Abstract
The Siamese fighting fish (Betta splendens) is well known as an aggressive fish with unique spawning and parental care behavior. During reproduction, male fish construct a bubble nest, court females, protect the brood, and defend the territory through aggressive displays. Aggression in male Siamese fighting fish has long been the subject of investigation; however, the kinematics of aggression during contests have been largely overlooked. Here we investigated how nest-holding, male Siamese fighting fish use two different types of displays, gill flaring and fin spreading, towards intruders during various reproductive phases; before (BB) and after bubble nest building, and after spawning (AS), and hatching (AH). Males were more aggressive towards male than female intruders and the level of aggression changed significantly between reproductive phases. Gill flaring, the more energetically costly display, was the dominant initial display towards male and female intruders in BB, AS, AH phases. However, defending males switched to fin spreading after prolonged exposure to intruders. The results suggest that Siamese fighting fish use gill flaring as an acute response in order to defend their territory; this response may be replaced by fin spreading as a chronic response, probably to reduce the energetic costs during the contest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Access of animals to critical resources is of particular importance and is usually achieved through aggression. Aggressive behaviors involved in reproduction are common in territorial defense, during breeding and the protection of offspring, all of which have effects on reproductive success (Tubert et al. 2012). Although the intensity of behaviors involved in aggression varies between individuals (Bell 2005), their expression may have significant effects on individuals fitness. For example, Jaroensutasinee and Jaroensutasinee (2003) found that successful males Siamese fighting fish (Betta splendens) may produce two to three successive broods in a single breeding season. Given the high level of individual variation in aggressiveness and the uncertainty of the natural environment, it is likely that the level of aggression displayed by an individual is a product of its internal state and the state of the surrounding environment.
Aggressive encounters in animals seldom lead to fighting. Rather a series of displays often ensue when opponents confront one another which gradually escalate until one opponent withdraws (e.g., Schroeder and Huber 2001). Only if both parties are very evenly matched will costly fights break out. The ethogram of the display process likely varies with risk perception, which in turn depends on the opponent and the context. For example, animals are often extremely aggressive when defending their young (e.g., Forsatkar et al. 2014) and thus might proceed through the aggression ethogram at a faster rate and may skip some low-level threat display components. Thus, the manner in which animals display during aggressive encounters provides insight into their level of perceived risk.
The male Siamese fighting fish has been long recognized for its territoriality and the aggressive displays it performs when confronting intruder conspecifics (Simpson 1968). This fish is a highly aggressive species; both males and females display some degree of aggression against conspecifics (Karino and Someya 2007), making it an ideal model species to study the process of aggressive displays during a contest. Studies examining social interactions of Siamese fighting fish (e.g., McGregor et al. 2001), suggest that gill flaring and fin spreading are the most common aggressive displays in male–male and male–female interactions. The duration of gill flaring is an indicator of fighting capacity and correlates with fighting abilities in this species (Evans 1985; Alton et al. 2013). Low-level aggression typically involves fin displays which can progress to gill flaring, charging and biting. Despite the large number of studies on courtship and aggressive behaviors of this species, no study has assessed how the nest-holding male behaves towards to a contestant in the early stages of the encounter and how this might vary across the breeding phases.
Conflict between individuals is energetically costly (Castro et al. 2006). Engaging in aggressive interactions causes significant increases in metabolic rate and oxygen requirements as well as the potential for physical harm (Alton et al. 2013). In Siamese fighting fish, gill flaring behavior is thought to be the most expensive behavior of the aggression display from an energetic perspective (Alton et al. 2013). The opercula coverings of the gills are actively used for respiration, thus during gill flaring displays, individuals need to cope with a reduction in respiratory gas exchange in addition to the extra energetic requirements associated with fighting (Dore et al. 1978). This suggests that this activity is associated with high metabolic costs during a contest. Coordinating investment in each of the aggressive display components, therefore, plays a key role during a contest. Presumably, individuals progress from the least costly modes of aggressive displays to the most expensive as a means of beating their component with the least amount of energetic expenditure (Kaufmann 1983). However, if the risk is perceived to be great, then individuals may immediately use the most aggressive display to ward off an intruder. Moreover, if a contest is prolonged, individuals may have to switch to less energetically costly displays.
