Abstract
This study tested the reverence of background color with growth, movement behavior, and some body physiological factors in Nile tilapia. Fish were first acclimatized for two weeks in 80 L glass aquaria. In the experimental design, three groups were maintained separately. In 1st group, glass aquaria were covered with black, 2nd with green charts, and 3rd remained uncovered and maintained as a control group. All groups were in three replicates and fed on a 30% protein diet. All fishes were closely observed for movement behavior and growth-related parameters. On the 28th day, fish were anesthetized and blood was drawn from the caudal fin to determine some hematological parameters, cortisol, and glucose level. The highest weight gain and the greatest number of movements throughout the aquarium were observed in the control group. Furthermore, aquarium color determined the body pigmentation color. Black aquarium had the lowest weight gain, glucose level, FCR, DFI, FCE, and CF, while green aquarium had the highest cortisol level. Histological changes observed in black aquaria fish included reduced lamellar size, deformed blood vessels, and cardiac muscle fascicle in the gills, liver, and heart, respectively, while green aquaria fish showed fused lamellae, necrosis, fibrosis, endomiocardiac hyperplasia, and cardiac muscle fascicle. It was concluded that background coloration has a significant effect on the growth performance, behavior, hematology, body color, histology, cortisol, and glucose levels of Oreochromis niloticus, with green background inducing significant stress while black background causes black pigmentation with less growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nile tilapia, Oreochromis niloticus, is a freshwater fish that belongs to genus Oreochromis, family Cichlidae, order Cichliformes, and class Actinopterygii. It is native to Africa, Lebanon, and Palestine (Hadiuzzaman et al. 2018; Snoeks et al. 2018). Nile tilapia can reach a maximum length and weight of 24 inches (60 cm) (Volstorf and Maia 2022) and 5 kg (Rakocy 2023), respectively. A higher growth rate is observed in male tilapia than in females. Individuals of both genders can tolerate temperatures between 8 and 42 °C. However, 27–30 °C is optimum for the growth of Nile tilapia in a controlled environment (Azaza et al. 2008). It faces temperature fluctuations at different places, but breeding only happens at 24 °C (Linnaeus 1758). Mostly, Nile tilapia is herbivorous, but at a young age, it may adapt as omnivorous (Hadiuzzaman et al. 2018). Tilapia feeds on hydrophytes, algae, and phytoplankton (Dina et al. 2020). Tilapia feeds in the daytime because light is the main factor affecting the feeding rate (Khanjani and Sharifinia 2021).
Tank coloration is the basic environmental factor that influences the behavior of fish in the aquarium (Barton 2002). Various studies have been conducted in which fishes were separated by the particular color of the tank to study the physiological and behavioral changes for a number of days, and it was observed that stress response, growth rate, survival, and skin color are all affected by the tank color (Ferosekhan et al. 2020; Ghavidel et al. 2020; Hur et al. 2018; Nawang et al. 2019; Saekhow et al. 2019; Santisathitkul et al. 2020; Sebesta et al. 2019; Sotoudeh et al. 2019).
The response of the fish toward the tank color changes according to age and species of fish (Papoutsoglou et al. 2005). The various tank colors including black may be troublesome for the growth performance of the model fish (Sumner and Doudoroff 1938). The coloration patterns affect the feeding of the fish because most fish are visual feeders, such as larval fish (Duray et al. 1996; Ostrowski 1989; Salze et al. 2008). Due to these color changes, stress is promoted that alters the behavior, feeding, and locomotion of the fish (Øverli et al. 2005).
The behavioral study is carried out for a better understanding of the nature of the organisms via observation of its behavior and the environmental factors that influence the behavior. The catchability of fish is a reflection of its efficiency, which is measured by the relationship between biomass abundance and fishing mortality (Arreguín-Sánchez 1996). The current study was designed to investigate the effects of background coloration on various aspects of tilapia, including feeding, movement behavior, hematology, body pigmentation, and hormonal changes.
Materials and methods
Experimental animal
Nile tilapia was obtained from Manga Fish Hatchery. The initial weight of the experimental fish was 24.91 ± 8.17 g, as determined by electronic balance (with a minimum range of 0.001 g). The animals were kept in plastic bags with fresh water in which oxygen had permeated, ensuring that no deaths occurred during transportation.
