Abstract
This study aimed to evaluate the effects of low-energy blue LED irradiation on the osteogenic differentiation of stem cells from the apical papilla (SCAPs). SCAPs were derived from human tooth root tips and were irradiated with 0 (control group), 1 J/cm2, 2 J/cm2, 3 J/cm2, or 4 J/cm2 blue light in osteogenic induction medium. Cell proliferation was analyzed using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. Osteogenic differentiation activity was evaluated by monitoring alkaline phosphatase (ALP), alizarin red staining, and real-time polymerase chain reaction (RT-PCR). The results of the MTT assay indicated that SCAPs in the LED groups exhibited a lower proliferation rate than those in the control group, and there were statistically differences between the 2 J/cm2, 3 J/cm2, and 4 J/cm2 groups and the control group (P < 0.05). The results of the ALP and alizarin red analyses showed that blue LED promoted osteogenic differentiation of the SCAPs. And 4 J/cm2 blue light upregulates the expression levels of the osteogenic/dentinogenic genes ALP, dentin sialophosphoprotein (DSPP), dentin matrix protein-1 (DMP-1), and osteocalcin (OCN) in SCAPs. Our results confirmed that low-energy blue LED at 1 J/cm2, 2 J/cm2, 3 J/cm2, and 4 J/cm2 could inhibit the proliferation of SCAPs and promotes osteogenic differentiation of SCAPs. Further in vitro studies are required to explore the mechanisms of the effects by low-energy blue LED.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental stem cells include periodontal ligament stem cells (PDLSCs), dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth (SHEDs), dental follicle progenitor cells (DFPCs), and stem cells from the apical papilla (SCAPs) [1]. Since Sonoyama W et al. successfully isolated SCAPs from the papilla of the root tip of young adult teeth, a large number of studies have proved that SCAPs have the abilities for self-renewal, high proliferation, and multidirectional differentiation to dentin, osteoblasts, and vascular nerve cells [2, 3]. SCAPs are usually derived from extracted orthodontic apical papilla tissues, forming root dentin. In the case of young permanent apical lesions, SCAPs can guide the development of the root, eventually forming the root and promoting the healing of periapical tissues [2, 4, 5]. Compared with other odontogenic stem cells, SCAPs have stronger proliferation and differentiation abilities in vitro [6]. SCAPs can stimulate the proliferation of stem cells in vitro to obtain a sufficient number of cells and promote the differentiation of stem cells for the treatment of clinical diseases, which has higher advantages in experiment and clinic and has important significance in tissue regeneration engineering.
Photobiomodulation (PBM) is a treatment modality based on the effects of low-level laser irradiation (LLLI) or light-emitting diode (LED) emissions on biological tissue. PBM has many benefits, such as pain relief, vasodilation [7], tissue healing [8], and promoting the proliferation [9, 10] and mineralization [11]. Blue LED is a safe and effective narrow spectrum light source. Studies have confirmed that irradiation of human skin stem cells with blue light increases cell differentiation and thereby reduces skin hyperplasia [12]. Blue LED can treat neonatal jaundice [13]. Blue LED promotes amniotic fluid–derived stem cell differentiation and inhibits cell proliferation more than red light does [14]. It can effectively promote cell proliferation and differentiation in vitro and is a potential tool in the process of regeneration and tissue engineering in vivo [15]. However, previous studies mainly focused on red LED and laser light, with less attention to blue LED and even less research on odontogenic stem cells. In particular, the effects of blue light on the proliferation and osteogenic differentiation of SCAPs were rarely investigated. In this study, we irradiated SCAPs with low-energy blue LED at various energy densities to elucidate the effects of light energy on the proliferation and osteogenic differentiation of SCAPs.
Materials and methods
The isolation of stem cells from the apical papilla (SCAPs) for this study was performed according to the Ethics Committee of the Affiliated Hospital of Stomatology Southwest Medical University Certificate (contract grant 20180314001). The eligibility criteria for patients were as follows: all donors were in good health without systemic diseases, human premolar teeth with immature roots were collected from 3 patients (10–18 years old). Each analysis in this study was repeated on three independent samples.
