Abstract
The effects of five aquarium backgrounds (transparent, white, red, blue, and black) on growth, feed utilization, digestive enzyme activities, skin coloration, muscle parameters, and carcass composition were investigated in solid red male Siamese fighting fish (Betta splendens). The fish (1.13 ± 0.01 g initial body weight) were distributed individually into glass aquaria (3.5 cm width × 8 cm length × 20 cm height) varying in color backgrounds (n = 15 per treatment). They were fed a commercial floating diet for small ornamental fish (35% crude protein) twice daily (0800 and 1700 h), and all measurements of fish were examined at the end of 8 weeks. The growth performance (specific growth rate = 0.97% body weight day−1) and feed utilization (feed conversion ratio = 1.60 g feed g gain−1 and protein efficiency ratio = 1.83 g gain g protein−1) of the fish reared on blue background were superior relative to the other treatments, followed by red background as next best (0.86% body weight day−1, 1.50 g feed g gain−1, and 1.71 g gain g protein−1, respectively). There were no significant differences in specific activities of digestive enzymes (pepsin, trypsin, chymotrypsin, lipase, and amylase) between the fish reared on blue and transparent backgrounds (P > 0.05), but significantly increased ratio of amylase to trypsin was observed in the blue treatment. Skin coloration (in terms of lightness and redness), muscle RNA concentration, and muscle RNA/protein ratio did not differ in the fish reared on blue background, while muscle actin/myosin ratio and carcass lipid increased, relative to the control with transparent background. Findings from the current study indicate that the blue-based aquarium background is appropriate for rearing male Siamese fighting fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male Siamese fighting fish (Betta splendens) are popular as ornamental aquarium fish around the world (Froese and Pauly 2017), whereas females are usually sold in bulk at low price, with some being kept by producers for breeding purposes (Monvises et al. 2009). Fin shape and body color are the main criteria used by culturists when choosing male fish for stocking aquaria, but the aggressive and territorial nature of the males means that they are usually held separately from each other (Forsatkar et al. 2017; Monvises et al. 2009). Therefore, improving the physical environment in an aquarium could serve the well-being of captive fish.
Background color is one factor affecting the fish production. This parameter has significant effects on survival, growth performance, feed utilization, and carcass proximate composition of various fish species (McLean et al. 2008; Papoutsoglou et al. 2000; Raghavan et al. 2013). These responses could be induced by direct effects of background color on fish neural/hormonal processes, behavior, and feeding success, or some combination thereof (Papoutsoglou et al. 2005). Therefore, the best background color depends on species and should be separately determined for any species that will be produced (McLean et al. 2008).
Background color can change light environments, in terms of intensity and color, causing stress and using energy available from food digestion (Hochachka and Somero 2002). In order to track feed utilization under chronic stress relating to background color, assessing physiological changes through the digestive enzyme activities is a reasonable approach. Digestive enzymes directly play an important role in transforming macronutrients into forms easily digested, as well as respond to physical parameters in an aquaculture system (Rungruangsak-Torrissen et al. 2006). Previous studies on the light parameters have reported changes in digestive enzyme activities, including protein-, carbohydrate-, and lipid-digesting enzymes (Ramzanzadeh et al. 2016; Shan et al. 2008). Therefore, observations of the digestive enzyme activities might reveal the homeostasis of nutrients under different background colors.
In addition, background color can influence the skin color of aquatic animals (Doolan et al. 2009; Papoutsoglou et al. 2000; Qin et al. 2012). This change is directly related to neural and hormonal response, especially the stress hormones caused by chronic stress, such as melanocyte-stimulating hormone (α-MSH) and melanin-concentrating hormone (MCH) (Lamers et al. 1992; Yamanome et al. 2005). In Siamese fighting fish, redness of the skin is controlled by carotenoids, and the female fish prefer red males over blue males, suggesting a sexual-selection advantage from being red (Clotfelter et al. 2007). Therefore, physiological induction by various backgrounds that may alter the skin coloration in captive fighting fish can impact sexual-selection advantage as well as commercial value of the fish.