Acclimatization of fish
Fishes were acclimatized for seven days in glass aquariums before the trial. The specimens were kept in water–glass aquariums with aerators and aquarium heaters to maintain oxygen (at 6–6.5 mg/L) and temperature levels. Each aquarium was set to a temperature of 27 to 28 °C for the best fish survival. The light intensity was kept constant at 40 watts m−2.
Experimental design
The animals were kept in semi-stable condition for 14 days after stabilization in nine experimental water–glass aquariums (10 fish in each tank) with rectangular dimensions (three replicates for each control, green- and black-colored group). Commercial food (30% protein diet) was provided to the fish twice a day. (Food was the same for all the treated and control groups.) The water was changed daily twice a day. The suction pump was used to remove the animal waste present in the water. After that, fresh water was introduced into the glass-water aquariums. The animals were taken one by one to a small water container at the end of a 14-day trial. Blood samples were obtained in EDTA vials by the use of BD syringes (Shahzad et al. 2021).
Analysis of hematological profile
A complete blood count (CBC) was determined for blood profile analysis using an automated hematology analyzer (Mindray, BC-6200). The CBC report was received from the laboratory to get the numbers of red blood cells, white blood cells, and platelets. The samples of blood were used to study hemoglobin, red blood cells, white blood cells, mean cell hemoglobin, platelets count, and cell hemoglobin concentration.
Mean corpuscular volume
The conventional formula, which is given below, was used to calculate mean corpuscular volume (MCV).
Mean cell hemoglobin
MCH was calculated using the formula
Mean cell hemoglobin concentration
Mean cell hemoglobin concentration (MCHC) was calculated using the following formula
Assessment of coloration effect
The aquariums were covered with colored charts on all sides of the aquariums. Two colors were studied (green and black). One tank was covered with green charts all around and the other tank with black charts to give artificial shade to the fish in the tank. Ten fish in each tank were reared for 14 days, and observations were taken in seconds at each side of the aquarium every day with the help of cameras that were adjusted on the top of each aquarium. On the 15th day, the fish from each aquarium were extracted to check the body weight, body color, and hematology.
FCR, FBW, TFI, DFI, and survival rate calculation
The weight of the fish was calculated at the beginning and end of the experiment. Food intake was calculated per day during the trial. Growth parameters, e.g., weight gain (WG), final body weight (FBW), daily feed intake (DFI), survival rate, feed conversion efficiency (FCE), and feed conversion ratio (FCR), were calculated by using the standard formula in each tank or of each fish (Li et al. 2009).
Cortisol level and blood glucose level
Ten blood samples from each group were collected to check cortisol and blood glucose level. After blood collection, blood was subjected to centrifugation at a temperature of 4 °C and at a speed of 3000 rpm for a time duration of 15 min. A Cortisol ELISA Kit (M-1850 made by Alpha Diagnostic International, USA) was used to measure the plasma cortisol level. All of the assayed samples were in duplicate. A microplate reader was used to read the plates at 415 nm. A standard curve was used to interpolate cortisol levels; 1.5 pg/ml was the minimum detectable level of the assay.
The blood glucose levels (mg/dL) in control and treated groups were measured using the Accu Check Performa (a hand-held one-touch glucose meter) and test strips (Roche USA).
Histological studies
The liver, heart, and gills were sampled after the 14th day and preserved in a 10% formalin solution. Dehydration steps were used for fixation. In the first step, 80% ethanol was used to place tissues, then 90%, and finally, 100% ethanol was used for the 2-h period in each dilution. For making the tissues clearer, they were placed in cedar wood oil. In the incubator, paraplast was used for placing the tissues at 60 °C for 30 min. After 30 min, the paraplast was changed and placed tissues again at 60 °C for 12 h. At the same conditions, the paraplast was the third time changed. Box blocks of each tissue were generated, and plastic casters were used to mount the tissues. The rotatory microtone was used to fix the embedded tissue, and 3–5 μm thick portions were slit for each tissue. After transferring each section to a clean slide, sections were straightened out on Fisher slides, heated, and kept on the slide for 24 h.
Hematoxyline-eosin staining
Xylene was used for de-parafinization, and 50 to 100% dilution of ethanol was used for rehydration. Tap water was used to wash the slides, stained with hematoxyline, bright coloration was ensured by again dipping in water, and eosin staining was done. Complete dehydration was done by placing the slides in absolute alcohol. Two drops of DPX (histology mountant) were placed on each slide and covered with a cover slip for ensuring complete spreading.
