Abstract
In response to anthropogenic eutrophication and global warming, deep-water oxygen depletion is expected to have large effects on freshwater lake biogeochemistry and resident communities. In particular, it has been observed that deep-water hypoxia may potentially lead to regime shifts of lake benthic communities. We explored such community shifts by reconstructing a high-resolution subfossil chironomid record from a sediment core collected in the sub-alpine lake Remoray in France. We identified an abrupt shift in chironomid composition triggered by the collapse of the dominant Sergentia coracina-type chironomids around 1980. We found that the collapse of Sergentia coracina type was coupled to a gradual increase in organic matter content in lake sediments caused by eutrophication. We concluded that the most probable cause for the collapse of Sergentia coracina type was a change in oxygen concentrations below the minimal threshold for larval growth. We also analyzed trends in variance and autocorrelation of chironomid dynamics to test whether they can be used as early warnings of the Sergentia collapse. We found that variance rose prior to the collapse, but it was marginally significant (Kendal rank correlation 0.71, p = 0.05), whereas autocorrelation increased but insignificantly and less strongly (Kendal rank correlation 0.23, p = 0.25). By combining reconstructions of ecosystem dynamics and environmental drivers, our approach demonstrates how lake sediments may provide insights into the long-term dynamics of oxygen in lakes and its impact on aquatic fauna.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypoxia has become a major ecological concern for lake ecosystems over the last 150 years. Lack of oxygen induces various impacts on lakes including increases in their contribution to greenhouse gas emissions (Gonzalez-Valencia et al. 2014), fish mortalities (Rao et al. 2014), and disappearance of benthic organisms (also called dead zones; Diaz and Rosenberg 2008). Oxygen depletion in the profundal zone is considered natural for some freshwater ecosystems and can be caused by morphological factors (e.g., Aeschbach-Hertig et al. 1999) or by thermal stratification (Jankowski et al. 2007). However, it is now widely accepted that anthropogenic disturbances are one of the main factors for decreasing oxygen concentrations in lakes leading to hypoxia (Rabalais et al. 2010; Frossard et al. 2013). Rising input of organic matter in lake sediments is the major driver of oxygen depletion in deep layers of lakes (Charlton 1980). The main process behind such depletion is the increase in bacterial degradation of detritus that consumes oxygen (den Heyer and Kalff 1998). This process is often fueled by anthropogenic nutrient enrichment (also called eutrophication; Downing et al. 2008; Anderson et al. 2014) that is driven by agricultural practices or urban point sources of pollution (Carpenter et al. 1998; Jenny et al. 2016). In the current context of the Anthropocene (Crutzen 2006) that is characterized by a growing impact of anthropogenic eutrophication, hypoxia and its effects on benthic life are expected to become widespread in freshwater ecosystems.
Abrupt changes in benthic fauna due to hypoxia are identified as one of the main regime shifts in marine ecosystems (Rocha et al. 2015). Regime shifts are usually characterized by abrupt and dramatic changes in community composition triggered by collapsing or changing abundances of single or multiple taxa (Conversi et al. 2015). Regime shifts driven by hypoxia can also be found in lakes. A gradual decrease in lake oxygen concentrations below a tolerance threshold may force macroinvertebrate larvae to migrate out of the hypoxic zone (Wülker 1961) and to become absent from these areas (Heinis and Davids 1993). It may even lead to the complete collapse of the benthic fauna (Brodersen et al. 2004) and to the establishment of a stable hypoxic state. Such shifts to a hypoxic state are often difficult to reverse (Millet et al. 2010). Frossard et al. (2013) found that the deep layers of the water column in a sub-alpine lake remained hypoxic despite a large reduction in nutrient input. The disappearance of benthic consumers (such as chironomid larvae; Diptera, Chironomidae) due to lack of oxygen may have considerable consequences for the functioning of the whole lake. This may disrupt the benthic–pelagic trophic coupling (Gratton and Vander Zanden 2009; Wagner et al. 2012) affecting the whole aquatic food web up to top-predators (Covich et al. 1999). Understanding and detecting the collapse of benthic communities would be of great value for a lake manager.
The detection of upcoming regime shifts has recently attracted a lot of attention, and indicators have been developed that can signal impending sudden ecosystem changes (Scheffer et al. 2009; Dakos et al. 2015). Such early-warning signals (EWS) quantify statistical changes in ecosystem dynamics that are caused by critical slowing down (Wissel 1984) or flickering (Scheffer et al. 2009). Critical slowing down means that temporal dynamics in a system become sluggish close to an impending transition, whereas flickering corresponds to alternative flips from one attraction basin to another in the vicinity of an impending shift (Dakos et al. 2013). Both phenomena lead to a rising variance (Carpenter and Brock 2006), whereas critical slowing down also causes an increase in autocorrelation (Held and Kleinen 2004). These two indicators were shown to signal trophic cascades in lakes (Carpenter et al. 2011), shifts to lake eutrophication (Wang et al. 2012) and plankton collapses in experimental populations (Drake and Griffen 2010; Veraart et al. 2011).
Despite recent modeling techniques (Carpenter et al. 2014) and analysis of remotely sensored lake variables (Batt et al. 2013), the practical use of EWS in ecosystem management for detecting upcoming regime shifts is still limited. Part of the reason is that EWS require long-term and high-resolution monitoring data (Dakos et al. 2015). Unfortunately, most natural ecosystems, including freshwater lakes, have been monitored for a too short time period and at low temporal resolution. Thus, our ability to evaluate the power and use of EWS in detecting lake regime shifts still lies in retrospective analysis of past records.