For improving the quality of Siamese fighting fish under captive individual rearing conditions, the effects of various aquarium background colors were investigated in the current study, using long-finned solid red male Siamese fighting fish as a model. The quality of reared fish was assessed based on growth performance, feed utilization, digestive enzyme activities, skin color, muscle parameters, and carcass composition. The optimal color from the current study might be used by culturists to improve the commercial value of the fish.
Materials and methods
Fish acclimatization and preparation
One-month-old solid red male Siamese fighting fish were collected from a local farm in Nakonpathom province of Thailand. They were individually acclimatized in transparent cylindrical plastic beakers (7.5 cm diameter × 12.5 cm height) containing 150 mL water, for 2 weeks with the natural 12L:12D cycle (lights-on 0600 to 1800 h). They were fed a commercial floating diet (10% moisture, 35% crude protein, 4% crude fat, 5% crude fiber, and 12% ash) for small ornamental fish (Sakura; U. Lek Trading Co., Ltd., Bangkok, Thailand) twice daily (0800 and 1700 h), with each meal being 1% of fish body weight (BW).
Fish rearing
Seventy-five screened fish of similar size (1.13 ± 0.01 g initial body weight) were distributed individually into glass aquaria (3.5 cm width × 8 cm length × 20 cm height) varying in color backgrounds (transparent, white, red, blue, and black). These color conditions were created by covering outside walls and bottom of aquaria using polypropylene flute board. These individual fish were designated as the experimental units (n = 15 per treatment). The fish were reared in static water and aeration was not supplied. De-chlorinated water was used to replace 80% of the water in each aquarium at 3-day intervals. The water quality during the experiment was maintained at pH 6.54 ± 0.07 and temperature 28.90 ± 0.13 °C. Survival of the reared fish was recorded daily before beginning the first feeding. Uneaten excess diet was siphoned off 30 min after feeding, dried at 60 °C until constant weight, and the value was used to calculate the feeding rate (FR), feed conversion ratio (FCR), and protein efficiency ratio (PER). At the end of 8-week experiment, all the fish were starved for 24 h and then were anesthetized by clove oil (30 mg mL−1). Measurement of BW and lengths (total and standard) of all the fish was performed, followed by determining the color (n = 15 per treatment). The dissection of fish was performed on ice using sterile scalpel and scissors. The abdominal wall was cut in order to remove visceral organs, weighed and used to calculate viscerosomatic index (VSI, n = 15 per treatment) prior to extracting digestive enzymes (n = 5 per treatment). Epaxial white muscle (below the dorsal fin, without scales) was removed and used for muscle analysis (n = 5 per treatment), while the whole body of five fish per treatment (including visceral organs) was minced and used for carcass composition analysis (n = 5 per treatment). All the samples were kept at − 20 °C and all assays were performed within 1 month after sampling. Survival, growth performance, and feed utilization parameters were calculated as described below:
where Wt = mean weight (g) at day t and W0 = mean weight (g) at day t0.
where C = daily feed consumption (g), W0 = initial body weight (g), and Wt = final body weight, (g), t = feeding duration (day).
Determination of digestive enzyme activity
Digestive enzyme extraction and protein quantification
The whole visceral organs were homogenized in 0.2 M Na2HPO4-NaH2PO4 buffer (pH 8) at a ratio of 1:15 (w/v), using a micro-homogenizer (THP-220; Omni International, Kennesaw GA, USA). The homogenates were centrifuged at 15,000×g for 30 min at 4 °C and supernatants were collected and kept at − 20 °C until use. The protein concentration of a crude enzyme extract was determined according to the standard method of Lowry et al. (1951), using bovine serum albumin as protein standard.