Cultivation of SCAPs
SCAPs were obtained according to the protocol of Shu Diao et al. [16]. The cells were taken from young premolar tooth with open root tips, which were extracted for orthodontic treatment in local oral hospitals. The usage of apical papilla tissue was allowed with the informed consent of the patients’ parents and approved by the biomedical science research ethics committee of the Affiliated Hospital of Stomatology Southwest Medical University. The tissue was digested with 3 mg/ml collagenase I (BioSharp Inc, Hefei, China) and 4 mg/ml dispase II (Gibco, Carlsbad, CA, USA). The cells were seeded into a tissue culture flask and incubated in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Shanghai, China) at 37 °C in a humidified atmosphere of 5% CO2. The culture medium was changed twice a week. When the cells had proliferated to approximately 80%, they were passaged at a ratio of 1:2 using 0.25% trypsin (AMRESCO, Solon, OH, USA). The obtained cells were subsequently identified as SCAPs.
Irradiation procedure
The light source device used in this study was a blue light LED (LUX VI; Zhuomuniao, China) with continuous output and wavelength of 420–480 nm (1-W output). The distance from the light source to the cell layer was 1 cm. The spot diameter at this distance was 3.5 cm. Under these conditions, the power density measured at the cell level was 100 mW/cm2. Based on past studies, the calculation formula (energy density = power density × irradiation time), and expected illumination energies, the study was divided into five groups: 1 J/cm2—irradiated for 10 s, 2 J/cm2—irradiated for 20 s, 3 J/cm2—irradiated for 30 s, 4 J/cm2—irradiated for 40 s, and the non-irradiated—control group. Therefore, the light exposure times for each group of cells were 10 s, 20 s, 30 s, 40 s, and 0 s, respectively, every other day. The first day of irradiation was denoted day 1. According to the experimental design and the number of days of testing, the corresponding irradiation and test were carried out. Non-irradiated cells were cultured under the same conditions as the irradiated cells. All irradiations were performed by the same operator.
MTT assay
The 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assays were performed according to the study of Pagin M T et al. [17]. The cells were plated in 96-well plates at a density of 4 × 103 cells with DMEM supplemented with 10% fetal bovine serum (FBS). On the second day, the culture medium was replaced with osteogenic differentiation media (ODM; Cyagen Biosciences, Guangzhou, China). The irradiation was performed on days 1, 3, 5, 7, and 9. Then, MTT analysis was carried out on the first, third, fifth, seventh, and ninth days after the irradiation of the cells. The cell growth curve was plotted as the average absorbance at each time point. Each experiment was performed three times and each point in sextuplicate.
Alkaline phosphatase activity
The osteogenic differentiation methods described in the previous study were followed [18]. Briefly, ALP staining and ALP activity were performed at 7 and 14 days.
Calcium quantitative analysis
Matrix mineralization was analyzed using the alizarin red staining assay after 28 days of culture. The procedure was performed as previously described [18]. To quantitatively determine the calcium mineral content, on day 28, the SCAPs were washed three times with PBS and 100 mM of cetylpyridinium chloride solution (Beijing Solarbio Science & Technology Co., Beijing, China) was added. Then, the dish was incubated for 30 min at room temperature. The supernatants were transferred to a 96-well plate, and the absorbance of each group measured at 562 nm (BioTek, USA). Cetylpyridinium chloride solution was taken as a blank control group.
