Abstract
We sampled modern chironomids at multiple water depths in Lake Annecy, France, before reconstructing changes in chironomid assemblages at sub-decadal resolution in sediment cores spanning the last 150 years. The lake is a large, deep (zmax = 65 m), subalpine waterbody that has recently returned to an oligotrophic state. Comparison between the water-depth distributions of living chironomid larvae and subfossil head capsules (HC) along three surface-sediment transects indicated spatial differences in the influence of external forcings on HC deposition (e.g. tributary effects). The transect with the lowest littoral influence and the best-preserved, depth-specific chironomid community characteristics was used for paleolimnological reconstructions at various water depths. At the beginning of the twentieth century, oxygen-rich conditions prevailed in the lake, as inferred from M. contracta-type and Procladius sp. at deep-water sites (i.e. cores from 56 to 65 m) and Paracladius sp. and H. grimshawi-type in the core from 30 m depth. Over time, chironomid assemblages in cores from all three water depths converged toward the dominance of S. coracina-type, indicating enhanced hypoxia. The initial change in chironomid assemblages from the deep-water cores occurred in the 1930s, at the same time that an increase in lake trophic state is inferred from an increase in total organic carbon (TOC) concentration in the sediment. In the 1950s, an assemblage change in the core from 30 m water depth reflects the rapid expansion of the hypoxic layer into the shallower region of the lake. Lake Annecy recovered its oligotrophic state in the 1990s. Chironomid assemblages, however, still indicate hypoxic conditions, suggesting that modern chironomid assemblages in Lake Annecy are decoupled from the lake trophic state. Recent increases in both TOC and the hydrogen index indicate that changes in pelagic functioning have had a strong indirect influence on the composition of the chironomid assemblage. Finally, the dramatic decrease in HC accumulation rate over time suggests that hypoxic conditions are maintained through a feedback loop, wherein the accumulation of (un-consumed) organic matter and subsequent bacterial respiration prevent chironomid re-colonization. We recommend study of sediment cores from multiple water depths, as opposed to investigation of only a single core from the deepest part of the lake, to assess the details of past ecological changes in large deep lakes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paleolimnological studies provide valuable information on environmental changes in lakes over extended time scales. The increasing influence of anthropogenic forcing on these systems has led to a growing body of studies that focus specifically on ecological changes that lakes have undergone over the last century (Battarbee et al. 2011). Among the numerous sediment variables that can be used as proxies to infer ecological changes in lakes, biological remains, especially chironomid head capsules (hereafter HC), have been used extensively to infer past climate and environmental variables such as temperature (Millet et al. 2012), oxygen concentration (Quinlan and Smol 2002; Brodersen and Quinlan 2006) and lake productivity (Woodward and Shulmeister 2006).
Most of these paleolimnological investigations were carried out in relatively small lakes. For example, Larocque et al. (2001) studied chironomid-temperature relationships in a set of lakes (n = 100) with surface areas <20 ha. Small, shallow lakes were favored in climatic and environmental reconstructions because chironomids respond more directly to environmental variables in these lakes than they do in large, deep lakes (Eggermont and Heiri 2011). In such small systems, a single sediment core from the deepest part of the lake has been hypothesized (Hofmann 1988) or shown (van Hardenbroek et al. 2011) to integrate biological remains from the entire lake basin. A detailed analysis of the spatial variability of chironomid remains in shallow Norwegian lakes (Heiri 2004), however, highlighted the existence of spatial patterns in the distribution of HC, although most taxa were present in sediment from the maximum depth.
Recent studies highlighted important depth-related differences in the composition of biological remains in samples from surficial sediment in shallow (Engels and Cwynar 2011; Cao et al. 2012) and deep lakes (Eggermont et al. 2008; Kurek and Cwynar 2009). In the large, deep lakes, results indicate the bathymetric structure of the chironomid community is effectively preserved in the sediment. These water-depth-dependent differences in assemblage composition are common in chironomid communities and are related to variability in environmental conditions such as oxygen availability (Quinlan and Smol 2002) or habitat structure, e.g. macrophyte cover (Langdon et al. 2010). Reconstructions using chironomid assemblages from different depths should therefore more accurately describe the chironomid assemblage composition at the whole-lake scale, and enable inference of temporal changes, which are both of primary interest in environmental policy (Water Framework Directive (WFD) 2000). Furthermore, anthropogenic effects on living organisms may differ along the depth gradient. For example, increased nutrient loading often induces collapse of deep-water chironomid assemblages as a consequence of hypolimnetic hypoxia, whereas in the littoral zone, assemblages can be richer and more abundant because of increased macrophyte coverage under moderate eutrophication (Langdon et al. 2010; Millet et al. 2010). These spatially variable responses, observed mainly in chironomid densities, have multiple effects on the lake ecosystem. Changes in chironomid densities influence sediment structure and mineralization processes (Olafsson and Paterson 2004) and also have implications for fish populations (Vander Zanden et al. 2003). Thus, the depth-specific dynamics of chironomid assemblages must be considered to better specify the ecological consequences of anthropogenic forcings on the structure and function of food webs in large, deep lakes (Free et al. 2009).
A prerequisite for multiple-depth assemblage reconstruction is that HC assemblages from cores collected at various depths accurately reflect the chironomids that lived at those depths all around the lake, i.e. there has been limited vertical HC transport. The suitable location for such chironomid reconstructions will differ depending on the lake in question, because of its morphometry, water circulation, tributaries and wind effects (Schmäh 1993; Brodersen and Lindegaard 1997). Comparison of the depth distributions of larvae and HC along depths transects can be used to test whether HC reflect accurately the bathymetric distribution of the live larvae. If the HC assemblage is similar to that of living larvae at multiple depths, the depth-specific chironomid assemblage reconstructions will be more reliable. Such an approach can substantially improve the robustness of chironomid-based reconstructions, but to our knowledge, this multiple-transect approach has not been used previously, coupled with a HC-living larvae comparison.
In this study, we evaluated chironomid assemblages at three different depths in re-oligotrophied Lake Annecy, a large, deep pre-alpine lake that underwent eutrophication during the second half of the twentieth century. Our objectives were to:
-
1.
Compare the bathymetric distributions of HC and living larvae along three water-depth transects to assess differences in chironomid assemblage composition and identify a suitable location within the lake to perform chironomid reconstructions.
-
2.
Evaluate the gain in ecological information provided by a multiple-depth coring approach (community composition, depth-specific temporal changes) compared with the ecological information obtained from a single core retrieved from the deepest region of the lake.
-
3.
Assess the extent of ecological changes on a whole-lake scale using depth-specific (i.e. local) chironomid assemblage changes. These changes were interpreted considering the direct influence of changes in the organic matter content of the sediment as well as oxygen constraints.