Digestive enzyme assay
The protocols for assaying activities of pepsin, trypsin, chymotrypsin, amylase, and lipase are summarized in Table 1. The optimal conditions for Siamese fighting fish were chosen from Thongprajukaew et al. (2010a, b). Each measured product was compared against the linear range of its standard curve. One unit (U) of enzyme is defined as the amount that catalyzed the conversion of 1 μmol of substrate per minute. Specific activities of enzymes are expressed as U (or mU) mg protein−1. Ratio of amylase to trypsin (A/T ratio) was quantified by dividing the specific activities from the same sample.
Color measurement
The unconscious fish were carefully cleaned by soft blotting paper and then the color was measured from the middle parts of bodies. Color measurements and means of expression followed Commission Internationale de l’Eclairage (CIE) standards (CIE 2004). A MiniScan EZ (Hunter Associates Laboratory, Reston VA, USA) was calibrated to white and black standards before measuring the color parameters. The values of skin color recorded were lightness (L*, scale from 0 to 100 represents a color spectrum from black to perfect white), redness (a*, scale describes redness when positive, gray when zero, and greenness when negative), and yellowness (b*, scale describes yellowness when positive, gray when zero, and blueness when negative). The redness index (a*/b*) was calculated from a* and b* separately for each subject.
Muscle analysis
Protein synthesis capacity and its turnover rate
Fifty milligrams of frozen epaxial white muscle was used to determine the concentrations of RNA and protein, as described in Rungruangsak-Torrissen (2007). The muscle was sonicated in TRIzol® reagent (Invitrogen, Carlsbad CA, USA) using ultrasonic processing (VCX; Sonic and Materials Inc., Newtown CT, USA). The mixtures were separated by adding chloroform to obtain upper aqueous phase (RNA) and lower organic phase (protein). The solution was precipitated by isopropanol, washed by ethanol, oven-dried, and dissolved in sodium acetate or sodium dodecyl sulfate (SDS), for RNA or protein determination, respectively. The absorbance was spectrophotometrically measured at 260 nm and 280 nm, and the extinction coefficients for calculation were E260 = 40 μg RNA mL−1 and E280 = 2.1 mg protein mL−1, in the same order. The RNA/protein ratio was calculated from the values from the same sample.
Enthalpy of myosin and actin
Ten milligrams of frozen epaxial white muscle was placed in an aluminum pan, sealed, allowed to equilibrate at room temperature, and then heated from 20 to 100 °C at a rate of 10 °C min−1 against an empty pan. Thermal characteristics (To, onset temperature; Td, denaturation temperature; Tc, conclusion temperature) recorded from a differential scanning calorimeter (DSC7; Perkin Elmer, Waltham, Massachusetts, USA) were used for identification of myosin and actin peaks (Matos et al. 2011; Thongprajukaew et al. 2015). The enthalpy (ΔH) of each protein, referring to amounts of native proteins, was automatically recorded from peak area under the endothermic curve.
Proximate chemical composition of carcass
The moisture and crude ash of the whole body were determined by oven drying at 105 °C for 24 h or until constant weight, and a muffle furnace at 600 °C for 2 h, respectively, according to standard methods of AOAC (2005). Crude protein was determined as described in protocol for muscle protein determination (Rungruangsak-Torrissen 2007). Crude lipid was determined by extracting dried samples with ethyl acetate for 2 h in a rotary mixer, as described in Supannapong et al. (2008). All values are expressed on a wet weight basis.
Statistical analysis
The five treatments followed a completely randomized design (n = 15 per treatment). All 15 replicate fish were used for analysis of BW, length, skin coloration, and VSI, whereas five replicate fish were for digestive enzyme assay, muscle analysis, and carcass composition. The data were subjected to analysis in Statistical Package for Social Science version 14 (SPSS Inc., Chicago, USA) for all the statistical evaluations. The arc sine transformation was applied to percentage values prior to analysis. The data are here expressed as means, with the standard error of mean (SEM) expressing the variability. Comparisons of means in the statistical analyses were carried out using Duncan’s multiple range test at α = 0.05 significance level (P ˂ 0.05).