Statistical analysis
Fish were observed in the aquarium under certain conditions, and the number of observations was noted according to the movement of fish at particular points. The average value of each side per day was considered, and the data was analyzed via Microsoft Excel. The data was presented as mean ± SD of both test and control groups. The range was also included in the final description’s table. F-value and p-value were calculated to compare both test and control groups with the help of GraphPad Prism (Version 9.0.0).
Results
Growth performance
A highly significant difference was observed in weight gain between the control and treated groups (Table 1). The control group has the highest weight gain when compared with the green and black groups.
Movement behavior
Highly significant differences in movements were observed by the comparison of the control group and treated groups, i.e., black and green colorations. When comparing the treated and control groups, the maximum movement was observed on the left side of the control group, while the least movement was observed in the column of the green group. The maximum movements were detected in the control group in comparison with the treated groups on the right side and column. The movement of the black group was the highest at the center when compared to the control and green groups. Non-significant differences in movements were noticed by the comparison of control and treated groups at the base, surface, and feeding rate (Table 2).
Hematology
Highly significant differences were observed in some hematological parameters when comparing the control and color-treated groups (Table 3). A highly significant difference (P < 0.01) was observed in WBCs, MID, GRA, HGB, MCV, and PLT. A significant difference (P < 0.05) was noticed in LYM and MCHC, while no significant differences were observed in RBCs, HCT, MCH, PCT, and MPV.
Assessment of alteration in body pigmentation due to background coloration
The aquariums were covered with charts to give the artificial coloration. One aquarium was covered with black and one with green charts that were compared with the control group with the natural reflection of light. Fish in the black aquarium attained the darkness of the aquarium’s coloration. On the other hand, fish in the green aquarium got green pigmentation due to the background coloration of the aquarium. Fish in the control group have the light or natural skin color of Nile tilapia (Fig. 1).
Assessment of FCR, DFI, FCE, CF, and survival rate due to background coloration
Highly significant differences were observed in the FCR of control and treated groups as FCR in control, green, and black aquarium was 1.72 ± 0.34, 3.630 ± 0.190, and 5.987 ± 0.1629, respectively (Fig. 2).
Highly significant differences in DFI, FCE, and CF were also observed among the three groups, while survival rates did not differ significantly among the control, black, and green groups (Table 4).
Assessment of cortisol and glucose level due to background coloration
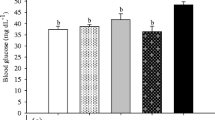
Cortisol levels differed significantly between the green and control groups, with the green group having the highest level of cortisol, whereas blood glucose levels differed significantly between the black and control group (Fig. 3).
Histology consequences due to green coloration
The comparison of gills, heart, and liver section of the control and green group showed fused and deformed lamella (Fig. 4), fibrosis, necrosis, and endomiocardiac hyperplasia with a proliferation of spindle shape cells (Fig. 5), and damaged hepatic tissue and necrosis of hepatic parenchyma tissue (Fig. 6), respectively.
Histology consequences due to black coloration
The comparison of gills, heart, and liver section of the control and black group showed a reduction in lamellar size and deformed cartilage (Fig. 7), deformation and cardiac muscle fascicle (Fig. 8), and deformed blood vessel (Fig. 9), respectively.
Discussion
The present study was conducted to investigate the effect of background coloration, i.e., black and green, on growth performance, behavior, blood hematology, cortisol level, glucose level, and feeding of Nile tilapia, purchased from Manga fish hatchery, District Lahore, Punjab, Pakistan.
One of the critical abiotic factors for the development and growth of Oreochromis niloticus is light. In order for sustainable Nile tilapia culture in an indoor environment, reasonable light intensity is required (Khanjani and Sharifinia 2021; Wang et al. 2020). A similar case was observed in our study as the order of weight gain was control > green > black, which was also supported by other studies (Jentoft et al. 2006; Mat Nawang et al. 2019; Okomoda et al. 2017). Moreover, FCR in different groups of our study was in the order black > green > control. The reason for the lowest growth in a black-colored aquarium is that light is unable to penetrate the aquarium due to the dark coloration, and the fish is unable to see and utilize the feed efficiently. Therefore, the lowest weight gain and the highest FCR were observed in the black group. This was similar to the report of Khanjani and Sharifinia (2021), who observed that a decrease in light intensity caused less weight gain and a higher FCR in Nile tilapia.