Study area
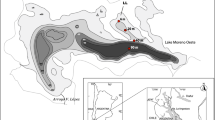
Lake Annecy is a large (2,740 ha), deep (65 m) hard-water lake located in the French pre-Alps (45°50′N, 6°40′E, 447 m a.s.l.) (Fig. 1). The lake basin is surrounded by calcareous rocks of Mesozoic age. It comprises two sub-basins, the “Grand Lac” to the north and the “Petit Lac” to the south, which are separated by a submerged bar reshaped by glaciers (Nomade 2005) (Fig. 1). The local climate is characterized by dry, snowy winters and warm summers associated with high rainfall (100–150 cm year−1). The lake is monomictic and mixing usually occurs from December to February (Balvay 1978). The land cover of the watershed is constrained by the strong altitudinal gradient (alt. max. = 2,351 m). Forests (63 % of the total watershed area) are mainly found on steep slopes, whereas pastures and cultivated areas (21 %) are restricted to flat lowlands. The urbanized area of the watershed (13 %) is mainly concentrated along the lake shore, where tourism has developed since the 1970s. The current total population in the catchment is 186,000.
Lake Annecy is currently oligotrophic and total phosphorus concentrations (TP) in the epilimnion have been about 7–8 μg L−1 during winter circulation since the 2000s (INRA/SILA data). Prior to the 1940s, the phytoplankton community of Lake Annecy was dominated by taxa such as Cyclotella spp., which are typical oligotrophic diatoms (Leroux 1908). A decrease in the oxygen content of the hypolimnion was first reported in 1937 by Hubault (1943), signaling the earliest signs of eutrophication, followed by algal blooms in the 1940s (Servettaz 1977). Monitoring data (INRA/SILA) indicate that Lake Annecy was in a mesotrophic state in the mid-1970s, with TP levels reaching approximately 15 μg L−1 during winter mixing (Perga et al. 2010). A remediation plan, including wastewater collection and treatment, was implemented in 1957 and completed in 1967. Since the 1970s, remediation has reduced TP in the water column, leading to a return to oligotrophy in the 1990s (TP < 10 μg L−1).
The fish community of the lake has been managed since the late ninetieth century, when Whitefish (Coregonus sp.) and Arctic char (Salvelinus alpinus) were introduced (Leroux 1908). The coregonid population was supported by sporadic introductions from 1900 until the 1930s and by annual introductions every winter from 1936 to 1997. Currently, the fish resources of the lake are exploited by two professional fishermen and ~2,000 recreational anglers.
Materials and methods
Coring and sampling of surface sediment
Living chironomid larvae were studied in 48 sediment samples retrieved from four water depths (2, 30, 56 and 65 m) along three transects (Fig. 1). The top 5 cm of sediment was sampled using an Ekman grab in spring 2008 and autumn 2009 (two samples/depth/transect/year). This sampling strategy was designed to avoid under-representation of chironomid taxa arising from different species phenologies. Sediment samples were preserved in the field using 60 % ethanol.
For the chironomid HC analysis (i.e. assemblage reconstructions), nine sediment cores were retrieved at the points on the transects where larvae samples were obtained, at 30, 56 and 65 m water depth, using a gravity corer (Uwitec, Austria) (Fig. 1). Cores were not retrieved at 2 m depth because not enough sediment had accumulated or sediments were disturbed by resuspension. An additional sediment core was obtained from the deepest area of the lake (65 m) in 2009 and devoted to chronology (Fig. 1).
Chironomid analysis
In the laboratory, surficial sediment obtained using the Ekman grab was sieved through a 250-μm-mesh sieve. Chironomid larvae were hand-sorted from the residue under a stereomicroscope at ×40 magnification. Chironomid larvae were treated with cold KOH solution (10 %) overnight and were mounted on slides ventral side up. Identification of larvae was performed using Wiederholm’s (1983) guide.
Head capsules were extracted from the top cm of the nine sediment cores and from samples taken at 0.5-cm intervals in cores from the selected transect “Sévrier” (Sev65, Sev56 and Sev30), spanning the last 150 years. The procedure involved treatment with HCl (10 %) and KOH (10 %) and sieving of the sediment through 100- and 200-μm mesh (Walker 2001). Chironomid HC were hand-sorted from the sieved residue under ×40–70 magnification and mounted ventral side up on microscope slides using Aquatex® mounting agent. Identification of specimens to the genus or species-group levels followed Wiederholm (1983) and Brooks et al. (2007) and was performed under ×100–1,000 magnification.
Core chronology
To establish a chronology for the cores, a reference chronology was developed on the LDAref core from the deepest part of the lake. The activities of 210Pb, 226Ra, 137Cs and 241Am were measured in approximately 1 g of dried sediment by gamma spectrometry, using very-low-background, high-efficiency well-type Ge detectors in the Modane Underground Laboratory (Reyss et al. 1995). Six standards were used to calibrate the gamma detectors (Cazala et al. 2003), and a 24–48 h count time was required to achieve a statistical errors <10 % for excess 210Pb in the deepest samples and for the 1963 peaks of 137Cs and 241Am. Excess 210Pb activity was calculated as the difference between total 210Pb activity and 226Ra activity (supported 210Pb).
Correlation between the cores from the selected transect (“Sévrier”) was achieved following different procedures. Cores Sev65 and Sev56 were correlated to the reference core using visible lithological tie points. The shallow-water core, Sev30, had no visible lithological markers. It was therefore correlated to the reference core using high-resolution spectrophotometry. The first derivative value at 555 nm was chosen as a proxy for clay mineral composition (Damuth and Balsam 2003). Clay minerals in Lake Annecy are derived from pedogenic processes in the catchment (Manalt et al. 2001), and because they represent the smallest grain-size fraction, they are easily transported far into the lake from the river mouth, via discharge of the main river. Spectrophotometric variations are assumed to be synchronous and were useful for core correlation throughout the lake.
Organic matter analysis
The total organic carbon (TOC) content and the hydrogen index (HI) were measured in the cores of the selected transect (Sev30, Sev56 and Sev65) using Rock–Eval pyrolysis (Espitalié et al. 1985) with a Model 6 device (Vinci Technologies). The sample resolution was set to 0.5-cm intervals for Sev65 (57 samples) and 1-cm intervals for Sev56 and Sev30 (29 and 30 samples, respectively). Analyses were carried out on 50–100 mg of crushed, dried samples. TOC (%) is indicative of the amount of organic matter (OM) in the sediment. Because OM content of the sediment is negatively correlated with oxygen concentrations at the sediment–water interface, TOC is a relevant proxy for a major environmental constraint on chironomid communities (Sæther 1979; Brodersen and Quinlan 2006) and considered to be a local driver of chironomid assemblages. HI is expressed as the H/C ratio of OM and represents the amount of hydrocarbon products released during pyrolysis (in mg HC g−1 TOC). HI provides insights into the origin of the OM in the sediment and the degree of OM degradation. High HI values are indicative of fresh, autochthonous OM, whereas low HI values are indicative of strongly degraded autochthonous or allochthonous OM, with high lignin content. HI was therefore used to assess temporal variability in the quality of sediment TOC (Meyers and Lallier-Vergès 1999).