Results
Survival, growth performance, and feed utilization
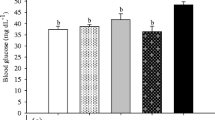
No fish died during the 8-week study period, and fish reared in tanks with black background had poorer growth and feed utilization than the fish in the other treatments (Table 2). Relatively high growth, low feed consumption, best feed conversion, and highest PER were observed in the fish reared on the blue and on the red backgrounds. The highest values of VSI were found for fish reared on the white background, whereas the highest CFs were recorded in fish in the blue and black background treatments (Table 2).
Specific activities of digestive enzymes
The aquarium background had significant effects on the specific activities of digestive enzymes, except for chymotrypsin (Table 3). Pepsin specific activity was higher in fish reared on black background than with red background; the other three treatments were intermediate. The fish reared on the white background had highest trypsin and lipase activities. The fish in the white and black background treatments had higher amylase activities than fish in the transparent (control) treatment, but did not differ significantly from fish reared on red or blue backgrounds. The A/T ratio was highest in the fish reared on the blue background (Table 3).
Color parameters
The a* values of the fish skin did not differ between the five treatments (Table 4). Fish reared on the blue and white backgrounds tended to have highest L*, b*, and a*/b* values, but few significant differences among treatments were observed.
Muscle analysis
Protein synthesis capacity and its turnover rate
The lowest RNA was achieved with black background (Table 5). This treatment also gave the lowest RNA/protein ratio relative to the other remaining treatments, except for the blue background.
Enthalpy of myosin and actin
The amounts of native myosin and actin were similar across the five treatments (Table 5). Fish reared on blue and red backgrounds had the highest and lowest actin/myosin ratio, respectively, while no significant differences among the remaining treatments were observed.
Carcass composition
Varying the background color did not affect crude ash (Table 6). Decreased moisture content was observed in the fish reared on blue and black backgrounds relative to control (transparent) treatment. There were no significant differences in crude protein between most actual treatments and the control. Crude lipid was the highest and lowest in fish reared on blue and transparent backgrounds, respectively, while no significant differences among the remaining treatments were observed.
Discussion
In the present study, growth performance and feed utilization of solid red fighting fish were not statistically different when reared on the blue background relative to control (transparent), while the black background had negative impact on standard length and SGR. Similar positive findings were reported for the white sea bream (Diplodus sargus) when reared on blue tank color (Karakatsouli et al. 2007) as well as a negative effect for scaled carp (Cyprinus carpio) (Papoutsoglou et al. 2000) or Eurasian perch (Perca fluviatilis) (Tamazouzt et al. 2000) when reared on black color.
Increased VSI indicates chronic stress, which leads to viscerosomatic energy storage and viscerosomatic mass growth processes in rainbow trout (Oncorhynchus mykiss) reared in high stocking density (Qun et al. 2016). Significantly increased VSI in the fish reared on white background suggests physiological change caused by the background colors. This phenomenon is contributed by MCH when rearing the fish on white background, leading to the promotion of somatic growth, and reducing locomotion activity as well as conserving energy (Takahashi et al. 2004; Yamanome et al. 2005). Regarding the body morphometrics, different background colors had significant effects on skeletal growth (length) per body mass increase. Lowest CF in fish reared on transparent, white, and red backgrounds indicated the rate of weight increase was faster than the rate of skeletal growth. Lamers et al. (1992) reported that Mozambique tilapia (Oreochromis mossambicus) reared on black background can have increased secretion of α-MSH. This hormone directly affects calcium metabolism and bone resorption (Ikari et al. 2018). Therefore, physiological change caused by the background color is postulated to have significant effects on CF through hormonal regulation.
Improved feed consumption due to sharp contrast between feed and background has been reported by a number of authors (Downing and Litvak 2000; El-Sayed and El-Ghobashy 2011; El-Sayed et al. 2013). This is in agreement with the highest FR in fish reared on white background. In contrast, fish reared on the red or black tanks were inferior in FR; one third of the feed (mixed red, green, and black pellets) has the same color as the tank. In some larval fish, rearing in a black walled tank and feeding by live diet (rotifers and Artemia nauplii) can improve the food perception due to sharper contrast between feed and background color (Hinshaw 1986; Martin-Robichaud and Peterson 1998). Differences in the above results occur due to the colors of water column, tank wall, or feed. However, lower FR with the blue treatment, as well as with red, was not negative, as there was also superior feed utilization in terms of FCR and PER.