In the present study we examined the influence of an intruder’s sex at different stages of the reproductive cycle on the aggressive displays of nest-holding male Siamese fighting fish. Territorial and reproduction status are both known to affect aggressive behavior of fighting fish (Jaroensutasinee and Jaroensutasinee 2003). We investigated the first encounter of nest-holding males in male–male and in male–female interactions and the duration of their displays was recorded. We were specifically interested in the progression of displays to determine when lower-cost displays (raised fins) were replaced by higher-cost displays (gill flaring). We hypothesized that males would show a greater response to male as opposed to female intruders and that the response would be most acute when eggs or fry were present in the nest. In high-risk situations, we predicted that territorial males would progress rapidly to high-cost display either by spending little time on low-cost displays or skipping them entirely. Moreover, we predicted that if a nest-holding male uses costly behavior as an acute response [i.e., gill flaring that induces hypoxic stress (Kuperberg et al. 2009)], it may be forced to behave in a less costly manner as a chronic response (i.e., fin display) in order to sustain the contest.
Materials and methods
Animals
Siamese fighting fish, Betta splendens, are small (ca 7.5 cm long) fish of the gourami family (Osphronemidae) native to slow-moving and stagnant, overgrown waters in Thailand, Vietnam, Cambodia and Laos. When agitated, wild Siamese fighting fish turn bright colors, and over years of selective breeding, strains have been bred to take on these colors permanently. The males build bubble nests at the surface of the water. After intense courtship displays, the male gathers fertilized eggs in his mouth and blows them into the nest. The male then tends the eggs until they hatch about 28–36 h later (Monvises et al. 2009). Females are the choosy sex, while the males compete with other males for territory and mates. The males are larger than the females due to the frequency of male–male combat. Larger males tend to win more fights and thus attract more females (Andersson 1994). Female fighting fish prefer large males with longer, undamaged fins because these traits indicate that the male is an excellent fighter and in good health (Allen and Nicoletto 1997).
Thirty adult mature male Siamese fighting fish were purchased from a local distributor and were kept individually in 1-L opaque containers to avoid priming of aggressive behavior. Fish were maintained at a water temperature of 26 ± 1 °C and under a 12-h:12-h light:dark photoperiod, optimal conditions for the initiation of nest building. Containers were maintained with dechlorinated municipal water, which was changed on every third day. In addition, 30 adult females were purchased from another local distributor. Female fish were kept as groups of 15 fish in two 20-L tanks under the same temperature and light conditions as the males. All fish were fed to satiation twice daily with 0.9-mm commercial pellets and frozen blood worms.
Spawning tank set-up
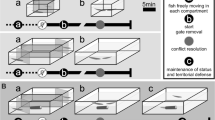
Seven identical copies of rectangular glass tanks with dimensions of 40 × 30 × 30 cm were used for the behavioral studies (Fig. 1). The water depth was 20 cm and the temperature maintained at 26 ± 1 °C with an aquarium heater. The apparatus was divided into two unequal compartments by a glass sheet. The smaller compartment (Fig. 1) was used for intruders. An 8 × 8-cm Styrofoam nest was situated slightly under the water surface in the corner of the larger compartment (Fig. 1). To induce spawning, a gravid female was introduced into the larger compartment following construction of the bubble nest. After spawning, the female was removed from the tank and did not take any further part in the study.
Testing procedure and behaviors
The following behaviors were measured for 14 male fish (mean length ± SD; 53.48 ± 3.13 mm) of 30 in our pool: gill flaring (defined as the raising of the gill covers), and fin spreading [defined as the quick expansion of the fins (Forsatkar et al. 2014)]. The general design of the behavioral observations is shown in Fig. 2. Aggressive behaviors were tested at four distinct reproductive phases. The first behavioral measurements were taken from 5 to 7 h following the male’s introduction to the tank before the construction of the bubble nest (BB). The second behavioral measurements were made after focal male fish had constructed their bubble nests (AB). The third measurement was made after spawning (AS), 1 h after the female was removed. The final measurements were performed after hatching of the larvae (AH). In each set of behavioral measurements, the subject male was netted from his housing tank and placed into the apparatus. The intruder compartment was covered by an opaque partition. After 5–7 h, a gravid female (37.54 ± 3.20 mm) was placed into the intruder’s compartment. After 10 min following the insertion of the female intruder, the partition was removed, allowing visual contact between the experimental fish and the intruder. The male–female interaction was recorded using a digital camera. Then, the partition was replaced in its original position, and the female intruder was removed and returned to its tank. After a 10-min interval, a male intruder (54.81 ± 2.41 mm) was placed into the compartment and kept there for a 10-min acclimation period. Then, the partition was removed again, and the male–male interaction was recorded. The intruder was removed at the end of the observation period and returned to its container. Video recordings were then converted to pictures (1 picture per second) using a KMPlayer (version 3.0.0.1438; http://www.kmplayer.com) and the behaviors displayed in each picture were counted. We did not balance the order of female and male intruders because our previous studies found that the order of presentation had no influence of the behaviors of male Siamese fighting fish (Forsatkar et al. 2015). The first behavior displayed in response to an intruder was identified and its duration was recorded (hereafter “first response”). Additionally, the total time each individual spent using the two displays over the duration of the 5-min exposure was noted (hereafter “total response”). To prevent pseudoreplication of behavioral responses between conspecifics, each intruder was tested only for one interaction (i.e., BB, or AB, or AS, or AH) with a focal male. However, the same intruders were used at each reproductive phase for all of the focal males [a total of four male and four female size-matched intruders (Forsatkar et al. 2015)].