Numerical and statistical analyses
For the living larval community, counts from the three transects were merged by depth and the abundance of each taxon was expressed relative to all counts. Chironomid counts from the three transects were merged to provide a robust assessment of the depth-distribution of larvae. Transect-specific patterns likely reflect, at least in part, a sampling effect of low replication, rather than true differences in environmental conditions.
For the chironomid HC, the bathymetric distributions (from 30 to 65 m) of the subfossil communities were defined for each transect. At each depth, the relative abundance of each subfossil taxon was obtained by dividing the number of HC from each taxon by the overall number of HC found on the transect.
For the chironomid assemblage reconstructions, HC counts were transformed into accumulation rates using the age-depth models. HC accumulation rates (HCAR) are expressed as number of HC 10 cm−2 year−1.
Chironomid assemblage zones (CAZ) were determined for each of the three water depths using the Bray-Curtis dissimilarity index and CONISS, a program for stratigraphically constrained cluster analysis (Grimm 2004). Taxa with <5 occurrences were excluded from the analysis. The number of statistically significant zones was assessed using the broken stick model (Bennett 1996). Only HC originating from deep-living chironomid species, according to Brooks et al. (2007), were utilized to define the CAZ in the deeper core and to assess depth-specific assemblage changes. Variability of HCAR and TOC content between CAZ was assessed in the cores using analysis of variance (ANOVA) followed by Tukey HSD post hoc tests.
An analysis of similarity (ANOSIM), using the “Bray-Curtis” dissimilarity index, and a correspondence analysis (CA) were performed to explore the influence of water depth on the live chironomid larvae and HC assemblages and select the most suitable transect for the chironomid assemblage reconstructions. Eighteen taxa were considered, after removal of unidentified specimens and taxa that were absent in either the larvae or HC datasets. This CA comprised 12 samples (3 depths × 3 transects for HC samples + 3 depths for the summed larvae samples). The larval sample from 2 m depth was included in the ordination as a passive sample. This prevents excessive influence of the 2-m larvae sample in the CA because of its high species richness. The littoral influence on the subfossil assemblage compositions at 30, 56 and 65 m was therefore discriminated using the 2-m species assemblage composition. A second CA was used to assess HCAR variability over time at the various depths.
All statistical analyses were performed in R (R Development Core Team 2011). The packages ade4 (Dray and Dufour 2007) and FactoClass (Pardo and DelCampo 2007) were used for the CA. Ade4 was also used to perform the inter-class analysis. Rioja (Juggins 2009) and vegan (Oksanen et al. 2011) were employed for CONISS and ANOSIM.
Results
Chronology
The 210Pbexc profile for Lake Annecy exhibited regular exponential decay with depth in the sediment core (Fig. 2a). The constant flux, constant sedimentation model (CFCS, Krishnaswami et al. 1971) was chosen for this study because recent sediments were characterized by constant sedimentation, shown by the linear relations between log activity of 210Pb and both cumulative mass (r2 = 0.98) and depth (r2 = 0.98). Regardless of the model used, 210Pb-based chronologies should be confirmed by independent methods (Smith 2001). Our 210Pb chronology is supported by 137Cs profiles (Robbins and Edgington 1975). The CFCS age model indicated a mean sedimentation rate of 2.04 ± 0.01 mm year−1 for Lake Annecy. The 137Cs activity shows a sharp peak at 4.2 cm without any increase in 241Am activity, thus corresponding to fallout from the 1986 Chernobyl accident. This yields an average sedimentation rate of 1.83 ± 0.01 mm year−1 for Lake Annecy between 1986 and 2009. The well-resolved 137Cs peak at 9.8 cm, coinciding with an 241Am peak, confirms that sediment at this depth was deposited during the period of maximum atmospheric fallout from nuclear weapons testing, in 1963 (Michel et al. 2001). This age-depth relationship yields an average sedimentation rate of 2.13 mm year−1 in Lake Annecy from 1963 to 2009. Good agreement among the mean sedimentation rates derived from 210Pbex, 137Cs and 241Am profiles enabled us to estimate a mean sedimentation rate of 2.00 mm year−1 over the last century (Fig. 1 ).
a Age-depth model of LDAref from radionuclides (210Pb, 137Cs and 241Am). Seven litho-stratigraphic markers (N°1 to N°7) were selected on LDAref to allow correlation with the other cores. b, c Age-depth models of the SEV65 and SEV56 cores were correlated to LDAref according to visible markers. d Age-depth model of the SEV30 core was correlated to SEV65 using spectrophotometry
Correlation of the three cores indicated that sedimentation rate in each core was roughly constant. Mean sedimentation rates in two of the cores were similar to the reference core (Sev65, 2.00 mm year−1; Sev30, 1.98 mm year−1) (Fig. 2b, d). Sev56, however, showed a lower mean sedimentation rate (1.39 mm year−1) (Fig. 2c).
Living larvae versus subfossil remains in surface sediment: transect selection
A total of 1,104 chironomid larvae and 645 HC were extracted from surface sediment samples that integrated ca. 5 years according to the age/depth models (Fig. 2). According to the ANOSIM, there were no significant differences with respect to water depth between larvae and HC on any transect (p > 0.05). However, there were clear differences among transects with respect to similarity of samples across depths. The “Sévrier” transect had the highest similarity (p = 0.8) followed by the “Annecy” transect (p = 0.2) and the “Saint Jorioz” transect (p = 0.1). This analysis indicates the “Sévrier” transect is the best for using chironomid assemblages from different depths to do reconstructions. The CA biplot depicts these differences in the larvae/HC distributions. The CA axis 1 (F1) and axis 2 (F2) captured 58.3 % of the variability in chironomid larvae and the subfossil assemblage (Fig. 3). Along CA axis 1 (F1), two chironomid groups were defined. Taxa such as Cladopelma, Dicrotendipes, Cladotanytarsus, Cricotopus, Cryptochironomus, Thienemanniella and Ablabesmyia showed high F1 scores. Close association of these taxa with the larval sample obtained from a depth of 2 m indicated that they were major components of the littoral larval community. In contrast, Micropsectra, Chironomus, Sergentia, Pagastiella and Parakiefferiella were associated by low F1 scores and were related to samples from deeper water (30, 56 and 65 m). CA axis 2 (F2) segregated the specific larval composition of the three studied depths. However, the larvae assemblage at 30 m had an intermediate F2 score compared to the larvae assemblages at 56 and 65 m, suggesting that a depth gradient was not expressed along the F2 axis. However, because the sampling depths effectively differed along this axis, F2 scores nonetheless allowed differentiating sampling depths according to their chironomid assemblages. The larvae assemblage at 56 m was mainly composed of Chironomus and Procladius, whereas the assemblage at 65 m was characterized by Micropsectra, Sergentia and Parakiefferiella (Fig. 3). The HC sample for the “Saint Jorioz” transect at 56 m was noteworthy in being segregated from other samples, with low F2 scores associated with Eukiefferiella and, to some extent, Polypedilum.