Increased FR with high specific activity of digestive enzymes in the fish reared on the white background suggest high capacity of the fish to digest excess food. Therefore, high FR may not indicate good rearing conditions in the current study, similar to the observations in Asian seabass (Lates calcarifer) by Wattanakul et al. (2017). Regarding the A/T ratio, this marker is linked to fish feeding habits (Hofer and Schiemer 1981; Thongprajukaew et al. 2011). Different trends in the fish reared on blue background, relative to the other enzymes, indicate high capacity to utilize carbohydrates per amount of protein. Therefore, increased proportion of carbohydrates in the artificial feed of this carnivorous fish is appropriate when reared on blue background relative to transparent or red background.
Based on the four measured color parameters from the current study, all background colors are suitable since lightness and redness of the skin (a* and a*/b*) were similar to the control fish, while red background had positive effects in skin yellowness. Diminishing lightness when reared on black background, relative to red background, is due to the dispersion of pigments in the dermal melanophores of the skin, caused by secretion of α-MSH (Baker et al. 1984; Rotllant et al. 2003; Szisch et al. 2002), while decreased a*/b* value in solid red male fish when reared on red background, relative to blue background, can improve the distinction of fish from its surrounding environment.
Protein synthesis capacity (RNA concentration) and protein turnover rate (RNA/protein ratio) have been used as indirect indices of fish condition and growth (Saekhow et al. 2018; Sunde et al. 2001; Thongprajukaew et al. 2019). Unchanged values in fish reared on white, red, and blue backgrounds indicate no negative effects on muscle parameter of Siamese fighting fish in the current study. However, some negative effects on protein synthesis capacity and protein turnover rate were observed in fish reared on black background.
In fish flesh, myofibrillar protein (myosin and actin) constituted the major component, followed by sarcoplasmic protein and connective tissue proteins (Chaijan et al. 2010). The amounts of these proteins left in their native state was investigated through ∆H from the thermal property study. The enthalpic response of myosin and actin in the current study indicated no significant effects by the color backgrounds on amounts of myosin and actin, which relate to the physiological exercise by the fish (Thongprajukaew et al. 2015). Coughlin et al. (2016) reported the effects of rearing conditions on myosin heavy chain expression, causing changes in swimming performance and muscle contractile properties. In addition, intense exercise can promote partial denaturation of muscle myosin, leading to lower ΔH actin/myosin ratio (Matos et al. 2011). Therefore, the elevated ΔH actin/myosin ratio with the blue background treatment suggests the color backgrounds decreased partial denaturation of myosin.
In the current study, some improvements in lipid deposition were observed with the blue treatment, while the amount of moisture was inversely affected, when compared to the other treatments. This finding is in agreement with the observations in Caspian kutum (Rutilus frisii kutum) when reared on yellow tank compared to black, white, red, yellow, or blue tanks (Imanpoor and Abdollahi 2011). Higher amount of lipid in fish reared on blue background might be caused by decreased swimming activity, reducing energy usage from lipid catabolism, which leads to accumulation in tissues (Karakatsouli et al. 2010). Differences in energy allocation caused by the background color should be of interest, although with species-specific details.
Conclusions
Based on our investigations of five alternative background colors (transparent, white, red, blue, and black), the solid red male Siamese fighting fish had generally superior growth performance, feed utilization, and muscle protein synthesis capacity and protein turnover rate, without negative effects on carcass composition and skin coloration, when reared on blue background. This treatment exhibited a protein-sparing effect, as indicated by the activity ratio of amylase to trypsin. This background color should be applied by the culturist since the fish are held individually after beginning aggressive behavior (1.5 months) until the fish are sold (3.5 to 4 months). Optimization of the other lighting factors, i.e., photoperiod, light spectral characteristics, light position, and light intensity (or their combined effects), is necessary for the optimal design of an intensive aquaculture system for this species. In addition, since Siamese fighting fish have varied body colors and these fish are significantly responsive to color backgrounds, specific backgrounds should be further investigated for the other color phenotypes, or to determine colors overall suited across the fighting fish.