Statistics
Data are reported as mean ± SE and all the statistical analyses were conducted using SPSS (version 22.0; IBM Statistics). We checked for homogeneity of variance with Levene’s test in all statistical tests and aggressive behavior scores were log transformed. The duration of time spent gill flaring and fin spreading as a first response and over the total observation period (total response) was compared using paired samples t-test. We used separate generalized linear mixed models to investigate the effect of intruder sex, phase of reproduction and their interaction on the display of gill flaring and fin spreading during the first response and the total response to the intruders. Individual identity was used as a random effect to account for multiple observations of the same individuals over time, and the fixed effects were sex of the intruders, phase of reproduction and their interaction. The size of the nest-holding males was initially used as a covariate but since there was no effect of length we removed it to simplify the models.
Results
No difference was found between length of focal and intruder males (independent samples t-test, t 26 = 0.73, p = 0.428). After watching the intruder, nest-holding males vigorously swam toward the intruder and moved their bony opercula forward and extended the branchiostegal membranes (Kuperberg et al. 2009). In the BB condition, only one of the 14 nest-holding males showed fin spreading as the first reaction towards both male and female conspecific intruders. However, for the AB condition, two and six nest-holding males pursued fin spreading against female and male intruders, respectively. In the two last stages of reproduction, AS and AH, all of the subjects displayed gill flaring as the first reaction towards female and male intruders.
Paired t-tests showed that the duration of gill flaring was significantly higher than the duration of fin spreading as the first response in the male–female interactions (Fig. 3), and male–male interactions (Fig. 4) in the BB, AS, and AH phases of reproduction (Fig. 5). However, fin spreading was used more often than gill flaring over the entire 5-min observation period towards both female (Fig. 3), and male (Fig. 4) intruders at all of the reproduction phases (Fig. 5). Males initially engaged in gill flaring behavior before switching to fin spreading. This switch occurred within 20–40 s of the appearance of a female intruder and the timing of the switch was significantly different between reproduction phases (F 3,55 = 152.26; p < 0.001). Similarly for male intruders, the switch occurred within 25–40 s and varied between reproductive phases (F 3,55 = 99.36; p < 0.001). Irrespective of the sex of the intruder, the switch occurred more rapidly during the AB observation than at other reproductive stages (<15 and <25 s after female and male intruder entry, respectively; Figs. 3, 4).
The mean (±SE) duration of time (s) spent gill flaring and fin spreading during each 5-s interval over 5 min by nest-holding male Siamese fighting fish in response to female intruders at four reproduction phases; before bubble nest (BB), after bubble nest (AB) building, after spawning (AS), and after hatching (AH). The grey areas represent the timing of the switch from gill flaring to fin display as the first response
The mean (±SE) duration of time (s) spent gill flaring and fin spreading during each 5-s interval over 5 min by nest-holding male Siamese fighting fish in response to male intruders at four reproduction phases: BB, AB, AS, and AH. The grey areas represent the timing of the switch from gill flaring to fin display as the first response
The mean (±SE) duration of time (s) spent gill flaring (GF) and fin spreading (FS) as the first response (A), and over the course of the total 5-min interaction (B) of nest-guarding male Siamese fighting fish towards male and female intruders at four reproduction phases: BB, AB, AS, and AH. *p < 0.05, **p < 0.01
Results from generalized linear mixed models showed main effects of intruder sex and reproduction phase on the duration of gill flaring and fin spreading as the first response (Table 1). However, the interaction between these two factors was not significant. Similar results were found for the analysis of the total response (Table 1).
In general, the response was stronger towards male intruders than towards female intruders as we predicted (Table 1; Fig. 5). Also, both forms of aggressive displays tended to be of greater duration when the male was protecting eggs and young (Table 2; Fig. 5).