Correspondence analysis (CA) biplot depicting the associations between chironomid genera and the relative compositions of both larvae and HC assemblages at three depths (30, 50 and 65 m) for the three transects studied. The larval sample taken from a depth of 2 m was included in the ordination as a passive sample. Larvae samples are indicated as “Larvae,” followed by the depth at which they were sampled. Subfossil samples are identified by the letter C, followed by the transect abbreviation (sev “Sévrier”, ann “Annecy”, jo “St Jorioz”) and the sampling depth
The F1 and F2 scores of the CA were extracted for both larval and HC samples and considered synthetic (both qualitative and quantitative) descriptors of the chironomid structure of the samples. At each depth, the difference in the CA scores between larval and HC samples was calculated and averaged for each transect (Table 1). Differences in the F1 scores between larvae and subfossil samples reflected the influence of littoral deposits on the subfossil assemblages at 30, 56 and 65 m (Fig. 3). Differences in F2 scores between larvae and subfossil samples are related to depth of the chironomid sample. The “Sévrier” transect had the lowest mean difference in F1 and F2 scores. Thus, it was the transect least influenced by littoral HC deposition and most suitable for reflecting the depth distribution of larvae. It was therefore selected to reconstruct chironomid assemblage changes over the last 150 years at three water depths: 30 m (Sev30), 56 m (Sev56) and 65 m (Sev65).
Downcore chironomid assemblage reconstructions and OM
Figure 4 illustrates the temporal dynamics of the main chironomid taxa at the three studied depths. For each depth, CAZs obtained from the constrained cluster-analysis (CONISS) highlight assemblage shifts.
Stratigraphy plots reflecting the temporal dynamics (HCAR) of the main chironomid taxa identified at 65 m (a), 56 m (b) and 30 m (c). TOC and HI are also presented, providing evidence for the relationship between chironomid assemblage changes and both qualitative and quantitative changes in the OM of the sediment. Low (one HC) and sporadic occurrences are indicated by black circles. Zonation used to identify the different CAZs for each of the studied depths was accomplished using the broken stick model following a CONISS analysis, considering the Bray-Curtis similarity index. At 65 and 56 m, only truly deep taxa, according to Brooks et al. (2007), were reported. At 30 m, the eight most abundant taxa that had clear temporal trends and accounted for 71.3 % of the overall HCAR are reported
Among the 52 taxa found in 67 samples from the core in 65 m of water (Sev65), five were found as living larvae inhabiting the deep zones of the lake, at 56 and/or 65 m (Chironomus, Sergentia, Micropsectra, Procladius, Paratendipes), and two others (H. grimshawi-type and Paracladopelma sp.) are associated with the deep zone in lakes (Wiederholm 1983; Brooks et al. 2007). Considering these seven taxa, including the two Micropsectra types, three CAZs were identified by cluster analysis (Fig. 4a). CAZ1 spanned from the late 1870s to the early 1930s. The mean HCAR was 7.9 ± 5.9 HC 10 cm−2 year−1 and was dominated by M. contracta-type and Procladius sp. Paracladopelma sp. and H. grimshawi-type also occurred sporadically during this time period. The onset of CAZ2 in the early 1930s was marked by a decrease of more than 50 % in M. contracta-type and Procladius sp. HCAR (ANOVA, p < 0.001) and exhibited variations such as the increase in M. contracta-type between the 1970s and the 1990s. During CAZ2, S. coracina-type HCAR increased significantly (ANOVA, p < 0.001). C. anthracinus-type appeared at the beginning of CAZ2 and Paratendipes at its end. Throughout CAZ3, from 2000 to 2008, S. coracina-type HCAR decreased, whereas HCAR of M. contracta-type, M. radialis-type and Procladius sp. fell to nearly zero. The total HCAR of profundal taxa declined to 0.85 ± 0.38 HC 10 cm−2 year−1 during CAZ3. TOC concentrations increased significantly between the CAZs (ANOVA, p < 0.001), ranging from 0.65 ± 0.23 % in CAZ1 to 1.61 ± 0.08 % in CAZ3. HI also increased significantly (ANOVA, p < 0.001) and ranged from 183.2 ± 58.6 in CAZ1 to 312.7 ± 6.0 in CAZ3.
In the core from 56 m water depth (Sev56), 40 taxa were identified in the 63 samples, and three CAZs were distinguished by cluster analysis (Fig. 4b). The chironomid assemblage of CAZ1, from ca. 1850 to 1915, mainly consisted of M. contracta-type, Procladius sp. and Paracladopelma sp. From ca. 1915 to 1945, CAZ2 was mainly characterized by an increase in M. contracta-type HCAR (ANOVA, p < 0.001). After ca. 1945, a sharp decrease in M. contracta-type HCAR, a disappearance of Paracladopelma sp. and an increase in the HCAR of S. coracina-type was observed in CAZ3. TOC concentrations differed significantly among the three CAZs (ANOVA, p < 0.001). TOC was lower during CAZ2 (0.90 ± 0.23 %) compared to CAZ1 (1.29 ± 0.37 %) and CAZ3 (1.87 ± 0.59 %). In the core from 65 m water depth, HI significantly increased over time, ranging from 88.3 ± 23.1 during CAZ1 to 282.3 ± 57.5 at the end of CAZ3.
In the core from 30 m water depth (Sev30), 79 genera or species groups were identified within the 48 samples. Cluster analysis indicated 2 CAZ, with a major change in the chironomid assemblage composition ca. 1950 (Fig. 4c). The total chironomid HCAR decreased from 123 ± 30 HC 10 cm−2 year−1 during the period between 1850 and 1950 (CAZ1) to 40 ± 16 HC 10 cm−2 year−1 during the period between 1950 and 2008 (CAZ2). The HCAR of all the taxa characteristic of CAZ1 (Paracladius sp., H. grimshawi-type, M. contracta-type) dramatically decreased at the CAZ1/CAZ2 transition. During CAZ2, Procladius sp., S. coracina-type and, to a lesser extent, M. contracta-type remained rather stable. TOC concentrations were significantly higher in CAZ2 than in CAZ1 (t test, p < 0.001) and ranged from 0.77 ± 0.08 % in CAZ1 to 1.26 ± 0.18 % in CAZ2. HI increased significantly from 283.05 ± 22.80 in CAZ1 to 387.43 ± 29.40 in CAZ2 (t test, p < 0.001).
The CAZs defined for each core were used to depict changes in the chironomid assemblage on a whole-lake basis over the last 150 years. The different CAZs and their transition points, which occurred in the 1910s, 1930s, 1950s or 2000s depending on water depth, were used to define five different whole-lake assemblage types (WLATs). A CA was performed using all the samples from the three cores, using taxa with at least five occurrences (167 samples, 49 taxa) to evaluate and test the influence of (1) sampling depth and, (2) the WLATs on whole-lake chironomid HCAR variability. The two first axes of the CA described 41.4 % of the chironomid HCAR variability (Fig. 5). Chironomid HCARs differed significantly both between sampling depths and between WLATs, explaining 19.82 and 16.80 % of the chironomid HCAR variability, respectively (permutation tests, p < 0.01).