References
AOAC (2005) Official methods of analysis of AOAC international, 18th edn. Association of Official Analytical Chemists (AOAC), Maryland
Areekijseree M, Engkagul A, Kovitvadhi U, Thongpan A, Mingmuang M, Pakkong P, Rungruangsak-Torrissen K (2004) Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture 234:575–587
Baker BI, Wilson JF, Bowley TJ (1984) Changes in pituitary and plasma levels of MSH in teleosts during physiological colour change. Gen Comp Endocrinol 55:142–149
Chaijan M, Jongjareonrak A, Phatcharat S, Benjakul S, Rawdkuen S (2010) Chemical compositions and characteristics of farm raised giant catfish (Pangasianodon gigas) muscle. LWT-Food Sci Technol 43:452–457
CIE (2004) Colorimetry, 3rd edn. Commission Internationale de l'Eclairage (CIE) Central Bureau, Vienna
Clotfelter ED, Ardia DR, Mcgraw KJ (2007) Red fish, blue fish: trade-offs between pigmentation and immunity in Betta splendens. Behav Ecol 18:1139–1145
Coughlin DJ, Shiels LP, Nuthakki S et al (2016) Thermal acclimation to cold alters myosin content and contractile properties of rainbow smelt, Osmerus mordax, red muscle. Comp Biochem Physiol 196A:46–53
Doolan BJ, Booth MA, Allan GL, Jones PL (2009) Changes in skin colour and cortisol response of Australian snapper Pagrus auratus (Bloch & Schneider, 1801) to different background colours. Aquac Res 40:542–550
Downing G, Litvak MK (2000) The effect of photoperiod, tank colour and light intensity on growth of larval haddock. Aquac Int 7:369–382
El-Sayed AFM, El-Ghobashy AE (2011) Effects of tank colour and feed colour on growth and feed utilization of thinlip mullet (Liza ramada) larvae. Aquac Res 42:1163–1169
El-Sayed AFM, El-Ghobashy AE, El-Mezayen MM (2013) Effect of feed colour on growth and feed utilization of Nile tilapia (Oreochromis niloticus L.) larvae and fingerlings. Aquac Nutr 19:870–876
Forsatkar MN, Nematollahi MA, Brown C (2017) Male Siamese fighting fish use gill flaring as the first display towards territorial intruders. J Ethol 35:51–59
Froese R, Pauly D (2017) Betta splendens Regan, 1910 Siamese fighting fish https://www.fishbase.org/summary/4768 Cited 10 Mar 2017
Hinshaw JM (1986) Factors affecting feeding, survival and growth of larval and early juvenile yellow perch (Perca flavescens Mitchill). Dissertation, North Carolina State University
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York
Hofer R, Schiemer F (1981) Proteolytic activity in the digestive tract of several species of fish with different feeding habits. Oecologia 48:342–345
Ikari T, Kobayashi Y, Kitani Y, Sekiguchi T, Endo M, Kambegawa A, Asahina K, Hattori A, Tabuchi Y, Amornsakun T, Mizusawa K, Takahashi A, Suzuki N (2018) α-Melanocyte-stimulating hormone directly increases the plasma calcitonin level and involves calcium metabolism in goldfish. Int Aquat Res 10:283–292
Imanpoor MR, Abdollahi M (2011) Effects of tank colour on growth, stress response and skin colour of juvenile Caspian kutum Rutilus frisii kutum. Glob Vet 6:118–125
Karakatsouli N, Papoutsoglou SE, Manolessos G (2007) Combined effects of rearingdensity and tank colour on the growth and welfare of juvenile white sea bream Diplodus sargus L. in a recirculating water system. Aquac Res 38:1152–1160
Karakatsouli N, Papoutsoglou SE, Sotiropoulos N et al (2010) Effects of light spectrum, rearing density and light intensity on growth performance of scaled and mirror common carp Cyprinus carpio reared under recirculating system conditions. Aquac Eng 42:121–127