Real-time polymerase chain reaction analysis
The results for the effect of blue light on SCAPs osteogenic differentiation showed that the osteogenic differentiation of SACPs following irradiation with 4 J/cm2 was stronger than other levels of light energy. Therefore, 4 J/cm2 was chosen as the experimental group for RT-PCR. The non-irradiated group served as the control group.On s 7 and 14, total RNA extraction was carried out using the Trizol kit (Beijing Baiao Science & Technology Co., Beijing, China) according to the manufacturer’s instructions. Real-time PCR reactions were performed with the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) and an IcycleriQ Multi-color Real-time PCR Detection System (Bio-Rad, USA). The expression of genes was calculated using the 2-ΔΔCT method as described previously. The primers for specific genes are shown in Table 1. Real-time polymerase chain reaction (RT-PCR) was performed to analyze the expression levels of ALP, DSPP, DMP-1, and OCN in each group of cells.
Statistical analysis
The results were statistically analyzed using SPSS 17.0 statistical software. The MTT results were analyzed using the Type III Tests of Fixed Effects. The ALP and calcium quantitative analyses were done using one-way ANOVA. The results of RT-PCR were compared using the independent sample T test. Significance was defined as P < 0.05.
Results
Effect of blue LED on cell proliferation
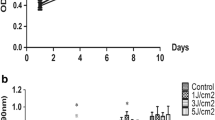
As shown in Fig. 1a, on the 3rd, 5th, 7th, and 9th days, the proliferation rates of the irradiated groups were lower than that of the control group. However, as shown in Fig. 1b, there were statistically significant differences between groups 2 J/cm2, 3 J/cm2, and 4 J/cm2 and the control group (P < 0.05). There were also differences in the proliferation rates between the light energy gradients on day 3 and day 5.
The influence of exposure to low-energy blue LED on the proliferation of SCAPs. a The graph shows the MTT curve. b The graph shows the statistical differences between groups. The data are expressed as the means ± standard deviation. αP < 0.05, light-exposed groups versus control group; βP < 0.05, 2 J/cm2, 3 J/cm2, 4 J/cm2 versus 1 J/cm2; χP < 0.05, 3 J/cm2, 4 J/cm2 versus 2 J/cm2; δP < 0.05, 4 J/cm2 versus 3 J/cm2
Blue LED enhances osteogenesis ALP staining
Early osteogenic differentiation was assessed using ALP staining and activity analysis on days 7 and 14. The results of the ALP staining are shown in Fig. 2a (day 7) and Fig. 2b (day 14). The levels of ALP after 7 and 14 days increased with time and were higher in experimental groups than in the control group. The level in the group that was irradiated with 4 J/cm2 of blue light was higher than that in other groups.
Photographs of the ALP staining of the control (a1, a2) and blue LED (b1, b2, c1, c2, d1, d2, e1, e2) conditions after 7 days (a) and 14 days (b) of culture. (a1–2) SCAPs ALP staining after 0 J/cm2 illumination; (b1–2) SCAPs ALP staining after 1 J/cm2 illumination; (c1–2) SCAPs ALP staining after 2 J/cm2 illumination; (d1–2) SCAPs ALP staining after 3 J/cm2 illumination; (e1–2) SCAPs ALP staining after 4 J/cm2 illumination. The naked eye view bars = 25 mm (a1, b1, c1, d1, e1). The microscopic view scale bars = 100 μm (a2, b2, c2, d2, e2)
ALP activity
The ALP activity was higher in each experimental group than in the control group (Fig. 3a, b). As shown in Fig. 3a, on day 7 after the SCAPs had been exposed to 1, 2, 3, or 4 J/cm2 of blue light, the ALP activities were 102%, 105%, 117%, and 157% of the control group activity, respectively. As shown in Fig. 3b, on day 14, the ALP activities of cells had increased to 105%, 112%, 144%, and 173% of the control group activity, respectively. There were statistically significant differences between the 3 J/cm2 and 4 J/cm2 groups and the control group (P < 0.05). Among the experimental groups, the values for 1 J/cm2 and 2 J/cm2 were statistically different from those for 3 J/cm2 and 4 J/cm2, respectively (P < 0.05), and the value for 3 J/cm2 was statistically different from that for 4 J/cm2 (P < 0.05).