Paleolimnological approaches use lake sediments as ecological archives to understand lake processes and to compensate for the lack of monitoring data in freshwater lakes (Smol 1992). Because of their high chitin content, the anterior part of the chironomid larval exoskeleton (the head capsule, HC) is well preserved in lake sediments, and analysis of chironomid remains serves as a powerful proxy to track long-term changes in oxygen conditions in the deepest part of lakes (Quinlan et al. 1998; Little et al. 2000; Brodersen et al. 2008). Oxygen demand varies widely among chironomid species (Brodersen et al. 2004; Luoto and Salonen 2010), and oxygen concentrations are one of the main factors that regulate chironomid communities (Verbruggen et al. 2011). At the same time, sedimentological analysis can be used to reconstruct environmental conditions for chironomid assemblages, such as organic carbon content in lake sediments that partly reflects the potential of bacterial oxygen consumption at the water/sediment interface (Meyers and Ishiwatari 1993), and sedimentary pigments that reflect lake nutrient concentrations (Guilizzoni et al. 2011). When combined, chironomid remains and sedimentological analyses allow us to reconstruct the dynamic of lake benthic functioning and to understand its controlling factors (Belle et al. 2016a).
Here, we study the abrupt collapse in chironomid assemblages driven by eutrophication using EWS on paleolimnological data. We first reconstructed subfossil chironomid assemblages, sediment organic carbon content and sedimentary pigments over the last 150 years in a sub-alpine lake. We then analyzed the temporal dynamics of chironomid assemblages to identify regime shifts in the chironomid assemblage composition and to explore their controlling factors. Lastly, we tested whether an abrupt shift in chironomid assemblages could be detected in advance by estimating changes in variance and autocorrelation in the paleolimnological records.
Methods
Study site and core chronology
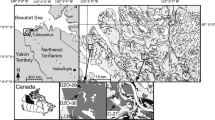
Lake Remoray (46°46′12″N; 6°15′49″E) is a sub-alpine lake (95 ha) located in the Jura Mountains (eastern France) with a maximal water depth of 27 m (Fig. 1a). The bedrock of its catchment is composed of a carbonate substratum. While the lake is dimictic, the profundal zone exhibits anoxic conditions at the end of summer and winter stratifications (Fig. 1b). Previous studies have revealed the absence of benthic organisms in the deepest part of the lake (Verneaux V. unpublished data). Belle et al. (2016b) had only suggested the disappearance of chironomid remains from the profundal zone at ca. AD 1980 (Anno Domini) due to agricultural activities, as there was no reconstruction of chironomid paleodiet after 1980.
a Location and bathymetric map of Lake Remoray. In the bathymetric map, black circle marks the coring position at the maximal water depth. b Oxygen profile measured on September 26, 2014, at the vertical of maximum water depth. c Age–depth relationship and standard uncertainty estimations of the Rem07_P1 core using a constant rate of supply (CRS) model. Gray bands indicate the two peaks of 137Cs contamination
We collected a short sediment core (196 cm) in the deepest part of the lake using a gravity corer in 2007 (UWITEC, 63 mm of diameter) at 27 m of water depth (Fig. 1a). The sediment dating of the last 150 years was performed using radiometric methods (based on combined measurements of 210Pb and 137Cs activities). 210Pb measurements were quantified using alpha spectrometry (MyCore Scientific Inc., Canada), and 137Cs measurements using gamma spectrometry (Radionuclide Unit, Chrono-Environment, France). The age–depth model and standard uncertainty estimations were then estimated with a constant rate of supply model (CRS; Appleby and Oldfield 1978). Reliability of the age–depth model was checked using the 137Cs contamination record. Two peaks can be easily identified in the Jura Mountains (Belle et al. 2014) corresponding to the Chernobyl nuclear accident (AD 1986) and the maximum fallout intensity of atmospheric nuclear weapon tests (AD 1963). These two characteristic peaks of 137Cs have been identified in the sediment core between 6 and 6.5 cm and between 9.5 and 10 cm depth in core corresponding to the contaminations of AD 1986 and AD 1963, respectively. The peak depths intercepted the uncertainty shell of the age–depth model confirming the reliability of the model (Fig. 1c). Hereafter, all ages were expressed in calendar years (AD).
Chironomid remains and sediment characteristics
For the chironomid analysis, the uppermost 21.5 cm was divided into 43 contiguous samples of 0.5 cm thickness. Samples were successively rinsed with NaOH (10%) and HCl (10%) solutions and sieved through a 100-µm mesh. Chironomid remains were hand-sorted from the sieving residue under a stereomicroscope and mounted in aqueous agent on microscope slides. Head capsules were identified under microscope using keys provided by Rieradevall and Brooks (2001) and Brooks et al. (2007). The composition of the chironomid assemblages was expressed as a percentage of each taxon of the total numbers of HC. Only taxa occurring in at least two samples and with a maximum relative abundance greater than 2.5% were included in the analysis (Walker 2001). To avoid any potential misleading interpretations due to changes in related morphotypes, chironomid assemblages were also expressed in terms of HC accumulation rates (in numbers of HC 100 cm−2 year−1).
Sediment core was also sliced on 11 contiguous samples of 2 cm thickness for sedimentological analysis. Organic carbon content in lake sediments was quantified using a vario TOC cube analyzer (Elementar; hereafter Corg and expressed in percentage %). Sediment samples were dried at 60 °C for 3 days prior to analysis and reduced to a fine powder using a mortar. Samples were then treated to remove any carbonate content by the gentle addition (100 µL) of HCl solution (3.7%) until the effervescence stopped. Sedimentary pigments were extracted from about 1 g of fresh sediments, overnight under nitrogen, with a solution of acetone and water (90:10) and were then centrifuged at 3000 rpm for 10 min. Total carotenoids (TC) were quantified from the extract via spectrophotometry and were expressed in milligrams per gram of organic matter (mg g−1 of OM; Guilizzoni et al. 2011).