Discussion
During reproduction, male Siamese fighting fish are faced with a complex decision-making process in which they must defend their territory against other males and attract females for spawning (Dzieweczynski and Leopard 2010). Here we found that the sex of the intruder had a significant impact on the intensity of the aggressive behavior of the host males. As predicted, we found that females represented less of a threat to nest-holding males than male intruders. Notably, the aggression towards females was very low during the bubble nest phase during which males try to attract females which will deposit their eggs. These results are consistent with previous findings (Simpson 1968; Doutrelant et al. 2001; Matos and McGregor 2002; Jaroensutasinee and Jaroensutasinee 2003). Interestingly, in the context of communication, Dzieweczynski et al. (2006) found that the highest 11-ketotestosterone concentration was present in fighting males which had a male audience. Males tend to reduce their aggression when a female is watching and switch to courtship displays (Doutrelant et al. 2001). We also found that the level of aggression varies greatly with reproductive phase, whereby males become increasingly aggressive when eggs and fry are present in the nest. These results are consistent with parental investment theory whereby individuals are motivated to defend their nest and the young within depending on their previous parental investment (Coleman and Gross 1991). Moreover, male Siamese fighting fish showed an initial costly, high-impact display, gill flaring, toward intruders, but this was gradually replaced with a less costly display, fin spreading, as the encounter was prolonged. The shift between the two display behaviors did not take long with the majority of focal males making the switch in the first 30 s of the interaction, but varied significantly between reproductive phases. This makes sense from an energetic perspective since fish cannot maintain an expensive gill flaring display for long periods of time. However, there are caveats to this interpretation since the experimental set-up insured that displays could not escalate into fights, nor could the intruder fully retreat from sight.
With the exception of the period of time after the bubble nest phase, gill flaring was displayed more often than fin spreading as the first encounter towards both male and female intruders in all reproductive phases. In general, host males responded acutely to the intruders. This result suggests that nest-holding males most often resort to a costly, full-strength display to defend their nest against another conspecific rather than gradually ramping up the display. This most likely occurs because the nesting male perceives all intruders as high risk when defending his nest, particularly if eggs of fry are present. Even females can represent a threat at most reproductive stages since they are known to eat eggs and fry (Jaroensutasinee and Jaroensutansinee 2001).
The intensity of aggressiveness during the bubble nest phase was lower than during the other stages of reproduction, and no significant differences were found in the propensity of fish to use gill flaring and fin spreading as the initial response in this phase. This result is not at all surprising; nest status is related to the levels of circulating hormones that are involved in aggression (e.g., Kleszczyńska et al. 2012), and Dzieweczynski et al. (2006) reported that the males with nests had lower 11-ketotestosterone in comparison to males without a nest. On the other hand, males with nests have more incentive to interact with conspecifics than males without a nest; they might increase their courtship display and aggressive behaviors directed towards female and male conspecifics, respectively (Dzieweczynski et al. 2005; Dzieweczynski and Leopard 2010; Forsatkar et al. 2014). The lower levels of aggression along with a lack of difference in the use of gill flaring or fin spreading behaviors after construction of a bubble nest might be indicative of courtship behaviors directed towards females. Although we did not measure courtship behaviors in the present study, Forsatkar (2012) showed that behaviors involved in reproduction, such as attracting a female for spawning, increased shortly after bubble nest creation relative to other reproductive phases.
In contrast with the initial response to intruders, the intensity of fin spreading over the entire observation period shown by nest-holding males against both types of intruder was higher than gill flaring. These results suggest that the expression of aggression in males with nests changes over time, and that acute responses are replaced by chronic displays. Our hypothesis that lower-cost behaviors replaced the higher-cost displays during a contest was supported. At the first encounter, nest-holding males choose gill flaring to implement the greatest potential initial impact at the beginning of the encounter with an intruder. Expression of this high-cost behavior decreased gradually and the duration of fin spreading increased to allow the defending males to sustain their aggressive displays. Only very high-quality males [in terms of body condition (Alton et al. 2013)] are capable of displaying long bouts of gill flaring because it interferes with the flow of water across the gills. Therefore, inevitably, males must switch to the alternative fin-spreading display if they are to maintain their defense for a long time. These results are the first descriptions of motivational changes in the aggression of nest-holding fighting fish. It should be noted that it is difficult to apply these results to the real world, where presumably the initial intense display would ward off most intruders. Here, however, intruders cannot flee and thus we simulate a (presumably) rare scenario where the intruder is highly motivated. Interestingly, the time for the fish to switch between the initial behavior displayed (gill flaring) and the secondary behavior (fin spreading) differed among reproduction phases. Focal males quickly switched from gill flaring to fin spreading after construction of the bubble nest than in any other reproductive phase against both females and males intruders. This illustrates the importance of the bubble nest in the reproductive behavior of fighting fish. Bubble nest quality is a reliable cue that females use during mate choice (Clotfelter et al. 2006), and generally males with nests have an incentive to transmit different information to receivers than males without nests (Dzieweczynski et al. 2005). As the bubble nest is only used in a reproductive context, male fish prioritize courtship behaviors during the bubble nest phase rather than aggression.