Correspondence analysis (CA) depicting the taxonomic changes in chironomid assemblages over time, reconstructed at the three studied depths. a HC samples grouped according to both sampling depths and WLATs defined by the stratigraphies. For each group, the numbers 30, 56 and 65 refer to the three studied depths, in metres. The numbers 1, 2, 3, 4 and 5 refer to the different WLATs. b Distribution of the 20 taxa with the highest scores on both the CA F1 and CA F2 axes
During the first three WLATs, from the 1850s to the 1930s, chironomid assemblages were mainly segregated along CA axis 1 (F1) according to depth. Sev30 was characterized by Paracladius sp. and H. grimshawi-type and differed from Sev56 and Sev65, which were characterized by M. contracta-type and Procladius sp. During the latter two WLATs, from the 1950s to the 2000s, the F1 scores of the chironomid assemblages from the various depths converged toward similar values (−1 to −0.5), indicating a progressive decrease in the depth-related specificity of chironomid assemblages. The increase in S. coracina-type HCAR in the chironomid assemblages at all depths was especially responsible for this uniformity in chironomid assemblage. During WLAT4 and WLAT5, the chironomid assemblages at all depths were also characterized by several taxa associated with the littoral zone, such as Dicrotendipes sp., Ablabesmyia sp., and Zalutchia sp. This increase in littoral influence during these two WLATs was related to the decrease in HCARs in the initial profundal community that occurred after the 1930s. The CA F1 therefore greatly reflected successive changes in chironomid assemblages, whereas CA F2 highlighted the increasing influence of littoral HC inputs over time resulting from the progressive extirpation of the original chironomid assemblages at the various depths.
Discussion
This study explored ways to improve analysis of changes in chironomid assemblages in large deep lakes, using the approach of multiple-depth reconstructions coupled with larvae/HC spatial comparisons. This approach was undertaken to better define the chironomid assemblage composition and its temporal dynamics at the whole-lake level, compared to reconstructions based on a single core from deep water.
Comparison of modern and subfossil chironomid assemblages: site selection
Our results highlighted transect-specific differences in the ability of subfossil samples to accurately represent the water-depth distribution of living larvae. Sampling of living larvae involved two seasons to account for species phenology. Spring sampling was completed before the massive emergences of most chironomid species. Autumn sampling improved the assessment of the living community and may have also improved determinations of species that might have been at early stages and thus unidentifiable during spring sampling. Our study did not aim to make an exhaustive assessment of the whole lake community, which would have required a larger sampling effort. Instead, our sampling strategy was designed to compare the water-depth distributions of larvae and HC, and we found that the dominant taxa in HC samples were also found as larvae. The “St Jorioz” transect displayed the greatest differences in the bathymetric distribution between HC and living larvae. The local influence of a tributary and the steep slope of the lake bottom at this location likely facilitate re-deposition of HC from the littoral to the profundal zone. This may explain the poor ability of this transect to provide a reliable picture of depth-specific chironomid assemblages or their temporal variability. In contrast, the bathymetric distributions of HC and larvae were more similar in the “Annecy” and “Sévrier” transects. Both were less influenced by HC export from the littoral zone and more representative of depth-specific chironomid assemblages. These two transects had lower slopes and no nearby tributary. The “Annecy” transect had a poorer match between HC and larvae, despite a gentler depth gradient than the “Sévrier” transect. The wind effect may explain this result, as the “Annecy” transect is located at the extreme end of the lake and is more subject to wind-induced HC redeposition than is the “Sévrier” transect.
The “Sévrier” transect was selected for chironomid paleo-reconstructions at various depths because its HC/larvae bathymetric distributions were most similar although differences were detected. For example, HC of littoral taxa such as Psectrocladius, Dicrotendipes and Cladopelma were found in sediment samples at 30 and 56 m, whereas larvae of these taxa were only found at a depth of 2 m. These findings are in agreement with results reported by Brodersen and Lindegaard (1997), who compared HC species composition from surface sediment samples with samples of both trapped adult midges and living larvae in Lake Stigsholm (Denmark) and found differences in chironomid assemblage composition depending on the sampling method used. These differences can perhaps be attributed to the passive behavior of HC compared to larvae. Living larvae may aggregate in lakes (i.e. patchy distribution, Lobinske et al. 2002), whereas the spatial distribution of HC, while conserving the composition of chironomid assemblages (Cao et al. 2012), is more likely to be subject to passive deposition, thus evenly distributing initially aggregated abundances of chironomid larvae. Furthermore, sediment samples reflect a time-integrated measure of the chironomid assemblage that, in a sense, averages inter-annual variations in chironomid densities. Finally, the temporal integration of sediment samples facilitates collection of rare species that are especially difficult to find as live larvae in the modern community and would require excessive efforts to document. The sampling effort here, which consisted of 48 samples and was undertaken to define the modern community, was reasonable for characterizing the water-depth distribution of this community. Use of relative abundance to compare larval and HC bathymetric distributions seems reasonable, until adequate conversion factors become available to translate HC abundances into living larvae abundances.
Palaeolimnological development of Lake Annecy
Pre-disturbance assemblages (1850–1930)
Based on detection of different CAZs at depths in the core, five WLATs were identified in Lake Annecy for the period of interest (Fig. 6). The first significant change in chironomid assemblages occurred in the 1910s, at 56 m. This change was restricted to a relatively short time interval associated with a decrease in TOC values. However, it was only characterized by an increase in M. contracta-type HCAR and if this relationship (M. contracta-type/TOC) seems relevant, the reason why this pattern was only found at 56 m remains obscure. Nevertheless, this pattern was far from being the dominant feature during the period studied and considering the CA of the subfossil samples, WLAT1 and WLAT2 remained rather stable from the 1850s to the 1930s. Prior to ~1930, chironomid assemblages at each of the depths examined in this study were typical for a pre-modern perturbation state and can be described as follows:
Synthesis of the gradual temporal changes in chironomid assemblages for each of the studied depths and the definition of the different WLATs. From left to right, progressive chironomid assemblage shifts (CAZs) for the different depths, definition of the chironomid changes at the whole-lake level (WLATs), CA F1 scores, summarizing the taxonomic changes for each studied depth over time and the assemblage composition convergence in recent years, gradual changes in TOC concentration, temporal variability in the trophic state of the lake, emphasizing the increase in the trophic state from historical data obtained in the 1940s and the re-oligotrophication achieved since the 1990s
-
M. contracta-type and Procladius sp. assemblages in the deepest zones with moderate densities, as inferred from the HCARs of the two taxa (2.55 ± 1.51 and 1.08 ± 0.61 HC 10 cm−2 year−1, respectively).
-
Paracladius sp., H. grimshawi-type and M. contracta-type assemblages in the littoral zone, with higher densities (5–10 fold higher for each dominant taxon).