Stable isotopic carbon composition of sedimentary organic carbon (δ 13COM) was analyzed using an isotope ratio mass spectrometer interfaced with an elemental analyzer (EA-IRMS). Prior to analysis, carbonate removal was necessary by acid fumigation (with a concentrated solution of HCl) following Belle et al. (2014). The results are expressed as the delta notation with Vienna Pee Dee Belemnite as the standard: δ 13C (‰) = (R sample/R standard − 1) × 1000, where R = 13C/12C. The replication of sample measurements from standards produced analytical errors (1σ) of ±0.24 ‰ (n = 10). Lastly, Corg, TC and δ 13COM values were interpolated using a linear regression to produce the same number of data per descriptors (n = 43) in order to allow statistical analyses.
Chironomid dynamics and driving factors
Two zones, defined by major patterns in chironomid assemblage composition (see Results), were determined by constrained hierarchical cluster analysis using a Bray–Curtis distance and a CONISS linkage method (Rioja package for R) on the selected assemblages (based on the minimum frequency criteria). The significance of the zones was assessed using the broken-stick model (Bennett 1996). Detrended correspondence analysis (DCA) was performed on the chironomid assemblages to assess the compositional turnover and to select the appropriate ordination method (Legendre and Birks 2012). As the length of the first axis of the DCA was short (less than about 2.5 SD) and implied a low assemblage variation, we used a linear approach (Euclidean distance) of principal component analysis (PCA) on the chironomid assemblage data using the ade4 package (Dray and Dufour 2007) in R v. 3.1.2 (R Core Team 2014). The broken-stick model (Bennett 1996) was used to check the importance of the first PCA axis (PCA1). The PCA1 scores (expressing the percent variance explained) were used as proxy of the chironomid temporal variability. Eigenvalues of PCA1 as well as the variable contributions to PCA1 were also calculated.
We only considered organic matter and temperature as the main drivers of chironomid assemblages. We did not consider precipitation or macrophyte presence because their effect on benthic assemblages in this kind of sub-alpine deep lakes can be considered minimal (Verbruggen et al. 2011). We estimated the relationships between Corg and climate data with PCA1 using a generalized additive model (Simpson and Anderson 2009), with forward selection (α < 0.05). Local climate over the last 150 years was described using the annual air temperature extracted from the gridded HISTALP dataset (Auer et al. 2007).
Early-warning signals
The chironomid time series were divided into two datasets according to the detected zones (see section “Chironomid dynamics and driving factors” above). Only data prior to the shift in the composition of the chironomid assemblages were considered for EWS analysis (corresponding to 26 samples). As variation in sedimentation rate can affect the constancy in resolution of the paleorecord over time and introduce bias in the estimation of EWS (Carstensen et al. 2013), we checked the evolution of the number of years per sample in our record. We found a temporal resolution of 2 years per sample prior to the shift. Thus, samples with a lower temporal resolution than 2 (±1 according to the chronological uncertainty) years per sample were removed from the time series. After this correction, six samples were removed and 20 contiguous samples (data points) were conserved for statistical analysis.
Variance (as standard deviation; SD) and temporal autocorrelation (as the coefficient of a first-order auto-regressive model; AR1) were measured within rolling windows (Dakos et al. 2012) of a time span of 16, 20 and 24 years (corresponding to 8, 10 and 12 samples, respectively). Indicators were calculated every 2 years (with a forward step of 1 sample). Indicators were measured from the relative abundances and accumulation rates of Sergentia coracina type (the dominant chironomid taxon) and from the PCA1 scores (as an indicator of the community response). SD and AR1 were measured after linear detrending along the time series (Dakos et al. 2012). The trend of the indicators values was quantified by a Kendall rank correlation test (McLeod 2011). We assessed the significance of the observed trends in variance and autocorrelation by fitting the best auto-regressive moving average model (ARMA) to the original data based on Akaïke information criterion (AIC) to generate 100 surrogate time series using the early warnings package for R (Dakos et al. 2012). We calculated Kendall τ correlation coefficients for each surrogate time series, and we determined significance by comparing the trend estimated in the empirical time series to the distribution of the surrogate trends (Dakos et al. 2012).
All statistical analysis and figures were done using R 3.1.2 statistical software (R Core Team 2014).
Results
Seven morphotypes met the minimum frequency criteria for inclusion in the statistical analysis: Glyptotendipes pallens type, Tanytarsus lugens type, Cladotanytarsus mancus type, Tanytarsus ssp., Procladius, Paratanytarsus and Sergentia coracina type (Fig. 2a). Tanytarsus lugens type, Procladius, Paratanytarsus and Sergentia coracina type are considered to be profundal taxa, whereas Glyptotendipes pallens type, Cladotanytarsus mancus type, Tanytarsus ssp. are assumed to be mainly littoral taxa and are physically eroded from the shallower part of Lake Remoray where large macrophyte areas were observed (Magnin 1904). Cluster analysis on these assemblages (Bray–Curtis distance and CONISS linkage method) revealed two significant zones, and the transition between these two zones occurred abruptly at ca. 1980 (Fig. 2a). Prior to the shift, the relative abundances of chironomid assemblages were quite constant over 100 years and were dominated by the Sergentia coracina type (with an average percentage around 67 ± 13%). After the shift, the relative abundance and accumulation rates of this dominant taxon collapsed and remained null until the top of the sediment core (Fig. 2a). The first axis of PCA (PCA1) accounted for 31% of the variability of chironomid assemblages. This was the sole important axis of the PCA according to the broken-stick model. Sergentia coracina type contributed the most to the PCA1 (37% of the overall variability; Fig. 2b). High relative abundance of Sergentia coracina type was associated with positive PCA1 samples scores, while negative PCA1 scores mainly corresponded to high relative abundance of Procladius, Tanytarsus ssp. and Cladotanytarsus mancus type (accounting for 19, 16 and 8% of the overall variability, respectively; Fig. 2c). The other littoral or sub-littoral taxa exhibited the lowest values of contribution to the PCA1 variability (< to 8%). PCA1 scores were positive from 1890 to ca. 1980, but drastically decreased and became mostly negative after ca. 1980 marking clearly the shift in the composition of the chironomid assemblages (Fig. 2a).