Lamers AE, Flik G, Atsma W, Wendelaar Bonga SE (1992) A role for di-acetyl α-melanocyte-stimulating hormone in the control of cortisol release in the teleost Oreochromis mossambicus. J Endocrinol 135:285–292
Lowry OH, Rosenbrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Martin-Robichaud DJ, Peterson RH (1998) Effects of light intensity, tank colour and photoperiod on swimbladder inflation success in larval striped bass, Morone saxatilis (Walbaum). Aquac Res 29:539–547
Matos E, Silva TS, Tiago T, Aureliano M, Dinis MT, Dias J (2011) Effect of harvesting stress and storage conditions on protein degradation in fillets of farmed gilthead seabream (Sparus aurata): a differential scanning calorimetry study. Food Chem 126:270–276
McLean E, Cotter P, Thain C et al (2008) Tank colour impacts performance of cultured fish. Croat J Fish 66:43–54
Monvises A, Nuangsaeng B, Sriwattanarothai N, Panijpan B (2009) The Siamese fighting fish: well-known generally but little-known scientifically. ScienceAsia 35:8–16
Papoutsoglou SE, Mylonakis G, Miliou H, Karakatsouli NP, Chadio S (2000) Effects of background colour on growth performances and physiological responses of scaled carp (Cyprinus carpio L.) reared in a closed circulated system. Aquac Eng 22:309–318
Papoutsoglou SE, Karakatsouli N, Chiras G (2005) Dietary L-tryptophan and tank colour effects on growth performance of rainbow trout (Oncorhynchus mykiss) juveniles reared in a recirculating water system. Aquac Eng 32:277–284
Qin G, Lin Q, Gu N et al (2012) Effect of broodstock origin, background and substrate colour on skin colouration of three-spotted seahorses Hippocampus trimaculatus leach, 1814. J Exp Mar Biol Ecol 416–417:129–134
Qun L, Zhishuai H, Haishen W et al (2016) Effect of stocking density on water quality and (growth, body composition and plasma cortisol content) performance of pen-reared rainbow trout (Oncorhynchus mykiss). J Ocean Univ China 15:667–675
Raghavan PR, Xiao-ming Z, Wu L et al (2013) Rearing tank colour influences survival and growth of the early larvae of the yellow catfish, Pelteobagrus fulvidraco, Richardson. Acta Hydrobiol Sin 37:177–184
Ramzanzadeh F, Yeganeh S, JaniKhalili K, Babaei SS (2016) Effects of different photoperiods on digestive enzyme activities in rainbow trout (Oncorhynchus mykiss) alevin and fry. Can J Zool 94:435–442
Rotllant J, Tort L, Montero D, Pavlidis M, Martinez M, Wendelaar Bonga SE, Balm PHM (2003) Background colour influence on the stress response in cultured red porgy Pagrus pagrus. Aquaculture 223:129–139
Rungruangsak K, Utne F (1981) Effect of different acidified wet feeds on protease activities in the digestive tract and on growth rate of rainbow trout (Salmo gairdneri Richardson). Aquaculture 22:67–79
Rungruangsak-Torrissen K (2007) Digestive efficiency, growth and qualities of muscle and oocyte in Atlantic salmon (Salmo salar L.) fed on diets with krill meal as an alternative protein source. J Food Biochem 31:509–540
Rungruangsak-Torrissen K, Moss R, Andresen LH, Berg A, Waagbø R (2006) Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol Biochem 32:7–23
Saekhow S, Thongprajukaew K, Phromkunthong W, Sae-khoo H (2018) Minimal water volume for intensively producing male Siamese fighting fish (Betta splendens Regan, 1910). Fish Physiol Biochem 44:1075–1085
Shan XJ, Xiao ZZ, Huang W, Dou S (2008) Effects of photoperiod on growth, mortality and digestive enzymes in miiuy croaker larvae and juveniles. Aquaculture 281:70–76