Except for Procladius sp., which tolerates high OM, the dominant taxa are indicative of oxygen-rich conditions (Brodersen and Quinlan 2006; Brooks et al. 2007) and low TOC content (i.e. <1 %) at all the studied depths. These taxa are also considered typical of oligohumic-oligotrophic lakes (Sæther 1979). These results corroborate the early work of Leroux (1908) on phytoplankton, which indicated oligotrophic conditions in Lake Annecy at the beginning of the time period studied. Before the 1930s, the oligotrophic state of Lake Annecy was associated with a well-oxygenated hypolimnion. At that time, Lake Annecy exhibited efficient trophic functioning, with most of the energy (i.e. OM) flowing through the food web, resulting in little OM accumulation in the sediment.
Substantial differences in chironomid assemblages at various water depths at the beginning of the time period studied, corroborate the work of Engels and Cwynar (2011) and Luoto (2012), which indicated that sediment samples collected from the deepest part of a deep lake display restricted spatial integration of HC. Furthermore, we found that the dominant taxon at 30 m (Paracladius) was poorly represented in the sediment from the deepest part of the lake (i.e. Sev65). This indicates that characteristics of shallower zones are not quantitatively reflected in sediment from the deep zone of this lake. Nevertheless, richness was higher at 65 m than at 56 m, suggesting that a larger fraction of the whole-lake chironomid assemblage is found in the deepest part of the lake. The bottom contour at 56 m enables HC to drift downward, partly explaining this result. Depth-specific assemblage differences likely reflect the large size and great depth of Lake Annecy (2,740 ha, zmax = 65 m).
H. grimshawi-type, considered a deep-living taxon (Sæther 1975), was rarely recorded in the deep-water cores (56 and 65 m), but was abundant in the core from 30 m. Furthermore, several taxa (M. contracta-type) were represented at all depths, despite the fact that they are commonly encountered in deep water (Brooks et al. 2007). These peculiarities of the chironomid fauna in Lake Annecy likely come from a combination of environmental factors that support the lake’s distinctive chironomid assemblage (Brodersen and Quinlan 2006).
1930–1950: Eutrophication-driven changes
The first major change in the chironomid assemblage occurred in the deep zone (i.e. Sev65) around the 1930s. This change was characterized by an increase in the HCAR of hypoxia-tolerant S. coracina-type, which coincided with a decrease in the HCAR of the oxyphilous M. contracta-type, indicating a decrease in oxygen at the lake bottom (Fig. 6). This inference is supported by monitoring data (Hubault 1943) that indicate anoxia at 60 m in mid-summer 1937. This hypoxic zone progressively expanded upward. The number of oxyphilous taxa decreased at 56 m (Sev56) in ca. 1945 and at 30 m (Sev30) in the 1950s. Expansion of the hypoxic layer was correlated with an increase in TOC concentrations and HI in sediment cores over time (Fig. 6). The increase in TOC concentrations in the sediment promoted microbial respiration and consequent oxygen depletion (Jones et al. 2008). From the 1930s until the 1960s, the lake received an increase in nutrients from wastewaters (Perga et al. 2010). During this period, chironomid assemblages were affected by a decrease in oxygen, driven by increasing trophic state of the lake (i.e. eutrophication).
From nutrient control to “pelagic functioning” control after 1950
Since the 1960s, local authorities have undertaken various measures to control nutrient inputs to Lake Annecy, including the establishment a wastewater treatment plant. Such initiatives reduced nutrient concentrations in the water column (SRP < 0.01 mg L−1), but no recovery is evident in the chironomid assemblages, at any depth. Paradoxically, chironomid assemblages have shifted over time to a more homogenous (“depth-independent”) state, dominated by the S. coracina-type (i.e. WLAT4, WLAT5); this was illustrated by the convergence of CA F1 scores at the three studied depths (Fig. 6). These recent assemblage shifts, to namely WLAT4 (30 m) and WLAT5 (65 m), provide insights into the spatio-temporal variability of oxygen constraint at the sediment interface, that is, development of hypoxic conditions at 30 m and an increase in oxygen constraint at 65 m.
The increase in oxygen constraint in the deep zone, following the return of oligotrophy, was unexpected. Measurements of the sediment indicated that neither TOC concentrations nor the HI has decreased since the 1960s; in fact, these variables have increased continuously to the present. Although maturation of OM during early diagenesis can influence temporal patterns of TOC, and especially HI (Espitalié et al. 1985), the strength of these patterns enhances our confidence in the inferred progressive increase in phytoplankton-derived input to the sediment since the 1960s. Our results are in agreement with those of Meriläinen et al. (2000), who reported a “decoupling” between pelagic conditions and benthic conditions after mitigation efforts went into effect. In the 1930s, oligotrophy in Lake Annecy was accompanied by efficient trophic functioning in the pelagic zone. Today, however, the oligotrophic state is characterized by less efficient trophic functioning, leading to OM accumulation in sediments and hypoxia. There is evidence that predation by stocked Coregonus in Lake Annecy has driven a decrease in the size of Daphnia over the last 60 years, with a consequent increase in export of pelagic primary producer biomass to the sediment (Perga et al. 2010). In this context, the structure of the chironomid assemblage, since the return of oligotrophy, appears to be strongly coupled with pelagic functioning. Our study points to an indirect, top-down cascade effect, from the pelagic to the benthic food web. This pelagic-benthic linkage is seldom reported in field studies (Hargrave 2006) because the effects of fish on chironomid communities are mostly considered direct effects of predation (Mousavi et al. 2002).
The stability of chironomid assemblages over the last two decades suggests that the oxygen constraint initially promoted by “nutrient enrichment” was progressively replaced by changes in “pelagic functioning.” This temporal succession of constraints (“constraint substitution”) indicates that a consideration of changes in the food web structure might help researchers understand unexpected lake trajectories following the return of oligotrophic conditions (Jeppesen et al. 2005). Beisner et al. (2003) assessed the factors controlling resilience in north-temperate, clear-water lakes and suggest that a non-negligible part of the variation in resilience predictions is likely related to the food-web structure.