a Chironomid record of Lake Remoray (Rem07_P1 core) over the last 150 years, and dendrogram based on chironomid assemblages, constructed by hierarchical clustering analysis (Bray–Curtis distance, CONISS linkage method). The dashed line indicates a significant change according to the broken-stick model. Accumulation rates of Sergentia coracina type (expressed in HC.100 cm2 years−1) b Principal component analysis (PCA) performed on the relative abundances of the chironomid assemblages. c Factorial map of the PCA (PCA2 vs. PCA1) where circles indicate the samples prior to the abrupt shift, whereas square symbols represent the samples after the shift. Bold labels represent the samples corresponding to the transition between the two zones
Local climate was characterized by a significant warming (mean air temperature) starting in the late 1980 s (Fig. 3a). Organic content in lake sediments (Corg) was stable at about 3% from 1890 to ca. 1950, but showed a constant and gradual increase from 1920 onwards reaching a maximum in the early 2000s (up to 5.7%; Fig. 3a). There was a steep rise around 1950 (Fig. 3a) that may be interpreted as a step-wise increase in organic content. However, the gradual trends in both sedimentary pigments (TC) and organic matter δ 13C data (δ 13COM) (Fig. 3a)—that were both correlated with Corg—do not support the idea that the organic content experienced some sort of a sudden event. Sedimentary pigments TC and δ 13COM were negatively correlated. The δ 13COM values ranged between −33.4 and −30.4‰, whereas TC values were comprised between 1.1 and 4.9 mg g−1 of OM (Fig. 3a). Finally, a generalized additive model showed a strong nonlinear relationship (F = 14.1, p < 0.001, edf = 3.82 corresponding to a simple sigmoid curve) between organic content in lake sediments (Corg) and chironomid assemblages (PCA1) explaining 64.2% of the overall variability (Fig. 3b).
a Trends in chironomid assemblages (PCA1), organic carbon content in lake sediments (Corg, %) and mean air temperature (°C). Temporal dynamics of total carotenoid concentrations (TC, expressed in mg g−1 of OM) and carbon isotopic composition of organic matter (δ 13COM; ‰). Solid line is a local polynomial regression (loess parameters: size of the neighborhood window = 60% of the data; polynomials degree = 2). Horizontal dashed line indicates the significant abrupt change in the composition of the chironomid assemblages. In panels of Corg, TC and δ 13COM, black squares correspond to the measured data, whereas white squares represent the interpolated data. b Fitted smooth function between organic carbon content (Corg) and chironomid assemblages (PCA1 scores) from a general additive model (GAM). Gray surface marks the 95% uncertainty interval of the fitted function. On the x-axis, ticks show the distribution of observed values for the two variables. Number in parenthesis on the y-axis (3.82) is the effective degrees of freedom (edf) of the smooth function
We estimated standard deviation (SD) and autocorrelation at-lag-1 (AR1) of the relative abundances and accumulation rates of Sergentia coracina type and PCA1 scores in 13 rolling windows of 8 samples (every rolling window spans a period of 16 years; Fig. 4a, c). We found increasing trends in SD for both detrended Sergentia relative abundances and PCA1 scores (Kendall rank correlation test; τ = 0.516; p < 0.05 and τ = 0.714; p < 0.05, respectively, Fig. 4b, d), whereas raw data (not detrended) revealed weaker trends (Kendall rank correlation test; τ = 0.098; p > 0.05 and τ = 0.473; p < 0.05 for Sergentia and PCA1, respectively). These positive trends were marginally significant for both Sergentia relative abundances and PCA1 scores (p = 0.08 and p = 0.05, respectively, ESM 1 and 3) when compared to trends estimated from random realizations of a best-fitted ARMA model to the empirical data (see Methods). The trend was also confirmed by the SD analysis of accumulation rates of Sergentia coracina type (τ = 0.67; p < 0.05). We confirmed trends in SD also when using a rolling window size of 10 and 12 samples (corresponding to a time span of 20 and 24 years, respectively; ESM 1 and ESM 3). Positive trends were also found for AR1 applied to the Sergentia relative abundances (ESM 2), but were far from significant when AR1 was estimated on the PCA1 scores (ESM 4). Estimates of AR1 turned from negative to positive close to the collapse in chironomid assemblages making their trends difficult to interpret.
a Time series of the relative abundance of Sergentia coracina type in the benthic community (%). Light marked dots are the samples not used for estimating early warnings because of their low temporal aggregation. b Temporal trend in variance (measured as standard deviation) of Sergentia coracina type after linear detrending within a rolling window of 16 years (8 samples). c Time series of PCA1 scores. Light marked dots are the samples not used for estimating early warnings because of their low temporal aggregation. d Temporal trend in variance (measured as standard deviation) of PCA1 scores after linear detrending within a rolling window of 16 years (8 samples). Solid line is a local polynomial regression (loess parameters: size of the neighborhood window = 80% of the data; polynomials degree = 2). Vertical dashed line indicates the significant abrupt change in the composition of the chironomid assemblages. Kendall τ reports the rank correlation trend of standard deviation and its significance p
Discussion
Deep-water hypoxia can drive numerous changes in lake functioning, including increases in greenhouse gas emissions, fish mortality or benthic community collapse. As oxygen depletion in lakes is forecasted to increase under current trends of eutrophication and global warming, here we studied the potential for detecting the collapse of chironomid assemblages caused by oxygen depletion using early-warning signals in a paleolimnological record from a sub-alpine lake.