The growth performance of the fishes in the green aquarium was higher than in the black aquarium, which shows similar results to the studies conducted by Pedreira et al. (2012). However, there were some contradictory results reported as increased body weight in the black aquarium in the study of Batzina and Karakatsouli (2014); no impact of coloration on growth performance reported by Ebrahimi (2011); and no significant difference in weight and length of fish in the green aquarium observed by Ekokotu and Nwachi (2014). Moreover, no effect on growth performance after changing the background coloration was also observed by Costa et al. (2017) and Martinez-Cardenas and Purser (2007).
The behavior study in this research reported the maximum movement in control, green, and black aquariums, respectively, which was supported by the results of Sabri et al. (2012) who reported that the fishes in the green aquarium showed maximum movement while the fishes in the black aquarium were slower in movement and behavior at each side of the aquarium to respond. The possible reason for less movement of fish in a black-colored aquarium might be due to less light penetration in the aquarium because the fish is unable to see the feed and move to intake it.
The FCR of the control group was calculated as 1.72 ± 0.34, which was supported by the results of Forsatkar et al. (2017), who found an FCR value of 1.38 ± 0.24. The FCR of fish in the green aquarium in this study was 3.630 ± 0.190, which was contradictory to the results of Üstündağ and Rad (2015) who found an FCR value of 0.95 ± 0.06 for the green aquarium. The calculated FCR of fishes in the black aquarium was 5.987 ± 0.1629, which was different from the outcomes of Eslamloo et al. (2015) who found a value of 2.97 ± 0.09.
If the value of the condition factor (CF) is higher than 1, this means the fish under observation is in good health condition and will show isometric growth, which is the most important target of fish farming (Ayoade 2011). The values of CF in all the studied groups of our research were as green (1.127 ± 0.03 g/cm3) > control (1.05 ± 0.02 g/cm3) > black (1.017 ± 0.02 g/cm3). Our hypothesis that fish in dark coloration (black group) will be less healthy as compared to green or control groups was also supported by Khanjani and Sharifinia (2021), who reported the lowest CF in Nile tilapia reared in darkness. Moreover, other factors such as DFI and FCE were also lowest in the black-colored aquarium in our investigation. The order of survival rate from high to low was as follows: control (96.67 ± 5.77%) > black (93.33 ± 5.77%) > green (86.67 ± 5.77%).
No existing literature was found for the hematology of fish due to background coloration. So, the changes in hematological variables due to background coloration (green and black) are reported for the first time in this research. Moreover, these values can be used as reference values for further studies in the future. The histological changes due to background coloration were not available in the existing literature and were also reported for the first time in this study.
The background color has a significant effect on the body color of fish, and body color is important in the commercial market as fish are sold on the basis of their body color apart from their size and price (McLean 2021). We found that background coloration had a significant effect on the body color of fish, as fish in the black aquarium were darker as compared to the green and control groups. This was also found to be true by Ngugi et al. (2014), who found fish in the black-colored aquarium were darker as compared to other colors (clear and blue). Moreover, at the end of the experiment in our study, fish in the green-colored aquarium had green-colored body pigmentation while the control group was in a lighter color (similar to the start of the trial).
Cortisol helps organisms adapt to changes in their environment or environmental conditions. An increase in cortisol has been linked to stressful situations in several fish studies (Schreck and Tort 2016). The cortisol level in the present study, from high to low, was in the order green > black > control. This means that background coloration induces stress in the fish, which was also reported by McLean et al. (2008) and Merighe et al. (2004). Cortisol stimulates gluconeogenesis, which becomes active at the time when energy is required by the body. This leads to the use of high blood glucose levels to detect stress in fish (Sopinka et al. 2016). However, season, age, diet, and, most importantly, feed are the main factors due to which the concentration of glucose varies. Moreover, the blood glucose levels in our study were as control > green > black, which indicates that hyperglycemia might not be dependent on the level of cortisol, as reported by Herrera et al. (2019).
