Abstract
Water volume is a key parameter affecting the individual rearing of male Siamese fighting fish (Betta splendens Regan, 1910). In this study, minimization of water volume was pursued by assessing growth, feed utilization, digestive enzyme activities, color coordinates, muscle quality, and carcass composition. One-month-old solid-red male fish (0.97 ± 0.01 g initial body weight) were distributed individually into glass aquaria with five alternative water volumes (100, 150, 200, 250, and 300 mL), comprising 15 fish per treatment (n = 15), over 8 weeks duration. No mortality of the reared fish was found during the study. Growth performance and feed utilization of the fish reared in 150 mL water were superior to the other treatments. The water volume significantly affected specific activities of the digestive enzymes (P ˂ 0.05), except for amylase, and no differences in enzyme activities were observed between fish reared in 150 and in 300 mL water. The preferred treatment maintained skin lightness (L*) and had the highest redness (a* and a*/b*) among the treatments. Protein synthesis (RNA concentration) and its turnover rate (RNA/protein ratio) and myosin and actin in muscle also benefited from this treatment. Carcass composition, in terms of moisture, crude protein, and crude ash, was maintained, but the amount of crude lipid fluctuated with water volume. Based on our experiments, the preferred minimal water volume for individual rearing of male Siamese fighting fish should be about 150 mL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Siamese fighting fish (Betta splendens Regan, 1910) is a commercially produced and exported species of ornamental fish in Thailand (Monvises et al. 2009). It inhabits shallow waters among dense emergent vegetation near the margins of hill streams, swamps, or paddy fields (Jaroensutasinee and Jaroensutasinee 2001) and is native to the Mekong basin of Laos, Cambodia, Vietnam, and Thailand (Froese and Pauly 2016). Only the male fish are popular for their brilliant colors, and solid red is preferred by the fish culturists with solid blue coming in the second place (Thongprajukaew et al. 2014).
In the nature, B. splendens has a mean population density of 1.7 fish m−2 in shallow (about 2–9 cm) waters (Jaroensutasinee and Jaroensutassinee 2001). Under captivity in aquaria, this fish can be reared in small containers with poor quality water (Jaroensutasinee and Jaroensutasinee 2001). Individual rearing of this species can depress its otherwise aggressive behavior under captivity in aquaria (Halperin et al. 1992). Various water volumes have been used for rearing B. splendens in laboratory studies (Karino and Someya 2007; Verbeek et al. 2008; Takeuchi et al. 2010), as well as during export logistics. However, the minimal water volume for rearing this species has not been examined, but needs to be assessed.
The stocking density has been demonstrated as a variable crucial to growth performance (Sanchez et al. 2010), survival (Jha and Barat 2005), and feed utilization (Niazie et al. 2013) of cultured fishes. The effects of density on these parameters vary by fish species. Negative effects from decreased water volume have been generally reported (Jha and Barat 2005; Ahmadivand et al. 2013); however, also positive effects have been found in some cases (Osofero et al. 2009; Sammouth et al. 2009; Niazie et al. 2013). Varying the stocking density can require adaptations in the fish lifestyle by changes in feed consumption, social interactions, water quality, and stress (Cristea et al. 2012). In addition, the density also affects digestive enzymes that relate to the mechanisms of digestion and the feed utilization (Bolasina et al. 2006; Kanghae et al. 2016).
Stocking denstity has been reported to affect skin pigmentation in various fish (Ali et al. 2006; Zeng et al. 2010). Therefore, the physiological induction under restricted water volume might alter the skin coloration of captive fighting fish. In addition, studies of the myofibrillar proteins, actin and myosin (Tyska and Warshaw 2002), and protein synthesis capacity (Sunde et al. 2001), appear necessary to observe as indicators of the physiological changes in muscles under captivity. Proximate composition of carcass may also be affected by the stocking density (Osofero et al. 2009; Cretu et al. 2014; Lui et al. 2016), due to accumulation or depletion of energy reserves (Cretu et al. 2014). Therefore, the objective of this study was to minimize the water volume for the individual rearing of male Siamese fighting fish, based on assessment of growth, feed utilization, digestive enzyme activities, skin color, muscle quality, and carcass composition. Only solid-red male fish were chosen as the models, because they have the relatively high market prices in comparison to other solid colorations (Thongprajukaew et al. 2014). Findings from the current study might be useful for farm management, as well as on developing systems for the intensive rearing of this species.