The enhanced propensity of males for aggression when approaching the late stages of reproduction might be related to changes of reproductive status (Jaroensutasinee and Jaroensutasinee 2003; Forsatkar et al. 2015). With the increase in average reproductive value of the young, a higher cost of parental effort will be favored in line with parental investment theory (Coleman and Gross 1991). Parental care is energetically costly and parents increase the intensity of aggressive behaviors as their offspring get older and thus become more “valuable” not only in terms of their potential survival but also with respect to the effort already invested in the clutch (Jaroensutasinee and Jaroensutasinee 2003). Thus, during parental care, nest-holding males protected their brood with increasingly aggressive displays towards intruders according to the developmental stage of their offspring. Similarly, subordinate convict cichlids, Amatitlania nigrofasciata, given access to a mate, fought more persistently (Keeley and Grant 1993). The initial display behavior used by males and their willingness to fight may also be correlated with the number of eggs in the nest (Clotfelter et al. 2006).
It is important to note that although we used the same intruders for all focal males at the same reproductive phase in an attempt to reduce variability in intruder behavior, we did not record the behavior of the intruder and this may account for some variation between subjects. Prior fighting experience can affect an individual’s probability of winning a later contest (Hsu and Wolf 2001) and thus might influence the behavior of both the intruders and the focal males. When using fighting fish in repeated encounters, however, Dzieweczynski et al. (2012) found that fighting experience did not affect any of the female- or male-directed gill flaring or fin spreading displays. In the present study nest-holding males were exposed to a new intruder at each phase of reproduction and thus never had the opportunity to become familiar with them. Thus, if anything, our results are conservative.
To conclude, we have shown that Siamese fighting fish are more aggressive in the later stages of reproduction when nest-holding males are more motivated to take care of the nest and the developing young within. Males present more of a threat to nest-holding males than females. During a contest, nest-holding males switch from an energetically costly display to a less costly one in a bid to save energy while still protecting their nests. Crucially, the timing of this switch varies depending on reproductive phase, and provides insight into the level of energetic investment a male is willing to make to protect his brood.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Allen JM, Nicoletto PF (1997) Response of Betta splendens to computer animations of males with fins of different length. Copeia 1997:195–199
Alton LA, Portugal SJ, White CR (2013) Balancing the competing requirements of air-breathing and display behavior during male–male interactions in Siamese fighting fish Betta splendens. Comp Biochem Physiol A Mol Integr Physiol 164:363–367
Bell AM (2005) Behavioral differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J Evol Biol 18:464–473
Castro N, Ros AF, Becker K, Oliveira RF (2006) Metabolic costs of aggressive behavior in the Siamese fighting fish, Betta splendens. Aggress Behav 32:474–480
Clotfelter ED, Curren LJ, Murphy CE (2006) Mate choice and spawning success in the fighting fish Betta splendens: the importance of body size, display behavior and nest size. Ethology 112:1170–1178
Coleman RM, Gross MR (1991) Parental investment theory: the role of past investment. Trends Ecol Evol 6:404–406
Dore F, Lefebvre L, Ducharme R (1978) Threat display in Betta splendens: effects of water condition and type of agonistic stimulation. Anim Behav 26:738–745
Doutrelant C, McGregor PK, Oliveira RF (2001) The effect of an audience on intrasexual communication in male Siamese fighting fish, Betta splendens. Behav Ecol 12:283–286
Dzieweczynski TL, Leopard AK (2010) The effects of stimulus type on consistency of responses to conflicting stimuli in Siamese fighting fish. Behav Process 85:83–89
Dzieweczynski TL, Earley RL, Green TM, Rowland WJ (2005) Audience effect is context dependent in Siamese fighting fish, Betta splendens. Behav Ecol 16:1025–1030