Chironomid collapse and eutrophication
The reconstruction of the chironomid dynamics in Lake Remoray revealed a sudden change in assemblages at ca. 1980 characterized by the disappearance of Sergentia coracina type, the dominant chironomid taxon (Fig. 2a). This abrupt change was not a consequence of some sort of strong disturbance but rather driven by a gradual increase in organic matter content in lake sediments (Fig. 3a, b). The negative δ 13COM trend before 1980 indicated that sediments became increasingly enriched in lake-produced organic matter (Fig. 3a, Meyers and Ishiwatari 1993), probably in response to a rise in nutrient availability in the water column as manifested by the positive trend in TC values (Fig. 3a, Guilizzoni et al. 2011). This observation is in line with Belle et al. (2016a) who have hypothesized that intensification of agricultural activities in the catchment of Lake Remoray during the middle of the twentieth century has probably caused an important nutrient runoff and an increase in lacustrine productivity. Thus, eutrophication appears to be the driver behind the shift in the chironomid assemblages. In this context, small increase in relative abundances of littoral taxa could be induced by an artifact caused by the disappearance of Sergentia coracina type and the rise in the littoral secondary production as a direct consequence of lake eutrophication.
The mechanics between the chironomid collapse and eutrophication are probably related to the oxygen dynamics in the lake. Alternative hypotheses, like susceptibility to a fish predator, seem improbable, as there was no information on benthic predator introduction at the time in the lake. Nonetheless, we cannot unequivocally exclude other mechanism, as unfortunately, we cannot reconstruct the oxygen dynamics based on the paleorecord. Nonetheless, organic matter can serve as proxy to hypothesize the chain of events leading to the chironomids collapse. The rise of organic matter content in the sediment may lead to an increase in bacterial respiration rates inducing oxygen depletion in the profundal zone (Charlton 1980; Matzinger et al. 2010; Müller et al. 2012). Sergentia coracina type (the dominant chironomid taxon) is known to be quite tolerant to hypoxic conditions (oxy-tolerant taxon; Brodersen and Quinlan 2006). Several studies define the tolerance threshold of oxygen concentrations for the genus Sergentia between 2.7 mg L−1 (Quinlan and Smol 2001) and <4 mg L−1 (Luoto and Salonen 2010). Our results indicate that oxygen concentrations in the profundal zone—driven by increased accumulation of organic matter—reached this critical threshold around 1980 causing the disappearance of Sergentia coracina type (Fig. 2a). Once this threshold was crossed, organic matter concentrations kept increasing (up to 5.7%; Fig. 3a) probably inducing further depletion in oxygen concentrations that led to the establishment of the current anoxic state in the deep part of lake (Fig. 1b). The rest of the chironomid taxa after the shift did not seem to be affected strongly by the oxygen limitation. This can be explained, first by the fact that the increase in the three littoral taxa is mostly due to transport from the littoral zone where a rise in secondary production as a consequence of eutrophication might have increased their abundance. The rest three profundal taxa most probably did suffer from the lack of oxygen, but their contribution to community composition did not change much (Fig. 2a), as the disappearance of Sergentia coracina type numerically strengthens their percentage contribution.
Rising variance as an early indicator of chironomid collapse
We estimated early-warning signals (i.e., variance and autocorrelation) on the relative abundances and accumulation rates of Sergentia coracina type as well as on the PCA1 scores to test whether we can detect the impeding collapse in chironomid assemblages. In principle, changes in the dynamics of the PCA1 score would provide the necessary information for detecting the approaching assemblage shift. As Sergentia coracina type, however, was the taxon that suffered the strongest change, we also explored the indicators in its dynamics to compare whether signals at the assemblage level were reflected predominantly in the Sergentia coracina taxon. We found rising trends in variance but weaker increasing trends in autocorrelation prior to the collapse. Despite the noisy patterns, variance consistently increases and the trends are close to significant (ESM 1 and ESM 3). Trends in autocorrelations are less consistent as they mark a shift from negative to positive values (ESM 2 and ESM 4), which might be consequence of a sparsely resolved record, or flickering like it has been also found elsewhere (Wang et al. 2012). Nonetheless, taken these results together, they are in line at least with the hypothesis of greater variability close to a regime shift (Hare and Mantua 2000; Carpenter and Brock 2006; Carpenter et al. 2011; Pace et al. 2013). Indeed, we do find a rise in variance already few decades before the observed chironomid collapse (Fig. 4b), as it has been shown in paleorecords prior to lake eutrophication (Wang et al. 2012) and in observational data before community shifts in marine environments (Wouters et al. 2015).
EWS as a tool for ecosystem managers
Although EWS are a promising tool for ecosystem managers to detect impending abrupt shifts, their robust interpretation most likely will suffer from incomplete and low-resolution time series. A recent study has shown that even in some of the best monitored lakes with well-documented regime shifts, indicator patterns like the ones we analyzed here do not consistently signal in advance the registered shifts (Gsell et al. 2016). More importantly, rising variance and autocorrelation may be found also prior to abrupt but continuous transitions in ecosystems without alternative stable states (Kefi et al. 2013). This means that in our study, it is difficult to discriminate whether the abrupt shift represents a transition with bistability (Fig. 5a), or not (Fig. 5b). Such difference has important implications when it comes to recovery to a well-oxygenated profundal zone state allowing the re-colonization by chironomid larvae. In the case of a transition without bistability (Fig. 5a), restoration back to the pre-collapse state will occur at the same environmental conditions that the chironomid collapse took place (Fig. 5a). However, in the case of a transition with bistability, the recovery trajectory becomes complicated due to hysteresis (Scheffer et al. 2001): Environmental conditions must be restored at even lower conditions than the ones at which the regime shift occurred (Fig. 5b). For Lake Remoray, we found a nonlinear but continuous relationship between PCA1 scores and organic matter (Fig. 3b) indicating a threshold response rather than bistability. Nonetheless, a reduction in organic matter content in lake sediments does not necessarily lead to immediate higher oxygen levels at the water/sediment interface. Oxygen control in lakes is multifactorial resulting in maintaining a hypoxic (or anoxic) state (Frossard et al. 2013). For example, warming (as we observed in the data from 1990; Fig. 3a) could increase thermal stratification and, thus, delay changes in hypolimnetic oxygen conditions (Jankowski et al. 2006; Wilhem and Adrain 2007). Such conditions would contribute to a high re-colonization time of deep lake sediments by benthic organisms and a potential hysteretic effect on recovering to a pre-collapse state.