Materials and methods
Fish preparation and experimental setup
One-month-old solid-red male Siamese fighting fish were purchased from a local farm in Nakonpathom province of Thailand. The fish were individually acclimatized in cylindrical plastic beakers (7.5 cm diameter × 12.5 cm height) containing 250 mL water, for 2 weeks. The fish with similar size (0.97 ± 0.01 g initial body weight) were distributed individually into glass aquaria (3.5 cm width × 8 cm length × 20 cm height) containing five alternative water volumes (100, 150, 200, 250, and 300 mL), comprising 15 fish per treatment (n = 15) as the experimental units. The fish were fed a commercial floating diet (MCT Aquarium, Nakonpathom, Thailand) for small ornamental fish (containing ˃ 10% moisture, ˃ 46% crude protein, ˃ 6% crude lipid, ˃ 5% crude fiber, and ˃ 12% crude ash) twice daily (08.00 and 17.00 h) at 2% of body weight (BW). The experiment was conducted for 8 weeks with the natural 12 h:12 h light/dark cycle. Survival was recorded daily before beginning the first feeding. Uneaten excess diet was siphoned off 30 min after feeding, dried at 60 °C until constant weight, and the determined weight was used to calculate the feeding rate (FR), feed conversion ratio (FCR), and protein efficiency ratio (PER). At the end of the experiment, all the fish were starved for 24 h and then were anesthetized by clove oil. Measurement of BW and standard length of all the fish was performed at the end of experiment. All these fish were used for subsequent analysis of digestive enzymes, muscle quality, color coordinates, and carcass composition.
Water monitoring
The water was 80% replaced by dechlorinated stock within 3 consecutive days. Water was sampled at the same time (07.30 h) for quality analysis. Water temperature (Hg thermometer) and pH (pH meter) were determined according to standard methods of APHA, AWWA, and WPCF (1998). Ammonia, nitrite, and alkalinity were determined using a commercial test kit (Merck KGaA, Darmstadt, Germany). Dissolved oxygen was determined by a water analyzer (Multiparameter Display System; YSI 650MDS, YSI Incorporated, Ohio, USA).
Determination of digestive enzyme activity
Extraction of digestive enzymes
The frozen whole visceral organs (n = 5) were extracted in 0.2 M Na2HPO4-NaH2PO4 buffer (pH 8) at a ratio of 1:15 (w/v), using a micro-homogenizer (THP-220; Omni International, Kennesaw GA, USA). The homogenates were centrifuged at 15,000×g for 30 min at 4 °C, and supernatants were collected, and aliquots were kept at – 20 °C until use. The protein concentration of a crude enzyme extract was compared to a standard curve of bovine serum albumin, according to the standard method of Lowry et al. (1951).
Digestive enzyme assay
The optimal conditions of assaying pepsin (EC 3.4.23.1), trypsin (EC 3.4.21.4), chymotrypsin (EC 3.4.21.1), amylase (3.2.1.1), and lipase (EC 3.1.1.3) in Siamese fighting fish were chosen from Thongprajukaew et al. (2010a, b). Pepsin activity was assayed according to the method of Rungruangsak and Utne (1981), using 2% casein as substrate. The activity was spectrophotometrically measured at 720 nm against the linear range of L-tyrosine standard. Trypsin and chymotrypsin activities were determined as described by Rungruangsak-Torissen et al. (2006), using 1.25 mM N-α-benzoyl-Arg-p-nitroanilide (BAPNA) and 0.10 mM N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (SAPNA) as substrates, respectively. The liberated product of each enzyme was spectrophotometrically measured at 410 nm against the linear range of p-nitroanilide standard. Lipase activity was assayed according to the method of Winkler and Stuckmann (1979), using 0.01 M p-nitrophenyl palmitate (p-NPP) as substrate. The absorbance at 410 nm was measured against p-nitrophenol standard. Amylase activity was assayed as described by Areekijseree et al. (2004), using 5% starch soluble as the substrate. The product was measured at 540 nm against maltose standard.
Color measurement
Quantitative values of skin color in terms of lightness (L*), redness/greenness (a*), and yellowness/blueness (b*) were measured using a MiniScan EZ (Hunter Associates Laboratory, Reston VA, USA) with small area view (6 mm port and 5 mm view diameters). The instrument was calibrated to white and black standards before measurement. Each unconscious fish (n = 15) was carefully cleaned by soft blotting paper and then the color was measured from the middle part of body. The redness index (a*/b*) was also calculated.