Photographs of the ALP activity after 7 days (a) and 14 days (b) and the quantitative analysis of calcium after 28 days (c) of culture. The results are expressed as the means ± standard deviation. *P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001 indicate significant differences between control and light-exposed groups
Calcium quantitative analysis
The quantitative results of total matrix mineralization are shown in Fig. 3c. Irradiation promoted the mineralization of SACPs. Specifically, the values for the groups that were irradiated with 3 J/cm2 of blue light were higher than those for other groups, with statistically significant differences between 2 J/cm2, 3 J/cm2, and 4 J/cm2 groups and the control group (P < 0.05). Following irradiation with 1, 2, 3, or 4 J/cm2 blue light, the alizarin red activities were equivalent to 101%, 109%, 126%, and 102% of the control respectively.
Blue LED enhanced the expression levels of osteo/dentinogenic differentiation genes of SCAPs
The relative expression levels of ALP, DSPP, DMP-1, and OCN are shown in Fig. 4 a and b. On day 7 (Fig. 4a), the cells that were exposed to 4 J/cm2 of blue light showed an increase in the expression of all four genes, with the differences in the expression of ALP and DSPP being statistically significant (P < 0.05). On day 14 (Fig. 4b), the 4 J/cm2 group showed a significant increase in the expression of all four genes (P < 0.05). However, the expression levels of OCN and DMP-1 were higher than those on day 7, while the expression levels of ALP and DSPP were lower than those on day 7.
Blue LED effects on ALP, DSPP, DMP-1, and OCN gene expression by SCAPs under osteogenic stimulation. a The image shows the amount of gene expression on day 7. b The image shows the amount of gene expression on day 14. *P ≤ 0.05 and **P ≤ 0.01 indicate significant differences between control and light-exposed groups
Discussion
The odontogenic stem cells are derived from the ectoblast mesenchyme and are believed to have characteristics of proliferation and differentiation similar to neural crest cells [19]. SCAPs are important for tooth development and regeneration. Previous research showed that SCAPs derive from early odontoblasts and play an important role in the formation of tooth root dentin and root development [2, 4]. In the process of young permanent tooth lesions, perfect root canal disinfection and drug sealing can promote the development of affected tooth roots, which indicates that SCAPs can survive during inflammation and have strong resistance to infection, and further confirms the importance of SCAPs in root development [4, 20,21,22,23,24]. At the same time, SCAPs can produce vascularized dentin-endodontic complex and osteoblast-like cells [25,26,27]. Therefore, SCAPs are of great significance in the regeneration of bone and tooth tissue.
In the clinic, PBM can be used as an adjuvant therapy to improve clinical outcomes by selecting the correct parameters and indications [28]. Blue LED has a broad spectrum of antibacterial effects [29]. Visible blue light promotes increases in human keratinocyte and endothelial cell differentiation markers [12]. Blue light with wavelength 470 nm can affect the proliferation and osteogenic differentiation of amniotic fluid mesenchymal stem cells [14]. Light doses ranging from 0.5 to 10 J/cm2 are thought to induce cell proliferation [30]. Light energy densities between 0.5 and 4 J/cm2 are more effective in stimulating stem cell growth [31,32,33,34]. The range of LLLI or LED sources is typically 1–500 mW [35, 36]. Studies have confirmed the positive effects of interval exposure during PBM treatment [28]. Therefore, low-energy blue LED with 420~480 nm at a power of 100 mW/cm2 and optical energy density of 0~4 J/cm2 was used in this experiment. The effect of blue LED irradiation on alternate days on SCAPs proliferation and differentiation was also studied. The results provided an experimental basis for clinical treatment of the oral cavity and stem cell engineering.In this experiment, the MTT assay results showed that the overall tendency for all of the groups was an “S”-shaped curve, indicating that the acquired SCAPs were in log phase with good cell activity and good growth ability. In the osteogenic induction environment, the proliferation rate of the control group was higher than that of the experimental groups. With the passage of time, the proliferation rates of irradiated groups were significantly lower than that of the control group, which indicated that blue LED could inhibit the proliferation of SCAPs under mineralization conditions. The inhibition by the light increased with time. Based on the antagonism of stem cell proliferation and differentiation [37], it was therefore speculated that low-energy blue LED promoted the differentiation of SCAPs, similar to the results reported by Robertson [12]. The results of this experiment are consistent with the results of our previous study on the effect of blue light on gingival mesenchymal stem cells [18]. In this experiment, the effective light energy parameters were 0~4 J/cm2. Compared with the control group, the proliferation rates of the light groups from 1 to 4 J/cm2 were altered, and the change at 2 J/cm2 was the most obvious.