a A regime shift to chironomid collapse explained as an abrupt (continuous) response without a bifurcation. b A regime shift to chironomid collapse explained as an abrupt (discontinuous) response with bistability (fold bifurcation). Letters refer to different positions along the ecological trajectory of Lake Remoray. Stability landscapes have been graphically drawn to show changes in the basins of attraction of the ecosystem states. Note that in both cases a, b, variance is expected to rise before the regime shift
It is difficult to unequivocally detect an approaching abrupt shift to an alternative state. Still, being able to identify such precursors in observational records can be of use for ecosystem management especially when it comes to understanding nonlinear ecosystem responses to stress. Combining reconstructed environmental conditions with changes in the pattern of ecological dynamics, as we show in this work, could provide insights into lake oxygen dynamics, their controlling factors and impact on lake communities.
References
Aeschbach-Hertig W, Hofer M, Kipfer R, Imboden DM, Wieler R (1999) Accumulation of mantle gases in a permanently stratified volcanic lake (Lac Pavin, France). Geochim Cosmochim Acta 63:3357–3372
Anderson NJ, Bennion H, Lotter AF (2014) Lake eutrophication and its implications for organic carbon sequestration in Europe. Glob Change Biol 20:2741–2751
Appleby PG, Oldfield F (1978) The calculation of 210Pb dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA 5:1–8
Auer I, Böhm R, Jurkovic A, Lipa W, Orlik A, Potzmann R, Schöner W, Ungersböck M, Matulla C, Briffa K, Jones P, Efthymiadis D, Brunetti M, Nanni T, Maugeri M, Mercalli L, Mestre O, Moisselin J-M, Begert M, Müller-Westermeier G, Kveton V, Bochnicek O, Stastny P, Lapin M, Szalai S, Szentimrey T, Cegnar T, Dolinar M, Gajic-Capka M, Zaninovic K, Majstorovic Z, Nieplova E (2007) HISTALP—historical instrumental climatological surface time series of the Greater Alpine Region. Int J Clim 27:17–46
Batt RD, Carpenter SR, Cole JJ, Pace ML, Johnson RA (2013) Changes in ecosystem resilience detected in automated measures of ecosystem metabolism during a whole-lake manipulation. P Nat Acad Sci USA 110:17398–17403
Belle S, Parent C, Frossard V, Verneaux V, Millet L, Chronopoulou P-M, Sabatier P, Magny M (2014) Temporal changes in the contribution of methane-oxidizing bacteria to the biomass of chironomid larvae determined using stable carbon isotopes and ancient DNA. J Paleolimnol 52:215–228
Belle S, Millet L, Verneaux V, Lami A, David E, Murgia L, Parent C, Musazzi S, Gauthier E, Bichet V, Magny M (2016a) 20th century human pressures drive reductions in deepwater oxygen leading to losses of benthic methane-based food webs. Quat Sci Rev 137:209–220
Belle S, Verneaux V, Millet L, Etienne D, Lami A, Musazzi S, Reyss J-L, Magny M (2016b) Climate and human land-use as a driver of Lake Narlay (Eastern France, Jura Mountains) evolution over the last 1200 years: implication for methane cycle. J Paleolimnol 55:83–96
Bennett KD (1996) Determination of the number of zones in a biostratigraphical sequence. New Phytol 132:155–170
Brodersen KP, Quinlan R (2006) Midges as palaeoindicators of lake productivity, eutrophication and hypolimnetic oxygen. Quat Sci Rev 25:1995–2012
Brodersen KP, Pedersen O, Lindegaard C, Hamburger K (2004) Chironomids (Diptera) and oxy-regulatory capacity: an experimental approach to paleolimnological interpretation. Limnol Oceanogr 49:1549–1559
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of Palaearctic Chironomidae larvae in palaeoecology. QRA Technical Guide No. 10. Quaternary Research Association, London, p 276
Carpenter SR, Brock WA (2006) Rising variance: a leading indicator of ecological transition. Ecol Lett 9:311–318
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Carpenter SR, Cole JJ, Pace ML, Batt R, Brock WA, Cline T, Coloso J, Hodgson JR, Kitchell JF, Seekell DA, Smith L, Weidel B (2011) Early warnings of regime shifts: a whole-ecosystem experiment. Science 332:1079–1082
Carpenter SR, Brock WA, Cole JJ, Pace ML (2014) A new approach for rapid detection of nearby thresholds in ecosystem time series. Oikos 123:290–297
Carstensen J, Telford RJ, Birks HJB (2013) Diatom flickering prior to regime shift. Nature 498:E11–E12
Charlton MN (1980) Hypolimnion oxygen consumption in lakes: discussion of productivity and morphometry effects. Can J Fish Aquat Sci 37:1531–1539
Conversi A, Dakos V, Gårdmark A, Ling S, Folke C, Mumby PJ, Greene C, Edwards M, Blenckner T, Casini M, Pershing A, Möllmann C (2015) A holistic view of marine regime shifts. Philos Trans R Soc Lond B Biol Sci 370:1659
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. Bioscience 49:119–127
Crutzen PJ (2006) The ‘anthropocene’. In: Ehlers PDE, Krafft DT (eds) Earth system science in the anthropocene. Springer, Berlin, pp 13–18
Dakos V, Carpenter SR, Brock WA, Ellison AM, Guttal V, Ives AR, Kéfi S, Livina V, Seekell DA, van Nes EH, Scheffer M (2012) Methods for detecting early warnings of critical transitions in time series illustrated using simulated ecological data. PLoS ONE 7:e41010
Dakos V, van Nes EH, Scheffer M (2013) Flickering as an early warning signal. Theor Ecol 6:309–317
Dakos V, Carpenter SR, van Nes EH, Scheffer M (2015) Resilience indicators: prospects and limitations for early warnings of regime shifts. Philos T R Soc B 370:20130263
den Heyer C, Kalff J (1998) Organic matter mineralization rates in sediments: a within-and among-lake study. Limnol Oceanogr 43:695–705