Muscle quality
Protein synthesis capacity
The expoxial white muscle excluding scale and skin (below dorsal fin) was carefully removed (n = 5). Then 50 mg frozen samples were sonicated (VCX; Sonic and Materials Inc., Newtown CT, USA) in TRIzol® reagent (Invitrogen, Carlsbad CA, USA) and the concentrations of RNA and protein were determined as described in Rungruangsak-Torrissen (2007). The extinction coefficients for calculating RNA and protein were E260 = 40 μg RNA per milliliter and E280 = 2.1 mg protein per milliliter, respectively. The concentration ratio (RNA/protein ratio) from each sample was calculated from the amounts of RNA and protein.
Actin and myosin
Thermal parameters, including onset (To), denaturation peak (Td), and conclusion (Tc) temperatures, and enthalpy (ΔH), were determined using a differential scanning calorimeter (DSC7, Perkin Elmer,Waltham, Massachusetts, USA). Approximately 10 mg of thawed fish muscle (n = 5) was placed in an aluminum pan, sealed, allowed to equilibrate at room temperature, and then heated from 20 to 100 °C at a rate of 5 °C min−1 against an empty pan. Identification of myosin and actin peaks was performed as described in Thongprajukaew et al. (2015).
Proximate chemical composition of carcass
The whole body (n = 5) was minced and then moisture and crude ash were determined according to standard methods of AOAC (2005). Crude protein was determined as described in Rungruangsak-Torrissen (2007). Crude lipid was determined by ethyl acetate extraction according to Supannapong et al. (2008).
Statistical analysis and calculations
The experiment followed a completely randomized design (CRD), comprising five treatments and 15 replications each. The data were recorded in Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) and then subjected to analysis in Statistical Package for Social Science (SPSS) Version 14 (SPSS Inc., Chicago, USA) for all the statistical evaluations. The data are here expressed as mean ± standard error of mean (SEM). Comparisons of means in the statistical analyses were carried out using Duncan’s multiple range test with significance threshold P ˂ 0.05. Survival, growth performance, and feed utilization parameters were calculated as described below:
where Wt = mean weight (g) at day t, W0 = mean weight (g) at day t0.
where C = daily feed consumption (g), W0 = initial body weight (g), Wt = final body weight (g), t = feeding duration (day)
Results
Water quality during experiment
There were no significant differences in water temperature, dissolved oxygen, nitrite, or alkalinity between the five treatments (P ˃ 0.05, Table 1). The pH values were elevated in the treatments with 250 and 300 mL water (P ˂ 0.05), followed by 150 and 200 mL (P ˃ 0.05), on comparing to the lowest volume. Ammonia concentration was highest in the 100 mL volume, although without significant difference to the 150 mL treatment.
Survival, growth performance, and feed utilization
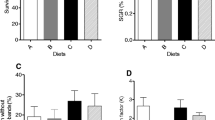
No mortality of reared fish was observed within the studied period (Table 2). Final body weight, body thickness, body depth, and SGR did not differ significantly between the five tratments. The highest and lowest weight gains were observed in the fish reared in 150 and 300 mL, respectively; the three remaining water volumes were intermediate between those two groups. Standard length was superior in the fish reared in 300 mL water relative to the lowest volume, while no significant differences were observed in the other groups. VSI was significantly decreased in the fish reared in 150 mL relative to the other four treatments. The highest CF values were observed in the fish reared in the two least water volumes. FCR and PER had superior values in the fish reared with 150, 200, or 250 mL water, while the FR showed no significant differences between the treatments.
Specific activities of digestive enzymes
The water volumes had significant effects on the specific activities of digestive enzymes, except for amylase (Table 3). The fish reared in the least water volume exhibited decreased enzyme activities, except for lipase and A/T ratio, relative to the other treatments. The fish reared in 200 or 250 mL had significant reductions in trypsin- and lipase-specific activities, respectively. There were no differences in any enzyme activity between rearing in 150 and 300 mL.
Color parameters
L* values of fish skin did not differ among the five treatments (Table 4). Color parameters indicating skin redness (a* and a*/b*) had their highest values in the fish reared in 150 mL water. This treatment also maintained the b* value, as did the treatments with 100, 200, and 250 mL, but the 300 mL treatment did not.
Muscle quality
Protein synthesis capacity
The highest RNA concentration was achieved by the fish reared in 200 and 250 mL (P ˂ 0.05) and followed by 150 mL (P ˃ 0.05) relative to 100 and 300 mL treatmentss (Table 5). There were no differences in protein concentration among the three least volume treatments, followed by statistically significant reduction with the 250 and 300 mL treatments. This trend was opposite to that observed for the RNA/protein ratio.