Ecological consequences: negative feedback loops
Detritivore diversity and abundance are important functional features of lake ecosystems and changes in the structure of the benthic food web may affect connections with higher-level food webs (Vander Zanden et al. 2005) and have dramatic repercussions on the recycling of OM (Olafsson and Paterson 2004). The dramatic decrease in chironomid abundance over time (~20-fold) suggests important changes in energy flow in this system over the last 150 years. More precisely, the contribution of the benthic food web to the higher trophic levels likely decreased. This pattern is similar to that described by Vander Zanden et al. (2003), who found a reduced contribution from the benthic food web to the higher trophic levels (i.e. fish) during the last century, following introduction of the freshwater shrimp Mysis relicta in Lake Tahoe, California. Furthermore, the lower abundance of chironomids in Lake Annecy likely facilitated maintenance of hypoxic conditions through negative feedback loops, despite re-oligotrophication, thus hampering the ecological recovery of chironomid assemblages. Simply, initial input of OM triggers a decrease in benthic fauna as a consequence of increased bacterial respiration. In this context, “benthic fauna” refers to chironomids and meiofauna involved in OM mineralization (Nascimento et al. 2012), taxa that are negatively impacted by hypoxic conditions (Kansanen 1981). The response is promotion of bacterial respiration of OM that would have been consumed by the vanished fauna. This phenomenon becomes amplified over time. Negative feedback loops that link benthic fauna and anoxic conditions have been reported mainly in marine “dead zones” (Diaz and Rosenberg 2008). However, Goedkoop and Johnson (1996) showed that chironomids and meiofauna in the profundal zone assimilated 2.4–6.0 % and 1.7–7.2 % of the deposited phytodetritus, respectively, from the spring bloom in Lake Erken. The invertebrate contribution to OM assimilation is rather high once the low chironomid densities (~300 ind m−2) and restricted period of activity are considered. Our results cannot provide a quantitative estimate of chironomid control over OM accumulation and consequent hypoxic conditions because conversion factors between HC abundances and larvae are not well established and the carbon requirements of each chironomid larval stage are lacking for many taxa. The dramatic decrease in chironomid HC abundance, especially at 30 m depth does, however, suggest the possible facilitation of hypoxic conditions by a decline in the invertebrates, perhaps explaining the time lag between responses in the pelagic and benthic communities.
Conclusions
Comparison of water-depth distributions of HC and larvae on transects highlighted differences that are likely a consequence of bottom slope, input streams and wind effects. Such a comparison should be undertaken to increase the robustness of reconstructions at multiple water depths. Before the twentieth century, oligotrophic conditions were inferred from two taxa (M. contracta-type at 65 m and Paracladius sp. at 30 m). These taxonomic differences highlight the influence of depth on the dominant taxon and support the usefulness of multiple-depth sampling to reach an assessment of the chironomid fauna on a whole-lake scale. Furthermore, the depth-specific temporal changes in chironomid assemblages suggested that the changes in environmental conditions do not constrain the chironomid fauna similarly at all depths. Hence, despite chironomid assemblages providing similar information about the trophic status of the lake, a multi-depth sampling approach is recommended to track the dynamics and extent of environmental changes, such as the expansion of hypoxic zones.
In Lake Annecy, chironomid assemblages were highly correlated with sediment TOC concentrations through time. If, however, chironomid assemblage shifts from the 1930s and 1950s were associated with eutrophication, it is possible that the top-down effects of Coregonus sp. on pelagic functioning may have cascaded down to the benthic food web from the 1960s until the 2000s. The loss of benthic consumers could have induced sustained hypoxia in the sediment. Therefore, changes in the structure of the food web might explain chironomid assemblage changes in Alpine lakes following re-oligotrophication. Our results highlight the difficulties in defining the ecological state of lakes according to the Water Framework Directive (WFD; European Union 2000), when using both pelagic and benthic indicators.
References
Balvay G (1978) Le régime thermique du lac d’Annecy (1966–1977). Revue de Géographie Alpine 66:241–261
Battarbee R, Morley D, Bennion H, Simpson G, Hughes M, Bauere V (2011) A palaeolimnological meta-database for assessing the ecological status of lakes. J Paleolimnol 45:405–414
Beisner B, Dent L, Carpenter S (2003) Variability of lakes on the landscape: roles of phosphorus, food webs, and dissolved organic carbon. Ecology 84:1563–1575
Bennett KD (1996) Determination of the number of zones in a biostratigraphical sequence. New Phytol 132:155–170
Brodersen K, Lindegaard C (1997) Significance of subfossile chironomid remains in classification of shallow lakes. Hydrobiologia 342–343:125–132
Brodersen K, Quinlan R (2006) Midges as palaeoindicators of lake productivity, eutrophication and hypolimnetic oxygen. Q Sci Rev 25:1995–2012
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of Palaearctic Chironomidae larvae in palaeoecology. QRA Technical Guide No. 10 Quaternary Research Association, London, p 276
Cao Y, Zhang E, Chen X, Anderson J, Shen J (2012) Spatial distribution of subfossil Chironomidae in surface sediments of a large, shallow and hypertrophic lake (Taihu, SE China). Hydrobiologia 691:59–70
Cazala C, Reyss JL, Decossas JL, Royer A (2003) Improvement in the determination of 238U, 228–234Th, 226–228Ra, 210Pb and 7Be by Gamma Spectrometry on evaporated fresh water samples. Environ Sci Technol 37:4990–4993
Damuth JE, Balsam WL (2003) Data report: spectral data from sites 1165 and 1167 including the HiRISC section from Hole 1165B. In: Cooper AK, O’Brien PE, Richter C (eds) Proceedings of ODP science results, p 188, College Station, TX (Ocean Drilling Program) 1–49
Diaz R, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Soft 22:1–20
Eggermont H, Heiri O (2011) The chironomid-temperature relationship: expression in nature and palaeoenvironmental implications. Biol Rev Camb Philos Soc 87:430–456
Eggermont H, Kennedy D, Hasiotis S, Verschuren D (2008) Distribution of larval Chironomidae (Insecta: Diptera) along a depth transect at Kigoma Bay, Lake Tanganyika (East Africa): implications for paleoecology and palaeoclimatology. Afr Entomol 16:162–184
Engels S, Cwynar L (2011) Changes in fossil chironomid remains along a depth gradient: evidence for common faunal thresholds within lakes. Hydrobiologia 665:15–38
Espitalié J, Deroo G, Marquis F (1985) La pyrolyse Rock Eval et ses applications 2de partie. Rev Inst Fr Pet 40:755–784
European Union (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 on establishing a framework for community action in the field of water policy. J Eur Commun L327:1–72
