen.2010.02.013
Hatakeyama J, Philp D, Hatakeyama Y, Haruyama N, Shum L, Aragon MA, Yuan Z, Gibson CW, Sreenath T, Kleinman HK, Kulkarni AB (2006) Amelogenin-mediated regulation of osteoclastogenesis, and periodontal cell proliferation and migration. J Dent Res 85:144–149. https://doi.org/10.1177/154405910608500206
Wang Y, Zhao Y, Ge L (2014) Effects of the enamel matrix derivative on the proliferation and odontogenic differentiation of human dental pulp cells. J Dent 42:53–59. https://doi.org/10.1016/j.jdent.2013.10.020
Paduano F, Marrelli M, Amantea M, Rengo C, Rengo S, Goldberg M, Spagnuolo G, Tatullo M (2017) Adipose tissue as a strategic source of mesenchymal stem cells in bone regeneration: a topical review on the most promising craniomaxillofacial applications. Int J Mol Sci 18:2140. https://doi.org/10.3390/ijms18102140
Karkehabadi H, Shahriari S, Najafi R, Khoshbin E, Abbaspourrokni H, Pakseresht Z (2019) Effect of Emdogain coated endodontic materials on viability of human dental pulp stem cells (HDPSCs). Giornale Italiano di Endodonzia 33.
Saberi EA, Karkehabadi H, Mollashahi NF (2016) Cytotoxicity of various endodontic materials on stem cells of human apical papilla. Iran Endod J 11:17–22. https://doi.org/10.7508/iej.2016.01.004
Mohamed DA, Fayyad DM (2017) The effect of different bioactive materials on the odontogenic differentiation potential of dental pulp stem cells using two different culture mediums. Tanta Dental J 14:120–128
Hwang YC, Hwang IN, Oh WM, Park JC, Lee DS, Son HH (2008) Influence of TGF-beta1 on the expression of BSP, DSP, TGF-beta1 receptor I and Smad proteins during reparative dentinogenesis. J Mol Histol 39:153–160. https://doi.org/10.1007/s10735-007-9148-8
Carnio J, Camargo PM, Kenney EB, Schenk RK (2002) Histological evaluation of 4 cases of root coverage following a connective tissue graft combined with an enamel matrix derivative preparation. J Periodontol 73:1534–1543. https://doi.org/10.1902/jop.2002.73.12.1534
Hammarström L, Heijl L, Gestrelius S (1997) Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J Clin Periodontol 24:669–677. https://doi.org/10.1111/j.1600-051x.1997.tb00248.x
Castellanos A, de la Rosa M, de la Garza M, Caffesse RG (2006) Enamel matrix derivative and coronal flaps to cover marginal tissue recessions. J Periodontol 77:7–14. https://doi.org/10.1902/jop.2006.77.1.7
Wu SM, Chiu HC, Chin YT, Lin HY, Chiang CY, Tu HP, Fu MM, Fu E (2014) Effects of enamel matrix derivative on the proliferation and osteogenic differentiation of human gingival mesenchymal stem cells. Stem Cell Res Ther 5:52. https://doi.org/10.1186/scrt441
Li G, Hu J, Chen H, Chen L, Zhang N, Zhao L, Wen N, Yang Y (2017) Enamel matrix derivative enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells on the titanium implant surface. Organogenesis 13:103–113. https://doi.org/10.1080/15476278.2017.1331196
Miller AA, Takimoto K, Wealleans J, Diogenes A (2018) Effect of 3 bioceramic materials on stem cells of the apical papilla proliferation and differentiation using a dentin disk model. J Endod 44:599–603. https://doi.org/10.1016/j.joen.2017.12.018
Min KS, Yang SH, Kim EC (2009) The combined effect of mineral trioxide aggregate and enamel matrix derivative on odontoblastic differentiation in human dental pulp cells. J Endod 35:847–851. https://doi.org/10.1016/j.joen.2009.03.014
Ching HS, Luddin N, Rahman IA, Ponnuraj KT (2017) Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review. Curr Stem Cell Res Ther 12:71–79. https://doi.org/10.2174/1574888x11666160815095733
Jue SS, Lee WY, Kwon YD, Kim YR, Pae A, Lee B (2010) The effects of enamel matrix derivative on the proliferation and differentiation of human mesenchymal stem cells. Clin Oral Implants Res 21:741–746. https://doi.org/10.1111/j.1600-0501.2009.01901.x
Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J, Simmer J, Macdougall M (2002) Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone 30:377–385. https://doi.org/10.1016/s8756-3282(01)00683-4
Asgary S, Nazarian H, Khojasteh A, Shokouhinejad N (2014) Gene expression and cytokine release during odontogenic differentiation of human dental pulp stem cells induced by 2 endodontic biomaterials. J Endod 40:387–392. https://doi.org/10.1016/j.joen.2013.09.017
Chen CC, Shie MY, Ding SJ (2011) Human dental pulp cell responses to new calcium silicate-based endodontic materials. Int Endod J 44:836–842. https://doi.org/10.1111/j.1365-2591.2011.01890.x
Rathinam E, Rajasekharan S, Chitturi RT, Martens L, De Coster P (2015) Gene expression profiling and molecular signaling of dental pulp cells in response to tricalcium silicate cements: a systematic review. J Endod 41:1805–1817. https://doi.org/10.1016/j.joen.2015.07.015
Hajizadeh N, Madani ZS, Zabihi E, Golpour M, Zahedpasha A, Mohammadnia M (2018) Effect of MTA and CEM on mineralization-associated gene expression in stem cells derived from apical papilla. Iran Endod J 13:94–101. https://doi.org/10.22037/iej.v13i1.17860
Saberi E, Farhad-Mollashahi N, Sargolzaei Aval F, Saberi M (2019) Proliferation, odontogenic/osteogenic differentiation, and cytokine production by human stem cells of the apical papilla induced by biomaterials: a comparative study. Clin Cosmet Investig Dent 11:181–193. https://doi.org/10.2147/CCIDE.S211893
Mahapatra D, Nikhade DP, Sukhtankar DS, Padmanabha DP (2020) Enamel matrix derivative in pulpal regeneration and repair. Eur J Mol Clin Med 7:1731–1737
Iwata T, Morotome Y, Tanabe T, Fukae M, Ishikawa I, Oida S (2002) Noggin blocks osteoinductive activity of porcine enamel extracts. J Dent Res 81:387–391. https://doi.org/10.1177/0810387
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625–13630. https://doi.org/10.1073/pnas.240309797
Funding
This study was financially supported by the School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran (Contract Number: 9811298972).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hamed Karkehabadi, Erfan Ahmadyani, Rezvan Najafi, Elham Khoshbin declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karkehabadi, H., Ahmadyani, E., Najafi, R. et al. Effect of biodentine coated with emdogain on proliferation and differentiation of human stem cells from the apical papilla. Mol Biol Rep 49, 3685–3692 (2022). https://doi.org/10.1007/s11033-022-07208-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07208-4