ALP and mineralized nodules are important markers of osteogenic differentiation of stem cells. Greater ALP expression is associated with a higher degree of cell osteogenic differentiation. On day 7 and day 14, the ALP expression in the light-exposed groups was higher than that in the control group, which is consistent with the positive effect of PBM therapy on cell differentiation reported by Turrioni et al. [38]. It can be speculated that blue LED can promote SCAPs differentiation. In this experiment, 4 J/cm2 has the most obvious promoting effect, which is consistent with previous studies that showed that 4 J/cm2 light is stimulatory [39] and can promote osteogenic differentiation of BMSCs [40]. The ALP activity of the SCAPs on the 14th day was higher than that on the 7th day, which may be due to either an increase in the ALP in the cells or proliferation of SCAPs, in which case the increase of cell number led to the increase in the ALP detection level. Studies have shown that an energy density of 3 J/cm2 LLLI has a positive impact on the biological characteristics of odontogenic stem cells [41, 42]. The results of the calcium quantitative analysis showed that mineralized nodules were formed in SCAPs in each group, and 2, 3, and 4 J/cm2 contributed to the differentiation of bone, but 3 J/cm2 resulted in a significant change in the expression of late osteoblast markers.
DSPP is a dentin-specific protein that plays an important role in the nucleation of hydroxyapatite and tooth development during the period of dentin calcification [6, 43]. DMP-1 and OCN are mineralized tissue-specific proteins that are highly expressed in bone or dentin matrix [6, 43, 44]. In this experiment, the expression of each gene in the light-exposed groups was higher than that in the control group, similar to the results of previous studies on the osteogenic differentiation of cells and the expression of related genes in the light-exposed group [14]. After exposure to 4 J/cm2, the expression of the ALP gene at day 7 and day 14 was statistically significant, which was consistent with the quantitative detection results of ALP. However, the expression of the ALP gene on day 14 was lower than that on day 7, which differed from the results for the determination of the ALP protein activity. The reason for these discrepant results may be that there are differences between protein and gene expression [6].
The expression levels of DSPP at day 7 and day 14 were also statistically different; however, the expression of DSPP in the later stage was lower than that on day 7, which may be due to the high expression of osteogenic genes and the inhibition of odontogenic differentiation [45]. On the 14th day, the DMP-1 and OCN genes were highly expressed, which indicated that SCAPs mineralization was increased. Thus, under certain conditions, 4 J/cm2 can promote the expression of ALP, DSPP, DMP-1, and OCN genes in SCAPs, which is of great significance for cell differentiation, tooth development, and bone formation.
Therefore, SCAPs proliferation and osteogenic/dentinogenic differentiation could be affected by blue LED under certain conditions. These results lay a solid foundation for MSC therapy and photobiomodulation, which is conducive to the development of SCAPs for tissue engineering. The results also provide a new method for the treatment of oral diseases. However, there are few studies on the mechanism of PBM therapy on the regulation of stem cell biological characteristics. The mechanism of blue LED light on the proliferation and differentiation of SCAPs needs further study. There are still some shortcomings in this experiment, and further exploration is needed regarding the later stages of the effects.
Conclusion
Our results confirmed that low-energy blue LED at 1 J/cm2, 2 J/cm2, 3 J/cm2, and 4 J/cm2 could inhibit the proliferation of SCAPs and promotes osteogenic differentiation of SCAPs. Further in vitro studies are required to explore the mechanisms of the effects by low-energy blue LED.