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Downing JA, Cole JJ, Middelburg JJ, Striegl RG, Duarte CM, Kortelainen P, Prairie YT, Laube KA (2008) Sediment organic carbon burial in agriculturally eutrophic impoundments over the last century. Global Biogeochem Cycle. doi:10.1029/2006GB002854
Drake JM, Griffen BD (2010) Early warning signals of extinction in deteriorating environments. Nature 467:456–459
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
Frossard V, Millet L, Verneaux V, Jenny J-P, Arnaud F, Magny M, Poulenard J, Perga M-E (2013) Chironomid assemblages in cores from multiple water depths reflect oxygen-driven changes in a deep French lake over the last 150 years. J Paleolimnol 50:257–273
Gonzalez-Valencia R, Sepulveda-Jauregui A, Martinez-Cruz K, Hoyos-Santillan J, Dendooven L, Thalasso F (2014) Methane emissions from Mexican freshwater bodies: correlations with water pollution. Hydrobiologia 721:9–22
Gratton C, Vander Zanden MJ (2009) Flux of aquatic insect productivity to land: comparison of lentic and lotic ecosystems. Ecology 90:2689–2699
Gsell AS, Scharfenberger U, Özkundakci D et al (2016) Evaluating early-warning indicators of critical transitions in natural aquatic ecosystems. PNAS 113:E8089–E8095. doi:10.1073/pnas.1608242113
Guilizzoni P, Marchetto A, Lami A, Gerli S, Musazzi S (2011) Use of sedimentary pigments to infer past phosphorus concentration in lakes. J Paleolimnol 45:433–445
Hare SR, Mantua NJ (2000) Empirical evidence for North Pacific regime shifts in 1977 and 1989. Progr Oceanogr 47:103–145
Heinis F, Davids C (1993) Factors governing the spatial and temporal distribution of Chironomid larvae in the Maarsseveen lakes with special emphasis on the role of oxygen conditions. Neth J Aquat Ecol 27:21–34
Held H, Kleinen T (2004) Detection of climate system bifurcations by degenerate fingerprinting. Geophys Res Lett 31:L23207
Jankowski T, Livingstone DM, Bührer H, Forster R, Niederhauser P (2006) Consequences of the 2003 European heat wave for lake temperature profiles, thermal stability, and hypolimnetic oxygen depletion: implications for a warmer world. Limnol Oceanogr 51:815–819
Jenny JP, Normandeau A, Francus P, Taranu ZE, Gregory-Eaves I, Lapointe F, Jautzy J, Ojala AEK, Dorioz J-M, Schimmelmann A, Zolitschka B (2016) Urban point sources of nutrients were the leading cause for the historical spread of hypoxia across European lakes. Proc Nat Acad Sci USA 113:12655–12660
Kéfi S, Dakos V, Scheffer M, Van Nes EH, Rietkerk M (2013) Early warning signals also precede non-catastrophic transitions. Oikos 122:641–648
Legendre P, Birks HJB (2012) From classical to canonical ordination. In: Birks HJB, Lotter AF, Juggins S, Smol JP (eds) Tracking environmental change using lake sediments. Developments in paleoenvironmental research. Springer, Berlin, pp 201–248
Little JL, Hall RI, Quinlan R, Smol JP (2000) Past trophic status and hypolimnetic anoxia during eutrophication and remediation of Gravenhurst Bay, Ontario: comparison of diatoms, chironomids, and historical records. Can J Fish Aquat Sci 57:333–341
Luoto TP, Salonen V-P (2010) Fossil midge larvae (Diptera: Chironomidae) as quantitative indicators of late-winter hypolimnetic oxygen in southern Finland: a calibration model, case studies and potentialities. Boreal Env Res 15:1–18
Magnin A (1904) Monographies botaniques de 74 lacs jurassiens suivies de considérations générales sur la végétation lacustre. Paul Klincksieck, Paris, p 426
Matzinger A, Müller B, Niederhauser P, Schmid M, Wüest A (2010) Hypolimnetic oxygen consumption by sediment-based reduced substances in former eutrophic lakes. Limnol Oceanogr 55:2073–2084
McLeod AI (2011) Kendall: Kendall rank correlation and Mann-Kendall trend test. R package version 2.2. http://CRAN.R-project.org/package=Kendall
Meyers PA, Ishiwatari R (1993) Lacustrine organic geochemistry—an overview of indicators of organic matter sources and diagenesis in lake sediments. Org Geochem 20:867–900
Millet L, Giguet-Covex C, Verneaux V, Druart J-C, Adatte T, Arnaud F (2010) Reconstruction of the recent history of a large deep prealpine lake (Lake Bourget, France) using subfossil chironomids, diatoms, and organic matter analysis: towards the definition of a lake-specific reference state. J Paleolimnol 44:963–978
Müller B, Bryant LD, Matzinger A, Wüest A (2012) Hypolimnetic oxygen depletion in eutrophic lakes. Environ Sci Technol 46:9964–9971
Pace ML, Carpenter SR, Johnson RA, Kurtzweil JT (2013) Zooplankton provide early warnings of a regime shift in a whole lake manipulation. Limnol Oceanogr 58:525–532
Quinlan R, Smol JP (2001) Chironomid-based inference models for estimating end-of-summer hypolimnetic oxygen from south-central Ontario shield lakes. Freshwr Biol 46:1529–1551
Quinlan R, Smol JP, Hall RI (1998) Quantitative inferences of past hypolimnetic anoxia in south-central Ontario lakes using fossil midges (Diptera: Chironomidae). Can J Fish Aquat Sci 55:587–596
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. ISBN: 3-900051-07-0. http://www.R-project.org
Rabalais NN, Diaz RJ, Levin LA, Turner RE, Gilbert D, Zhang J (2010) Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7:585–619
Rao YR, Howell T, Watson SB, Abernethy S (2014) On hypoxia and fish kills along the north shore of Lake Erie. J Great Lakes Res 40:187–191
Rieradevall M, Brooks SJ (2001) An identification guide to subfossil Tanypodinae larvae (Insecta: Diptera: Chrironomidae) based on cephalic setation. J Paleolimnol 25:81–99