Sunde J, Taranger GL, Rungruangsak-Torrissen K (2001) Digestive protease activities and free amino acids in white muscle as indicators for feed conversion efficiency and growth rate in Atlantic salmon (Salmo salar L.). Fish Physiol Biochem 25:335–345
Supannapong P, Pimsalee T, A-komol T, Engkagul A, Kovitvadhi U, Kovitvadhi S, Rungruangsak-Torrissen K (2008) Digestive enzymes and in vitro digestibility of different species of phytoplankton for culture of the freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus. Aquac Int 16:437–453
Szisch V, Van der Salm AL, Wendelaar Bonga SE et al (2002) Physiological colour changes in the red porgy, Pagrus pagrus, following adaptation to blue lighting spectrum. Fish Physiol Biochem 27:1–8
Takahashi A, Tsuchiya K, Yamanome T, Amano M, Yasuda A, Yamamori K, Kawauchi H (2004) Possible involvement of melanin-concentrating hormone in food intake in a teleost, barfin flounder. Peptides 25:1613–1622
Tamazouzt L, Chatain B, Fontaine P (2000) Tank wall colour and light level affect growth and survival of Eurasian perch larvae (Perca fluviatilis L.). Aquaculture 182:85–90
Thongprajukaew K, Kovitvadhi U, Engkagul A et al (2010a) Characterization and expression levels of protease enzymes at different developmental stages of Siamese fighting fish (Betta splendens Regan, 1910). Agric Nat Resour 44:411–423
Thongprajukaew K, Kovitvadhi U, Engkagul A et al (2010b) Temperature and pH characteristics of amylase and lipase at different developmental stages of Siamese fighting fish (Betta splendens Regan, 1910). Agric Nat Resour 44:210–219
Thongprajukaew K, Kovitvadhi U, Kovitvadhi S et al (2011) Effects of different modified diets on growth, digestive enzyme activities and muscle compositions in juvenile Siamese fighting fish (Betta splendens Regan, 1910). Aquaculture 322–323:1–9
Thongprajukaew K, Rodjaroen S, Yoonram K, Sornthong P, Hutcha N, Tantikitti C, Kovitvadhi U (2015) Effects of dietary modified palm kernel meal on growth, feed utilization, radical scavenging activity, carcass composition and muscle quality in sex reversed Nile tilapia (Oreochromis niloticus). Aquaculture 439:45–52
Thongprajukaew K, Pettawee S, Muangthong S, Saekhow S, Phromkunthong W (2019) Freeze-dried forms of mosquito larvae for feeding of Siamese fighting fish (Betta splendens Regan, 1910). Aquac Res 50:296–303
Wattanakul W, Thongprajukaew K, Songnui A, Satjarak J, Kanghae H (2017) Pre-soaking feed pellet significantly improved feed utilization in Asian seabass (Lates calcarifer). Aquaculture 471:106–112
Winkler UK, Stuckmann M (1979) Glycogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol 138:663–670
Yamanome T, Amano M, Takahashi A (2005) White background reduces the occurrence of staining, activates melanin-concentrating hormone and promotes somatic growth in barfin flounder. Aquaculture 244:323–329
Acknowledgments
Funding was provided by the budget revenue (contract no. SCI590420S) of the Prince of Songkla University (PSU). We acknowledge Assoc. Prof. Dr. Seppo Karrila and the Publication Clinic, Research and Development Office, PSU, for advice in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saekhow, S., Thongprajukaew, K. & Phromkunthong, W. Blue aquarium background is appropriate for rearing male Siamese fighting fish (Betta splendens). Aquacult Int 27, 891–903 (2019). https://doi.org/10.1007/s10499-019-00374-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00374-6