It is concluded that there is a significant effect of background coloration on the growth performance, movement behavior, hematology, histology, and body physiology of Nile tilapia. Moreover, body pigmentations changed according to the background coloration of the aquarium. Further studies should be carried out to check the influence of background coloration on the genetic makeup of Nile tilapia in the future.
Data availability
Not applicable.
References
Arreguín-Sánchez F (1996) Catchability: a key parameter for fish stock assessment. Rev Fish Biol Fisheries 6:221–242. https://doi.org/10.1007/BF00182344
Ayoade A (2011) Length-weight relationship and diet of African carp Labeo ogunensis (Boulenger, 1910) in Asejire Lake Southwestern Nigeria. J Fish Aquat Sci 6:472–478. https://doi.org/10.3923/jfas.2011.472.478
Azaza MS, Dhraïef MN, Kraïem MM (2008) Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. J Therm Biol 33:98–105. https://doi.org/10.1016/j.jtherbio.2007.05.007
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525. https://doi.org/10.1093/icb/42.3.517
Batzina A, Karakatsouli N (2014) Is it the blue gravel substrate or only its blue color that improves growth and reduces aggressive behavior of gilthead seabream Sparus aurata? Aquac Eng 62:49–53. https://doi.org/10.1016/j.aquaeng.2014.06.004
Costa DC, Mattioli CC, Silva WS, Takata R, Leme FO, Oliveira AL, Luz RK (2017) The effect of environmental colour on the growth, metabolism, physiology and skin pigmentation of the carnivorous freshwater catfish Lophiosilurus alexandri. J Fish Biol 90:922–935. https://doi.org/10.1111/jfb.13208
Dina R, Haryani G, Nasution S, Samir O (2020) Fish distribution in Ranggeh and Batang Air Stream, Tanjung Raya, Agam District, West Sumatra Province. In: IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, vol 1. IOP Publishing, p 012062
Duray MN, Estudillo CB, Alpasan LG (1996) The effect of background color and rotifer density on rotifer intake, growth and survival of the grouper (Epinephelus suillus) larvae. Aquaculture 146:217–224. https://doi.org/10.1016/S0044-8486(96)01375-0
Ebrahimi G (2011) Effects of rearing tank background color on growth performance in juvenile common carp, Cyprinus carpio L. Agric J 6:213–217. https://doi.org/10.3923/aj.2011.213.217
Ekokotu P, Nwachi O (2014) The growth performance of Clarias gariepinus fry raised in varying coloured receptacles. J Res Biol 4:1287–1292
Eslamloo K, Akhavan SR, Eslamifar A, Henry MA (2015) Effects of background colour on growth performance, skin pigmentation, physiological condition and innate immune responses of goldfish, Carassius auratus. Aquac Res 46:202–215. https://doi.org/10.1111/are.12177
Ferosekhan S, Sahoo SK, Radhakrishnan K, Velmurugan P, Shamna N, Giri SS, Pillai BR (2020) Influence of rearing tank colour on Asian catfish, magur (Clarias magur) and pangas (Pangasius pangasius) larval growth and survival. Aquaculture 521:735080. https://doi.org/10.1016/j.aquaculture.2020.735080
Forsatkar MN, Safari O, Boiti C (2017) Effects of social isolation on growth, stress response, and immunity of zebrafish. Acta Ethologica 20:255–261. https://doi.org/10.1007/s10211-017-0270-7
Ghavidel S, Kochanian P, Salati AP (2020) The effects of the tank colour on growth performance and physiological responses in fingerling grouper, Epinephelus coioides. Aquac Res 51:276–281. https://doi.org/10.1111/are.14373
Hadiuzzaman M, Ali MF, Habib MAB (2018) Review on etymological history of exotic cultured and aquarium fishes of Bangladesh. Int J Progress Sci Technol 9:132–138
Herrera M, Mancera JM, Costas B (2019) The use of dietary additives in fish stress mitigation: comparative endocrine and physiological responses. Front Endocrinol 10 https://doi.org/10.3389/fendo.2019.00447
Hur S, Hyeon J, Moon K, Kim H, Jeong H, Lim B (2018) Effects of background tank color on the growth and feeding-related gene expression of olive flounder, Paralichthys olivaceus. J Aquac Mar Biol 7:296–304