Thermal properties of actin and myosin
The amount of native myosin was relatively high in the fish reared in 150, 200, and 250 mL water volumes (Table 5). The amount of actin showed no significant differences between the five treatments.
Carcass composition
Varying the water volumes did not affect carcass moisture, crude protein, or crude ash (Table 6). Crude lipid was the highest in fish reared in 100 mL water, followed by 150 mL and the other three treatments.
Discussion
The water quality was significantly affected by the water volume, in the current study. The decrease of pH and increase of total ammonia with higher stocking density (lower water volume per fish) were similar to prior observations in koi carp, Cyprinus carpio (Jha and Barat 2005) and seabass, Dicentrarchus labrax (Sammouth et al. 2009). The pH in all treatments was higher than in the natural habitat of the wild fighting fish, which is pH 5.28–5.80 (Jaroensutasinee and Jaroensutasinee 2001). However, under laboratory conditions, the pH in all these treatments was within the range for rearing fighting fish, namely pH 6.5–7.5 (Verbeek et al. 2007), except for the slightly low pH with 100 mL water volume (pH 6.44 ± 0.04). Total ammonia was significantly elevated with 100 mL water relative to the other treatments; high-rearing density can cause dramatic changes in water quality from the excreted ammonia wastes. This observed trend in ammonia has been previously reported on rearing some fish (Jha and Barat 2005; Sammouth et al. 2009; Lui et al. 2016). Based on two above parameters, rearing the fighting fish individually in 100 mL water volume appears to be inappropriate, since it provides relatively poor water quality when compared to larger water volumes. However, the water exchange rate naturaly affects water quality, and in intensive aquaculture systems, the water is typically changed more often or a recirculating system is used. Regarding the other water quality parameters, no significant differences between the treatments were observed, and these parameters were within good levels, close to the ranges reported for B. splendens aquaculture (Jaroensutasinee and Jaroensutasinee 2001; Mandal et al. 2010).
Stocking density is among the most important rearing parameters affecting fish growth (Jha and Barat 2005; Sanchez et al. 2010; Niazie et al. 2013). Generally, food competition limits fish growth and leads to poor weight gain with high-density rearing (Stickney 1994). On the other hand, the decreased weight increment in the largest water volume of the current study might be associated with increasing swimming activity (Karakatsouli et al. 2010) as well as bubble nest building by this male fish, reducing the energy for growth. A minimal water volume can significantly decrease skeletal growth. Regarding morphometry, only CF was affected by the water volume while body thickness and depth were not. The relatively high CF value observed in the current study is due to the calculation of body weight per standard length, which reduces the variations from fin shape phenotypes. Lambert and Dutil (2001) have reported negative effects of increasing stocking density on the CF of Atlantic cod (Gadus morhua). This conflicts with the results found in this current study, where the male fighting fish were individually reared, reducing the social interactions between the fish. However, similar CF has been reported across different stocking densities when rearing fish (Saoud et al. 2007; Tolussi et al. 2010). The CF and VSI can relate to body energy storage, as observed by Goede and Barton (1990). Increased CF and VSI in the fish reared in the least water volume might be due to viscerosomatic energy storage and to viscerosomatic mass growth processes that reduce growth performance (Lui et al. 2016).
The SGR of fighting fish in this investigation was similar to that in data reported by James and Sampath (2006). Based on our investigation of growth parameters, the minimal water volume for rearing male fighting fish should be about 150 mL. This level had 5.36 cm water depth, which matches well the depth in the preferred natural habitat, 2–9 cm (Jaroensutasinee and Jaroensutassinee 2001). In addition, this volume is below the 250 mL used by the supplying farm and below the volumes reported by various researchers: 250 mL (Verbeek et al. 2008), 1000 mL (Takeuchi et al. 2010), and 2000 mL (Karino and Someya 2007).
Superior feed utilization (FR, FCR, and PER) was also observed in the fish reared in 150 mL water. This finding matches well the increased protease (pepsin, trypsin, chymotrypsin) and lipase activities. Since B. splendens are carnivorous fish, up-regulation of the protease activities can improve digestion and utilization of dietary proteins (Chakrabarti et al. 1995) and ditto to lipids and lipase activity. Since glucose is an essential energy source for a number of tissues (Romijn et al. 1990), maintaining the amylase activity is necessary for metabolic homeostasis. This finding is in agreement with the response of turbot, Scophthalmus maximus, when subjected to four rearing density levels (Xian et al. 2013). However, feeding habits, as indicated by the A/T ratio (Hofer and Schiemer 1981), were influenced by the water volume. Rearing the fish in 100 mL water can increase the energy requirements, increasing carbohydrate catabolism per amount of protein.