References
Dixin C, Hongyu L, Mian W et al (2018) The origin and identification of mesenchymal stem cells in teeth: from odontogenic to non-odontogenic. Current Stem Cell Research & Therapy 13(1):39–45
Sonoyama W, Liu Y, Fang D et al (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1:e79
Nada OA, El BRM (2018) Stem cells from the apical papilla (SCAP) as a tool for endogenous tissue regeneration. Frontiers in Bioengineering and Biotechnology 6:103
Huang TJ, Sonoyama W, Liu Y et al (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34(6):645–651
Shiehzadeh V, Aghmasheh F, Shiehzadeh F et al (2014) Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: new method and three case reports. Indian J Dent Res 25(2):248
Bakopoulou A, Leyhausen G, Volk J et al (2011) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56(7):709–721
Pereira MC, de Pinho CB, Medrado AR et al (2010) Influence of 670 nm low-level laser therapy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B Biol 98(3):188–192
Basso FG, Oliveira CF, Kurachi C et al (2013) Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med Sci 28(2):367–374
Pasternak-Mnich K, Ziemba B, Szwed A et al (2019) Effect of photobiomodulation therapy on the increase of viability and proliferation of human mesenchymal stem cells. Lasers Surg Med
Kim JE, Woo YJ, Sohn KM et al (2017) Wnt/β-catenin and ERK pathway activation: a possible mechanism of photobiomodulation therapy with light-emitting diodes that regulate the proliferation of human outer root sheath cells. Lasers Surg 49(10):940–947
Oliveira FA, Matos AA, Santesso MR et al (2016) Low intensity lasers differently induce primary human osteoblast proliferation and differentiation. J Photochem Photobiol B 163:14–21
Konig CJ, Buhner M, Murling G (2010) Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Investig Dermatol 130(1):259
Arnolda G, Chien TD, Hayen A et al (2018) A comparison of the effectiveness of three LED phototherapy machines, single- and double-sided, for treating neonatal jaundice in a low resource setting. PLoS One 13(10):e0205432
Higuchi A, Shen PY, Zhao JK et al (2011) Osteoblast differentiation of amniotic fluid-derived stem cells irradiated with visible light. Tissue Eng A 17(21–22):2593–2602
Ginani F, Soares DM, Alexandre DORH et al (2018) Low-level laser irradiation induces in vitro proliferation of stem cells from human exfoliated deciduous teeth. Lasers Med Sci 33(1):95–102
Diao S, Lin X, Wang L et al (2017) Analysis of gene expression profiles between apical papilla tissues, stem cells from apical papilla and cell sheet to identify the key modulators in MSCs niche. Cell Prolif 50(3):e12337
Pagin MT, de Oliveira FA, Oliveira RC et al (2014) Laser and light-emitting diode effects on pre- osteoblast growth and differentiation. Lasers Med Sci 29(1):55–59
Zhu T, Wu Y, Zhou X et al (2019) Irradiation by blue light-emitting diode enhances osteogenic differentiation in gingival mesenchymal stem cells in vitro. Lasers Med Sci 34(7):1473–1481
Huang TJ, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88(9):792–806
Chueh LH, Huang TJ (2006) Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod 32(12):1205–1213
Estrela C, Alencar AH, Kitten GT et al (2011) Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J 22(2):91–98
Diogenes A, Hargreaves KM (2017) Microbial modulation of stem cells and future directions in regenerative endodontics. J Endod 43(9):S95–S101
Chrepa V, Pitcher B, Henry MA et al (2017) Survival of the apical papilla and its resident stem cells in a case of advanced pulpal necrosis and apical periodontitis. J Endod 43(4):561–567
Lin LM, Kim SG, Martin G et al (2018) Continued root maturation despite persistent apical periodontitis of immature permanent teeth after failed regenerative endodontic therapy. Australian Endodontic Journal the Journal of the Australian Society of Endodontology Inc 44(3):292–299
Huang GTJ, Yamaza T, Shea LD et al (2010) Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng A 16(2):605–615