Jentoft S, ØXnevad S, Aastveit AH, Andersen Ø (2006) Effects of tank wall color and up-welling water flow on growth and survival of Eurasian perch larvae (Perca fluviatilis). J World Aquac Soc 37:313–317. https://doi.org/10.1111/j.1749-7345.2006.00042.x
Khanjani MH, Sharifinia M (2021) Production of Nile tilapia Oreochromis niloticus reared in a limited water exchange system: the effect of different light levels. Aquaculture 542:736912. https://doi.org/10.1016/j.aquaculture.2021.736912
Li K, Wang Y, Zheng ZX, Jiang RL, Xie NX, Bureau DP (2009) Replacing fish meal with rendered animal protein ingredients in diets for Malabar grouper, Epinephelus malabaricus, reared in net pens. J World Aquaculture Soc 40:67–75. https://doi.org/10.1111/j.1749-7345.2008.00227.x
Linnaeus (1758) Cultured aquatic species information programme Oreochromis niloticus. Food Agric Orgination U N
Martinez-Cardenas L, Purser GJ (2007) Effect of tank colour on Artemia ingestion, growth and survival in cultured early juvenile pot-bellied seahorses (Hippocampus abdominalis). Aquaculture 264:92–100. https://doi.org/10.1016/j.aquaculture.2006.12.045
Mat Nawang SUS, Ching FF, Senoo S (2019) Comparison on growth performance, body coloration changes and stress response of juvenile river catfish, Pangasius hypophthalmus reared in different tank background colour. Aquac Res 50:2591–2599. https://doi.org/10.1111/are.14215
McLean E (2021) Fish tank color: an overview. Aquaculture 530:735750. https://doi.org/10.1016/j.aquaculture.2020.735750
McLean E, Cotter P, Thain C, King N (2008) Tank color impacts performance of cultured fish. Croat J Fish Ribarstvo 66:43–54
Merighe GKF, Pereira-da-Silva EM, Negrão JA, Ribeiro S (2004) Effect of background color on the social stress of Nile tilapia (Qreochromis niloticus). Rev Bras Zootec 33:828–837. https://doi.org/10.1590/s1516-35982004000400002
Nawang SUSM, Ching FF, Senoo S (2019) Comparison on growth performance, body coloration changes and stress response of juvenile river catfish, Pangasius hypophthalmus reared in different tank background colour. Aquac Res 50:2591–2599. https://doi.org/10.1111/are.14215
Ngugi C, Opiyo M, Rasowo J (2014) Combined effects of stocking density and background colour on growth performance and survival of Nile tilapia (Oreochromis niloticus, L.) fry reared in aquaria. J FisheriesSciencescom 8:228–237
Okomoda VT, Tiamiyu LO, Wase G (2017) Effects of tank background colour on growth performance and feed utilization of African catfish Clarias gariepinus (Burchell, 1822) fingerlings. Croat J Fish 75:5–11. https://doi.org/10.1515/cjf-2017-0002
Ostrowski AC (1989) Effect of rearing tank background color on early survival of dolphin larvae. Prog Fish-Cult 51:161–163. https://doi.org/10.1577/1548-8640(1989)051%3C0161:EORTBC%3E2.3.CO;2
Øverli Ø, Winberg S, Pottinger TG (2005) Behavioral and neuroendocrine correlates of selection for stress responsiveness in rainbow trout—a review. Integr Comp Biol 45:463–474. https://doi.org/10.1093/icb/45.3.463
Papoutsoglou SE, Karakatsouli N, Chiras G (2005) Dietary L-tryptophan and tank colour effects on growth performance of rainbow trout (Oncorhynchus mykiss) juveniles reared in a recirculating water system. Aquac Eng 32:277–284. https://doi.org/10.1016/j.aquaeng.2004.04.004
Pedreira MM, Sampaio EV, Santos JCEd, Pires AV (2012) Larviculture of two neotropical species with different distributions in the water column in light-and dark-colored tanks. Neotrop Ichthyol 10:439–444. https://doi.org/10.1590/S1679-62252012005000011
Rakocy JE (2023) Oreochromis niloticus. Cultured Aquatic Species Information Programme. https://www.fao.org/fishery/en/culturedspecies/oreochromis_niloticus/en. Accesed 3 January 2023
Sabri DM, Elnwishy N, Nwonwu F (2012) Effect of environmental color on the behavioral and physiological response of Nile tilapia, Oreochromis niloticus. Glob J Sci Front Res Biol Sci 12:11–20