Skin color is another important factor influencing the commercial value of fish. In B. splendens, skin redness is controlled by carotenoids and the female fish prefer to associate with red males over blue males, suggesting a sexually selected advantage to being red (Clotfelter et al. 2007). In addition, within the red phenotype groups, the female fish prefer to associate with the vermilion males over those that are pale red (Blakeslee et al. 2009). In the current study, the skin redness parameters (a* and a*/b*) were the highest in fish reared individually in 150 mL water, suggesting that this treatment contributes to a valuable ornamental trait as well as to courting competency of the male fish. Metusalach et al. (1997) reported stocking density has an effect on carotenoid uptake in Arctic charr (Salvelinus alpinus); the highest pigment deposition was observed in the fish stocked at 50 kg m−3, when the other alternatives were lower (40 kg m−3) or higher (75 kg m−3) levels. Similar findings were also reported in darkbarbel catfish, Pelteobagrus vachelli (Zeng et al. 2010), and in rainbow trout, Oncorhynchus mykiss (Çagiltay et al. 2015). These responses are similar to our observations, but the amout of pigments in B. splendens feed and its feeding rate were fixed during this study. Stress is a more important factor affecting skin color than the dietary carotenoid administration, even though carotenoids are important pigments contributing to the red skin of B. splendens (Metusalach et al. 1997; Clotfelter et al. 2007). Fluctuations in skin redness might be associated with environmental cues, water volume, and with generating striped patterns of chromatophores (Kelsh 2004).
Increased muscle protein concentration during growth has been observed in rainbow trout (Rungruangsak-Torrissen et al. 2009). In Atlantic salmon (Salmo salar), SGR was negatively correlated with muscle RNA and RNA/protein ratio (Sunde et al. 2001). Similarly, significantly lower RNA concentration (protein synthesis) and RNA/protein ratio (protein turnover rate) were also observed in the faster growing males than in the slower growing females of Siamese fighting fish (Thongprajukaew et al. 2013). Based on these previous reports, reduced protein sythesis capacity and protein turnover rate while the protein concentration was maintained suggest continuous growth of the fish individually reared under the preferred conditions, i.e., in 150 mL water. The measured ∆H is linked to the amount of proteins left in their native state and may relate to the physiological exercise by the fish (Thongprajukaew et al. 2015) in a restricted water volume. Myofibrillar protein is the major component of fish muscle (39–56%), followed by sarcoplasmic protein (21–25%) and stroma or connective tissue proteins (6–21%) (Chaijan et al. 2010); myosin contributes 50–60% of the total (Shahidi 1994). The comparatively elevated ∆H of myosin in fish reared in 150, 200, or 250 mL of water may indicate its increased amount in muscle. This is similar to the findings of Coughlin et al. (2016) that the rearing conditions can directly affect myosin heavy chain expression, causing changes in swimming performance and muscle contractile properties. However, no differences were observed in the ∆H of actin between the five treatment groups of fish, in the current study.
Previous studies have reported effects of fish rearing density on carcass composition, with varying trends (Toko et al. 2007; Osofero et al. 2009; Karakatsouli et al. 2010). In the current study, no significant differences in carcass moisture, protein, and ash content were found between the preferred treatment and the other treatments. This indicates that the fighting fish can maintain their proximate composition in the face of restricted water volume. Similarly, no effects of stocking density on meat composition (moisture, CP, lipid, ash, and nitrogen free extract) were observed in rainbow trout (Cretu et al. 2014). Some improvements in lipid deposition were observed with the preferred treatment when compared to the baseline volume used by the farmer, 250 mL, or to other higher volumes. Higher amount of lipid in fish reared in 100 mL might be caused by decreased swimming activity, reducing energy usage from lipid catabolism (Karakatsouli et al. 2010). This finding is in agreement with the lipid contents observed in African catfish (Clarias gariepinus) and in vundu catfish (Heterobranchus longifilis), when subjected to increased stocking density (Toko et al. 2007).
Conclusions
Based on our investigation, the preferred minimal water volume for individual rearing of male Siamese fighting fish was 150 mL. This water level gave superior growth performance, feed utilization, skin redness, and muscle quality and had no negative effects on carcass composition. This volume should be applied since the male fish begins aggressive behavior at 1.5 months of age, and it continues until the fish is sold at approximately 4 months of age. Optimization of the other known factors in the physical circumstances, i.e., photoperiod, light intensity, aquarium shape, and water exchange rate, is necessary before designing an intensive aquaculture system for this species. Subsequent experiments on the effects of aquarium color background on the male fish are currently underway.