Dzieweczynski TL, Eklund AC, Rowland WJ (2006) Male 11-ketotestosterone levels change as a result of being watched in Siamese fighting fish, Betta splendens. Gen Comp Endocrinol 147:184–189
Dzieweczynski TL, Sullivan KR, Forrette LM, Hebert OL (2012) Repeated recent aggressive encounters do not affect behavioral consistency in male Siamese fighting fish. Ethology 118:351–359
Evans CS (1985) Display vigour and subsequent fight performance in the Siamese fighting fish, Betta splendens. Behav Process 11:113–121
Forsatkar MN (2012) Change in reproductive behavior of fighting fish, Betta splendens by fluoxetine exposure. Master’s thesis, University of Tehran. (in Persian)
Forsatkar MN, Nematollahi MA, Amiri BM, Huang WB (2014) Fluoxetine inhibits aggressive behavior during parental care in male fighting fish (Betta splendens, Regan). Ecotoxicology 23:1794–1802
Forsatkar MN, Dadda M, Nematollahi MA (2015) Lateralization of aggression during reproduction in male Siamese fighting fish. Ethology 121:1039–1047
Hsu Y, Wolf LL (2001) The winner and loser effect: what fighting behaviors are influenced? Anim Behav 61:777–786
Jaroensutasinee M, Jaroensutansinee K (2001) Bubble nest habitat characteristics of wild Siamese fighting fish. J Fish Biol 58:1311–1319
Jaroensutasinee M, Jaroensutasinee K (2003) Type of intruder and reproductive phase influence male territorial defence in wild-caught Siamese fighting fish. Behav Process 64:23–29
Karino K, Someya C (2007) The influence of sex, line, and fight experience on aggressiveness of the Siamese fighting fish in intrasexual competition. Behav Process 75:283–289
Kaufmann JH (1983) On the definitions and functions of dominance and territoriality. Biol Rev 58:1–20
Keeley ER, Grant JW (1993) Visual information, resource value, and sequential assessment in convict cichlid (Cichlasoma nigrofasciatum) contests. Behav Ecol 4:345–349
Kleszczyńska A, Sokołowska E, Kulczykowska E (2012) Variation in brain arginine vasotocin (AVT) and isotocin (IT) levels with reproductive stage and social status in males of three-spined stickleback (Gasterosteus aculeatus). Gen Comp Endocrinol 175:290–296
Kuperberg ES, Brown AC, Clotfelter ED (2009) Body condition in male Betta splendens does not predict their ability to perform opercular displays under hypoxic conditions. Ethology 115:1182–1189
Matos RJ, McGregor PK (2002) The effect of the sex of an audience on male-male displays of Siamese fighting fish (Betta splendens). Behavior 139:1211–1221
McGregor PK, Peake TM, Lampe HM (2001) Fighting fish Betta splendens extract relative information from apparent interactions: what happens when what you see is not what you get? Anim Behav 62:1059–1065
Monvises A, Nuangsaeng B, Sriwattanarothai N, Panijpan B (2009) The Siamese fighting fish: well-known generally but little-known scientifically. Sci Asia 35:8–21
Schroeder L, Huber R (2001) Fight strategies differ with size and allometric growth of claws in crayfish, Orconectes rusticus. Behavior 138:1437–1449
Simpson MJA (1968) The display of the Siamese fighting fish, Betta splendens. Anim Behav Monogr 1:i–73
Tubert C, Nostro FL, Villafañe V, Pandolfi M (2012) Aggressive behavior and reproductive physiology in females of the social cichlid fish Cichlasoma dimerus. Physiol Behav 106:193–200
Acknowledgments
We are grateful to the Aquaculture Department of the University of Tehran for providing us with the laboratory space to perform these experiments. This work was partly funded by the University of Tehran. We thank the editor and two anonymous reviewers for their constructive and insightful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no conflict of interest.
Ethical approval
Currently there are no laws regarding animal research in Iran; however, protocols were approved by the Scientific Committee of the Department of Fisheries of the University of Tehran (no. 2688508; April 2013). Animal handling and testing techniques were designed using guidance from the Association for the Study of Animal Behavior and the Animal Behavior Society (ASAB/ABS 2012).
About this article
Cite this article
Forsatkar, M.N., Nematollahi, M.A. & Brown, C. Male Siamese fighting fish use gill flaring as the first display towards territorial intruders. J Ethol 35, 51–59 (2017). https://doi.org/10.1007/s10164-016-0489-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-016-0489-1