Saekhow S, Thongprajukaew K, Phromkunthong W (2019) Blue aquarium background is appropriate for rearing male Siamese fighting fish (Betta splendens). Aquacult Int 27:891–903. https://doi.org/10.1007/s10499-019-00374-6
Salze G, McLean E, Schwarz M, Craig S (2008) Dietary mannan oligosaccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 274:148–152. https://doi.org/10.1016/j.aquaculture.2007.11.008
Santisathitkul N, Thongprajukaew K, Saekhow S, Sandos P, Buntomnimit S, Kanghae H (2020) Optimal background colour for rearing Asian seabass (Lates calcarifer). Aquac Res 51:1743–1752. https://doi.org/10.1111/are.14521
Schreck CB, Tort L (2016) 1 - The concept of stress in fish. In: Schreck CB, Tort L, Farrell AP, Brauner CJ (eds) Fish Physiology, vol 35. Academic Press, pp 1–34. https://doi.org/10.1016/B978-0-12-802728-8.00001-1
Sebesta R, Stejskal V, Matousek J, Lundova K (2019) The effect of light intensity and tank wall colour on survival and growth of peled Coregonus peled Gmelin 1788 larvae. Turk J Fish Aquat Sci 19:541–549
Shahzad K, Khan MN, Jabeen F, Chaudhry AS, Ara C, Khan MKA, Khan MS (2021) Study of some toxicological aspects of titanium dioxide nanoparticles through oxidative stress, genotoxicity, and histopathology in tilapia, Oreochromis mossambicus (Preprint). Res Square. https://doi.org/10.21203/rs.3.rs-388947/v1
Snoeks J, Freyhof J, Geelhand D, Hughes A (2018) Oreochromis niloticus. The IUCN Red List of Threatened Species 2018
Sopinka NM, Donaldson MR, O’Connor CM, Suski CD, Cooke SJ (2016) 11 - Stress indicators in fish. In: Schreck CB, Tort L, Farrell AP, Brauner CJ (eds) Fish Physiology, vol 35. Academic Press, 405–462. https://doi.org/10.1016/B978-0-12-802728-8.00011-4
Sotoudeh E, Feizollahi S, Banan A, Morshedi V, Hosseini A (2019) Oxidative status and digestive histology of barramundi perch in response to different tank colors. J Aquat Anim Health 31:139–146. https://doi.org/10.1002/aah.10060
Sumner F, Doudoroff P (1938) The effects of light and dark backgrounds upon the incidence of a seemingly infectious disease in fishes. Proc Natl Acad Sci U S A 24:463–466. https://doi.org/10.1073/2Fpnas.24.10.463
Üstündağ M, Rad F (2015) Effect of different tank colors on growth performance of rainbow trout juvenile (Oncorhynchus mykiss Walbaum, 1792). J Agric Sci 21:144–151
Volstorf J, Maia CM (2022) Oreochromis niloticus (farm: findings). FishEthoBase. https://fishethobase.net/db/31/farm/findings/. Accesed 3 January 2023
Wang K, Li K, Liu L, Tanase C, Mols R, van der Meer M (2020) Effects of light intensity and photoperiod on the growth and stress response of juvenile Nile tilapia (Oreochromis niloticus) in a recirculating aquaculture system. Aquac Fish. https://doi.org/10.1016/j.aaf.2020.03.001
Author information
Authors and Affiliations
Contributions
Writing – original draft preparation, reviewing, editing, and data analysis were done by Ahmad Waheed and Hina Naz. Conceptualization and editing were done by Muhammad Saleem Khan. Muhammad Wajid was involved in supervision and critically analyzed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was taken from the University of Okara ‘s Ethical Committee for the current research work (Reference no. UO/ETH/2022/misc.).
Informed consent
Informed consent was taken from all the authors for publication of this work.
Competing interests
The authors declare no competing interests.
Research involving human participants and/or animals
All the work carried out in this research was in accordance with the Declaration of Helsinki for animal ethics, and ethical approval was taken from the Ethical committee of the University of Okara (Reference number: UO/ETH/2022/misc.).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Waheed, A., Naz, H., Wajid, M. et al. Impact of background colorations on growth, movement behavior, and some body physiological factors of Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem 49, 275–287 (2023). https://doi.org/10.1007/s10695-023-01180-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01180-x