References
Ahmadivand S, Eagderi S, Imanpour MR (2013) Effects of stocking density on hematological parameters, growth and survival rate of Caspian roach (Rutilus rutilus caspicus) larvae. J Chem Biol Phys Sci 3:1320–1326
Ali MS, Stead SM, Houlihan DF (2006) Effects of socking density on ammonia excretion and the growth of Nile tilapia (Oreochromis niloticus L.). Bangladesh J Fish Res 10:13–24
AOAC (2005) Official methods of analysis of AOAC international, 18th edn. Association of Official Analytical Chemists, Maryland
APHA, AWWA, WPCF (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington, DC
Areekijseree M, Engkagul A, Kovitvadhi U, Thongpan A, Mingmuang M, Pakkong P, Rungruangsak-Torrissen K (2004) Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture 234:575–587
Blakeslee C, McRobert SP, Brown AC, Clotfelter ED (2009) The effect of body coloration and group size on social partner preferences in female fighting fish (Betta splendens). Behav Proc 80:157–161
Bolasina S, Tagawa M, Yamashita Y, Tanaka M (2006) Effect of stocking density on growth, digestive enzyme activity and cortisol level in larvae and juveniles of Japanese flounder, Paralichthys olivaceus. Aquaculture 259:432–443
Chaijan M, Jongjareonrak A, Phatcharat S, Benjakul S, Rawdkuen S (2010) Chemical compositions and characteristics of farm raised giant catfish (Pangasianodon gigas) muscle. LWT-Food Sci Technol 43:452–457
Chakrabarti I, Gani MDA, Chaki KK, Sur R, Misra KK (1995) Digestive enzymes in 11 freshwater teleost fish species in relation to food habit and niche segregation. Comp Biochem Physiol 112A:167–177
Clotfelter ED, Ardia DR, Mcgraw KJ (2007) Red fish, blue fish: trade–offs between pigmentation and immunity in Betta splendens. Behav Ecol 18:1139–1145
Coughlin DJ, Shiels LP, Nuthakki S, Shuman JL (2016) Thermal acclimation to cold alters myosin content and contractile properties of rainbow smelt, Osmerus mordax, red muscle. Comp Biochem Physiol 196A:46–53
Cretu M, Cristea V, Dediu L, Petrea SM (2014) The influence of different stocking densities on biochemical composition of rainbow trout meat reared in a recirculating aquaculture system. Anim Sci Biotechnol 47: 200–204
Cristea V, Mocanu MC, Antache A, Docan A, Dediu L, Ion S, Coada MT (2012) Effect of stocking density on leuckocyte reaction of Oncorhynchus mykiss (Walbaum, 1792). Anim Sci Biotechnol 45:31–36
Çagiltay F, Erkan N, Ulusoy Ş, Selcuk A, Özden Ö (2015) Effects of stock density on texture-colour quality and chemical composition of rainbow trout (Oncorhynchus mykiss). Iranian J Fish Sci 14:687–698
Froese R, Pauly D (2016) Betta splendens Regan 1910 Siamese fighting fish. http://www.fishbase.org/summary/4768. Accessed 10 March 2017
Goede RW, Barton BA (1990) Organismic indices andan autopsy-based assessment as indicators of health and condition of fish. Am Fish Soc Symp 8:93–108
Halperin JRP, Dunham DW, Ye S (1992) Social isolation increases social display after priming in Betta splendens but decreases aggressive readiness. Behav Proc 28:13–32
Hofer R, Schiemer F (1981) Proteolytic activity in the digestive tract of several species of fish with different feeding habits. Oecologia 48:342–345
James R, Sampath K (2006) Effect of dietary administration of methyltestosterone on the growth and sex reversal of two ornamental fish species. Indian J Fish 53:283–290
Jaroensutasinee M, Jaroensutasinee J (2001) Bubble nest habitat characteristics of wild Siamese fighting fish. J Fish Biol 58:1311–1319
Jha P, Barat S (2005) The effect of stocking density on growth, survival rate, and number of marketable fish produced of koi carps, Cyprinus carpio vr. koi in concrete tanks. J Appl Aquac 17:89–102
Kanghae H, Thongprajukaew K, Jatupornpitukchat S, Kittiwattanawong K (2016) Optimal-rearing density for head-starting green turtles (Chelonia mydas Linnaeus, 1758). Zoo Biol 35:454–461
Karakatsouli N, Papoutsoglou ES, Sotiropoulos N, Mourtikas D, Stigen-Martinsen T, Papoutsoglou SE (2010) Effects of light spectrum, rearing density and light intensity on growth performance of scaled and mirror common carp Cyprinus carpio reared under recirculating system conditions. Aquac Eng 42:121–127