Rocha J, Yletyinen J, Biggs R, Blenckner T, Peterson G (2015) Marine regime shifts: drivers and impacts on ecosystems services. Philos Trans R Soc B 370:20130273
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596
Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, van Nes EH, Rietkerk M, Sugihara G (2009) Early-warning signals for critical transitions. Nature 461:53–59
Simpson GL, Anderson NJ (2009) Deciphering the effect of climate change and separating the influence of confounding factors in sediment core records using additive models. Limnol Oceanogr 54:2529–2541
Smol JP (1992) Paleolimnology: an important tool for effective ecosystem management. J Aquat Ecosys Health 1:49–58
Veraart AJ, Faassen EJ, Dakos V, van Nes EH, Lürling M, Scheffer M (2011) Recovery rates reflect distance to a tipping point in a living system. Nature 481:357–359
Verbruggen F, Heiri O, MeriläInen JJ, Lotter AF (2011) Subfossil chironomid assemblages in deep, stratified European lakes: relationships with temperature, trophic state and oxygen. Freshw Biol 56:407–423
Walker IR (2001) Midges: chironomidae and related diptera. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments. Springer, Netherlands, pp 43–66
Wagner A, Volkmann S, Dettinger-Klemm PMA (2012) Benthic–pelagic coupling in lake ecosystems: the key role of chironomid pupae as prey of pelagic fish. Ecosphere 3:1–17
Wang R, Dearing JA, Langdon PG, Zhang E, Yang X, Dakos V, Scheffer M (2012) Flickering gives early warning signals of a critical transition to a eutrophic lake state. Nature 492:419–422
Wilhem S, Adrain R (2007) Impact of summer warming on the thermal characteristics of a polymictic lake and consequences for oxygen, nutrients and phytoplankton. Freshw Biol 53:226–237
Wissel C (1984) A universal law of the characteristic return time near thresholds. Oecologia 65:101–107
Wouters N, Dakos V, Edwards M, Serafim MP, Valayer PJ, Cabral HN (2015) Evidencing a regime shift in the North Sea using early-warning signals as indicators of critical transitions. Estuar Coast Shelf Sci 152:65–72
Wülker W (1961) Studien zur morphologie, biologie und verbreitung der gattung Sergentia kieff. (dipt., chironomidae). Arch Hydrobiol 25:307–331
Acknowledgements
The authors gratefully acknowledge the two anonymous reviewers and Michael Monagan for their constructive comments on this manuscript. We thank Laurent Millet and Valérie Verneaux (Chrono-Environnement, Besançon) for constructive discussions and Vincent Bichet and Laurie Murgia (Chrono-Environnement, Besançon) for technical help during coring survey and dating methods. We thank Christian Hossann (INRA Nancy, Champenoux) for assistance in the stable isotope analysis of carbon. Financial support was provided by Conseil Régional de Franche-Comté. The PTEF facility is supported by the French National Research Agency through the Laboratory of Excellence ARBRE (ANR-11-LABX-0002-01). VD is supported by an ETH fellowship through the Center for Adaptation to a Changing Environment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic Supplementary Material 1
A: Trend in standard deviation of the relative abundances of Sergentia coracina-type using rolling windows of n = 16, 20 and 24 years. Vertical dashed line indicates the significant abrupt change in the composition of the chironomid assemblages. B: Distribution of the Kendall τ estimates of the 100 surrogate time series after fitting an ARMA model to the data (see Methods). The red square indicates the Kendall τ estimate of the original data and p is the actual p-value (PDF 374 kb)
Electronic Supplementary Material 2
A: Trend in autocorrelation (as the coefficient of a first order Auto-Regressive model; AR1) of the relative abundances of Sergentia coracina-type using rolling windows of n = 16, 20 and 24 years (Dakos et al., 2012). Vertical dashed line indicates the significant abrupt change in the composition of the chironomid assemblages. B: Distribution of the Kendall τ estimates of the 100 surrogate time series after fitting an ARMA model to the data (see Methods). The red square indicates the Kendall τ estimate of the original data and p is the actual p-value (PDF 378 kb)
Electronic Supplementary Material 3
A: Trend in standard deviation of PCA1 scores using rolling windows of n = 16, 20 and 24 years. Vertical dashed line indicates the significant abrupt change in the composition of the chironomid assemblages. B: Distribution of the Kendall τ estimates of the 100 surrogate time series after fitting an ARMA model to the data (see Methods). The red square indicates the Kendall τ estimate of the original data and p is the actual p-value (PDF 455 kb)
Electronic Supplementary Material 4
A: Trend in autocorrelation (as the coefficient of a first order Auto-Regressive model; AR1) of PCA1 scores using rolling windows of n = 16, 20 and 24 years (Dakos et al., 2012). Vertical dashed line indicates the significant abrupt change in the composition of the chironomid assemblages. B: Distribution of the Kendall τ estimates of the 100 surrogate time series after fitting an ARMA model to the data (see Methods). The red square indicates the Kendall τ estimate of the original data and p is the actual p-value (PDF 362 kb)
Rights and permissions
About this article
Cite this article
Belle, S., Baudrot, V., Lami, A. et al. Rising variance and abrupt shifts of subfossil chironomids due to eutrophication in a deep sub-alpine lake. Aquat Ecol 51, 307–319 (2017). https://doi.org/10.1007/s10452-017-9618-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-017-9618-3