Na S, Zhang H, Huang F et al (2013) Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J Tissue Eng Regen Med 10(3):261–270
Yan M, Wang L, Lei G et al (2013) Proliferation and osteo/odontoblastic differentiation of stem cells from dental apical papilla in mineralization-inducing medium containing additional KH2PO4. Cell Prolif 46(2):214–222
Marques, Márcia Martins, Diniz, Ivan a Márcia Alves et al (2016) Photobiomodulation of dental derived mesenchymal stem cells: a systematic review. Photomed Laser Surg 34(11):500–508
Lipovsky A, Nitzan Y, Gedanken A, Lubart R (2010) Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers Surg Med 42(6):467–472
Alghamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249
Barboza CAG, Ginani F, Soares DM et al (2014) Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells. Einstein (Sao Paulo) 12(1):75–81
Soares D M , Ginani F , Henriques, ág uida Gomes et al (2015) Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med Sci 30(3):1171–1174
Mvula B, Mathope T, Moore T et al (2008) The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med Sci 23(3):277–282
Zaccara IM, Ginani F, Mota-Filho HG et al (2015) Effect of low-level laser irradiation on proliferation and viability of human dental stem cells. Lasers Med Sci 30(9):2259–2264
Whelan HT, Smits RL, Buchmann EV et al (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19(6):305–314
Posten W, Wrone DA, Dover JS et al (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31(3):334–340
Owen TA, Aronow M, Shalhoub V et al (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143(3):420–430
Turrioni APS, Basso FG, Montoro LA et al (2014) Phototherapy up-regulates dentin matrix proteins expression and synthesis by stem cells from human-exfoliated deciduous teeth. J Dent 42(10):1292–1299
Li WT, Leu YC (2007) Effects of low level red-light irradiation on the proliferation of mesenchymal stem cells derived from rat bone marrow. Conf Proc IEEE Eng Med Biol Soc 2007:5830–5833
Soleimani M, Abbasnia E, Fathi M et al (2012) The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts—an in vitro study. Lasers Med Sci 27(2):423–430
Ballini A, Mastrangelo F, Gastaldi G et al (2015) Osteogenic differentiation and gene expression of dental pulp stem cells under low-level laser irradiation: a good promise for tissue engineering. J Biol Regul Homeost Agents 29(4):813–822
Borzabadi-Farahani A (2016) Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J Photochem Photobiol B Biol 162:577–582
Li R, Peng L, Ren L et al (2009) Hepatocyte growth factor exerts promoting functions on murine dental papilla cells. J Endod 35(3):382–388
Feng JQ, Huang H, Lu Y et al (2003) The dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res 82(10):776–780
Wang S, Mu J, Fan Z et al (2012) Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res 8(3):346–356
Funding
This work was supported by the Luzhou Municipal People’s Government-Southwest Medical University science and technology strategic cooperation projects of China (no. 2017LZXNYD-T03), Luzhou Municipal Science and Technology Bureau of China (no. 2016-R-70(13/24)). The reagents of this study were supported by these funds that all came from Southwest Medical University.
Author information
Authors and Affiliations
Contributions
Yaoyao Yang and Tingting Zhu designed the research, conducted the experiments, and wrote the paper. Yan Wu and Chunxia Shu conducted the experiments. Qiang Chen, Juan Yang, and Xiang Luo performed the data analyses and edited the manuscript. Yao Wang designed the research, supervised the study, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study were in accordance with the Ethics Committee of the Affiliated Hospital of Stomatology Southwest Medical University Certificate (contract grant 20180314001) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Zhu, T., Wu, Y. et al. Irradiation with blue light-emitting diode enhances osteogenic differentiation of stem cells from the apical papilla. Lasers Med Sci 35, 1981–1988 (2020). https://doi.org/10.1007/s10103-020-02995-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02995-3