Karino K, Someya C (2007) The influence of sex, line, and fight experience on aggressiveness of the Siamese fighting fish in intrasexual competition. Behav Proc 75:283–289
Kelsh RN (2004) Genetics and evolution of pigment patterns in fish. Pigment Cell Res 17:326–336
Lambert Y, Dutil J (2001) Food intake and growth of adult Atlantic cod (Gadus morhua L.) reared under different conditions of stocking density, feeding frequency and size-grading. Aquaculture 192:233–247
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Lui Q, Hou Z, Wen H, Li J, He F, Wang J, Guan B, Wang Q (2016) Effect of stocking density on water quality and (growth, body composition and plasma cortisol content) performance of pen-reared rainbow trout (Oncorhynchus mykiss). J Ocean Univ China 15:667–675
Mandal SC, Sahu NP, Kohli MPS, Das P, Gupta SK, Munilkumar S (2010) Replacement of live feed by formulated feed: effect on the growth and spawning performance of Siamese fighting fish (Betta splendens, Regan, 1910). Aquac Res 41:1707–1716
Metusalach J, Brown A, Shahidi F (1997) Effects of stocking density on colour characteristics and deposition of carotenoids in cultured Arctic charr (Salvelinus alpinus). Food Chem 59:107–l14
Monvises A, Nuangsaeng B, Sriwattanarothai N, Panijipan B (2009) The Siamese fighting fish: well-known generally but little-known scientifically. ScienceAsia 35:8–16
Niazie EHN, Imanpoor M, Taghizade V, Zadmajid G (2013) Effects of density stress on growth indices and survival rate of goldfish (Carassius auratus). Global Veterinaria 10:365–371
Osofero SA, Otubusin SO, Daramola JA (2009) Effect of stocking density on tilapia (Oreochromis niloticus Linnaeus 1757) growth and survival in bamboo-net cages trial. Afr J Biotechnol 8:1322–1325
Romijn JA, Godfried MH, Hommes MJT, Endert E, Sauerwein HP (1990) Decreased glucose oxidation during short-term starvation. Metabolism 39:525–530
Rungruangsak K, Utne F (1981) Effect of different acidified wet feeds on protease activities in the digestive tract and on growth rate of rainbow trout (Salmo gairdneri Richardson). Aquaculture 22:67–79
Rungruangsak-Torrissen K (2007) Digestive efficiency, growth and qualities of muscle and oocyte in Atlantic salmon (Salmo salar L.) fed on diets with krill meal as an alternative protein source. J Food Biochem 31:509–540
Rungruangsak-Torrissen K, Moss R, Andresen LH, Berg A, Waagbo R (2006) Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.) Fish Physiol Biochem 32:7–23
Rungruangsak-Torrissen K, Stien LH, Daae BS, Vågseth T, Thorsheim GB, Tobin D, Ritola O (2009) Different dietary levels of protein to lipid ratio affected digestive efficiency, skeletal growth, and muscle protein in rainbow trout families. Scholarly Research Exchange 2009:1–13. https://doi.org/10.3814/2009/709529 Article ID 709529
Sammouth S, D’Orbcastel ER, Gasset E, Breuil G, Marino G, Coeurdacier JL, Fiveistad S, Blancheton JP (2009) The effect of density on sea bass (Dicentrarchus labrax) performance in a tank-based recirculating system. Aquac Eng 40:72–78
Sanchez P, Ambrosio PP, Flos R (2010) Stocking density and sex influence individual growth of Senegalese sole (Solea senegalensis). Aquaculture 300:93–101
Saoud IP, Ghanawi J, Lebbos N (2007) Effects of stocking density on the survival, growth, size variation and condition index of juvenile rabbitfish Siganus rivulatus. Aquac Int 16:109–116
Shahidi F (1994) Seafood proteins and preparation of protein concentrates. In: Shahidi F, Botta JR (eds) Seafood: chemistry, processing technology and quality. Blackie Academic & Professional, London, pp 3–9
Stickney RR (1994) Principles of aquaculture. John Wiley & Sons, New York
Sunde J, Taranger GL, Rungruangsak-Torrissen K (2001) Digestive protease activities and free amino acids in white muscle as indicators for feed conversion efficiency and growth rate in Atlantic salmon (Salmo salar L.) Fish Physiol Biochem 25:335–345
Supannapong P, Pimsalee T, A-komol T, Engkagul A, Kovitvadhi U, Kovitvadhi S, Rungruangsak-Torrissen K (2008) Digestive enzymes and in vitro digestibility of different species of phytoplankton for culture of the freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus. Aquac Int 16:437–453
Takeuchi Y, Hori M, Myint M, Kohda M (2010) Lateral bias of agonistic responses to mirror images and morphological asymmetry in the Siamese fighting fish (Betta splendens). Behav Brain Res 208:106–111
Thongprajukaew K, Kovitvadhi S, Kovitvadhi U, Rungruangsak-Torrissen K (2014) Pigment deposition and in vitro screening of natural pigment sources for enhancing pigmentation in male Siamese fighting fish (Betta splendens Regan, 1910). Aquac Res 45:709–719
Thongprajukaew K, Kovitvadhi U, Engkagul A, Rungruangsak-Torrissen K (2010a) Characterization and expression levels of protease enzymes at different developmental stages of Siamese fighting fish (Betta splendens Regan, 1910). Agric Nat Res 44:411–423
Thongprajukaew K, Kovitvadhi U, Engkagul A, Rungruangsak-Torrissen K (2010b) Temperature and pH characteristics of amylase and lipase at different developmental stages of Siamese fighting fish (Betta splendens Regan, 1910). Agric Nat Res 44:210–219
Thongprajukaew K, Kovitvadhi U, Kovitvadhi S, Engkagul A, Rungruangsak-Torrissen K (2013) Evaluation of growth performance and nutritional quality of diets using digestive enzyme markers and in vitro digestibility in Siamese fighting fish (Betta splendens Regan, 1910). African J Biotechnol 12:1689–1702
Thongprajukaew K, Rodjaroen S, Yoonram K, Sornthong P, Hutcha N, Tantikitti C, Kovitvadhi U (2015) Effects of dietary modified palm kernel meal on growth, feed utilization, radical scavenging activity, carcass composition and muscle quality in sex reversed Nile tilapia (Oreochromis niloticus). Aquaculture 439:45–52
Toko I, Fiogbe ED, Koukpode B, Kestemont P (2007) Rearing of African catfish (Clarias gariepinus) and vundu catfish (Heterobranchus longifilis) in traditional fish ponds (whedos): effect of stocking density on growth, production and body composition. Aquaculture 262:65–72
Tolussi CE, Hilsdorf AWS, Caneppele D, Moreira RG (2010) The effects of stocking density in physiological parameters and growth of the endangered teleost species piabanha, Brycon insignis (Steindachner, 1877). Aquaculture 310:221–228
Tyska MJ, Warshaw DM (2002) The myosin power stroke. Cell Motil Cyt 51:1–15
Verbeek P, Iwamoto T, Murakami N (2007) Differences in aggression between wild-type and domesticated fighting fish are context dependent. Anim Behav 73:75–83
Verbeek P, Iwamoto T, Murakami N (2008) Variable stress-esponsiveness in wild type and domesticated fighting fish. Physiol Behav 93:83–88
Winkler UK, Stuckmann M (1979) Glycogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol 138:663–670
Xian L, Ying L, Blancheton JP (2013) Effect of stocking density on performances of juvenile turbot (Scophthalmus maximus) in recirculating aquaculture systems. Chin J Oceanol Limnol 31:514–522
Zeng W, Li Z, Ye S, Xie S, Liu J, Zhang T, Duan M (2010) Effects of stocking density on growth and skin color of juvenile darkbarbel catfish Pelteobagrus vachelli (Richardson). J Appl Ichthyol 26:925–929
Acknowledgements
We acknowledge Assoc. Prof. Dr. Seppo Karrila and the Publication Clinic, Research and Development Office, PSU, for advice in manuscript preparation.
Funding
Funding was provided by the budget revenue (Contract No. SCI590420S) of the Prince of Songkla University (PSU). The fish samples were kindly provided by Boonrueang Plakat Farm in Nakonpathom province of Thailand. Research facilities were supported by Kidchakan Supamattaya Aquatic Animal Health Research Center, Faculty of Natural Resources, PSU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saekhow, S., Thongprajukaew, K., Phromkunthong, W. et al. Minimal water volume for intensively producing male Siamese fighting fish (Betta splendens Regan, 1910). Fish Physiol Biochem 44, 1075–1085 (2018). https://doi.org/10.1007/s10695-018-0495-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0495-z