Free G, Solimini A, Rossaro B, Marziali L, Giacchini R, Paracchini B, Ghiani M, Vaccaro S, Gawlik BM, Fresner R, Santner G, Schönhuber M, Cardoso AC (2009) Modelling lake macroinvertebrate species in the shallow sublittoral: relative roles of habitat, lake morphology, aquatic chemistry and sediment composition. Hydrobiologia 633:123–136
Goedkoop W, Johnson R (1996) Pelagic-benthic coupling: profundal benthic community response to spring diatom deposition in mesotrophic Lake Erken. Limnol Oceanogr 41:636–647
Grimm EC (2004) TGView version 2.0.2. Illinois State Museum, Research and Collections Center, Springfield
Hargrave C (2006) A test of three alternative pathways for consumer regulation of primary productivity. Oecologia 149:123–132
Heiri O (2004) Within-lake variability of subfossil chironomid assemblages in shallow Norwegian lakes. J Paleolimnol 32:67–84
Hofmann W (1988) The significance of chironomid analysis (Insecta: Diptera) for paleolimnological research. Palaeogeogr Palaeoclimatol Palaeoecol 62(501):509
Hubault E (1943) Les grands lacs subalpins de Savoie sont-ils alcalitrophes? Arch Hydrobiol 40:240–249
Jeppesen E, Søndergaard M, Mazzeo N, Meerhoff M, Branco C, Huszar V, Scasso F (2005) Lake restoration and biomanipulation in temperate lakes: relevance for subtropical and tropical lakes, Chapter 11. In: Reddy MV (ed) Tropical eutrophic lakes: their restoration and management, pp 331–359
Jones RI, Carter CE, Kelly A, Ward S, Kelly DJ, Grey J (2008) Widespread contribution of methane cycle bacteria to the diets of lake profundal chironomid larvae. Ecology 89:857–864
Juggins S (2009) Rioja: analysis of quaternary science data, R package version 0.5-6, http://cran.r-project.org/package=rioja
Kansanen PK (1981) Effects of heavy pollution gradient on the zoobenthos in lake Vanajavesi, southern Finland, with special references to meiozoobenthos. Ann Zool Fennici 18:243–251
Krishnaswami D, Lal JM, Martin M, Meybeck M (1971) Geochronology of lake sediments. Earth Planet Sci Lett 11:407–414
Kurek J, Cwynar L (2009) Effects of within-lake gradients on the distribution of fossil chironomids from maar lakes in western Alaska: implications for environmental reconstructions. Hydrobiologia 623:37–52
Langdon P, Ruiz Z, Wynne S, Sayer K, Davidson T (2010) Ecological influences on larval chironomid communities in shallow lakes: implications for palaeolimnological interpretations. Freshw Biol 55:531–545
Larocque I, Hall R, Grahn E (2001) Chironomids as indicators of climate change: a 100-lake training set from a subarctic region of northern Sweden (Lapland). J Paleolimnol 26:307–322
Leroux M (1908) Recherches biologiques sur le lac d’Annecy. Ann Biol Lacu 2:220–387
Lobinske R, Arshad A, Frouz J (2002) Ecological studies of spatial and temporal distributions of larval Chironomidae (Diptera) with emphasis on Glyptotendipes paripes (Diptera: Chironomidae) in three central Florida Lakes. Commun Ecosyst Ecol 31:637–647
Luoto T (2012) Intra-lake patterns of aquatic insect and mite remains. J Paleolimnol 47:141–157
Manalt F, Beck C, Disnar JR, Deconinck JF, Recourt P (2001) Evolution of clay mineral assemblages and organic matter in the late glacial-Holocene sedimentary infill of Lake Annecy, (northwestern Alps): paleoenvironmental implications. J Paleolimnol 25:179–192
Meriläinen J, Hynynen J, Teppo A, Palomäki A, Granberg K, Reinikainen P (2000) Importance of diffuse nutrient loading and lake level changes to the eutrophication of an originally oligotrophic boreal lake: a palaeolimnological diatom and chironomid analysis. J Paleolimnol 24:251–270
Meyers P, Lallier-Vergès E (1999) Lacustrine sedimentary organic matter records of late quaternary paleoclimates. J Paleolimnol 21:345–372
Michel H, Barci-Funel G, Dalmasso J, Ardisson G, Appleby PG, Haworth E, El-Daoushy F (2001) Plutonium, americium and cesium records in sediment cores from Blelham Tarn, Cumbria (UK). J Radioanal Nucl Chem 247:107–110
Millet L, Giguet-Covex C, Verneaux V, Druart JC, Adatte T, Arnaud F (2010) Reconstruction of the recent history of a large deep prealpine lake (Lake Bourget, France) using subfossil chironomids, diatoms, and organic matter analysis: towards the definition of a lake-specific reference state. J Paleolimnol 44:963–978
Millet L, Rius D, Galop D, Heiri O, Brooks SJ (2012) Chironomid-based reconstruction of Lateglacial summer temperatures from the Ech palaeolake record (French western Pyrenees). Palaeogeogr Palaeoclimatol Palaeoecol 315–316:86–99
Mousavi K, Sandring S, Amundsen PA (2002) Diversity of chironomid assemblages in contrasting subarctic lakes—impact of fish predation and lakes size. Arch Hydrobiol 154:461–484
Nascimento F, Näslund J, Elmgren R (2012) Meiofauna enhances organic matter mineralization in soft sediment ecosystems. Limnol Oceanogr 57:338–346
Nomade J (2005) Chronologie et sédimentologie du remplissage du lac d’Annecy depuis le Tardiglaciaire: implications paléoclimatologiques et paléohydrologiques. Thèse doctorale. Université de Savoie (France), p 197
Oksanen J, Blanchet G, Kindt R, Legendre P, O’Hara R, Simpson G, Solymos P, Stevens H, Wagner H (2011) Vegan: community ecology package. R package version 1.17-11, http://CRAN.R-project.org/package=vegan
Olafsson J, Paterson D (2004) Alteration of biogenic structure and physical properties by tube-building chironomid larvae in cohesive sediments. Aquat Ecol 38:219–229
Pardo CE, DelCampo PC (2007) Combinacion de metodos factoriales y de analisis de onglomerados en R: el paquete FactoClass. Revista Colombiana de Estadistica 3:235–245
Perga ME, Desmet M, Enters D, Reyss JL (2010) A century of bottom-up- and top-down-driven changes on a lake planktonic food web: a paleoecological and paleoisotopic study of Lake Annecy, France. Limnol Oceanogr 55:803–816
Quinlan R, Smol J (2002) Regional assessment of long-term hypolimnetic oxygen changes in Ontario (Canada) shield lakes using subfossil chironomids. J Paleolimnol 27:249–260
Reyss J-L, Schmidt S, Legeleux F, Bonté P (1995) Large, low background well-type detectors for measurements of environmental radioactivity. Nucl Instrum Methods Phys Res Sect A 357:391–397
Robbins J, Edgington D (1975) Determination of recent sedimentation rates in Lake Michigan using 210Pb and 137Cs. Geochim Cosmochim Acta 39:285–304
Sæther OA (1975) Nearctic and palaearctic Heterotrissocladius (Diptera: Chironomidae). Bull Fish Res Board Canada 193:67
Sæther OA (1979) Chironomid communities as water quality indicators. Holarctic Ecol 2:65–74
Schmäh A (1993) Variation among fossil chironomid assemblages in surficial sediments of Bodensee-Untersee (SW-Germany): implications for paleolimnological interpretation. J Paleolimnol 9:99–108
Servettaz PL (1977) Eau, la vie d’un lac alpin: chronique de la sauvegarde du lac d’Annecy. p 280
Smith JN (2001) Why should we believe 210Pb sediment geochronologies? J Environ Radioact 55:121–123
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org
van Hardenbroek M, Heiri O, Wilhelm M, Lotter A (2011) How representative are subfossil assemblages of Chironomidae and common benthic invertebrates for the living fauna of Lake De Waay, the Netherlands? Aquat Sci 73:247–259
Vander Zanden J, Chandra S, Allen B, Reuter J, Goldman C (2003) Historical food web structure and restoration of native aquatic communities in the Lake Tahoe (California-Nevada) basin. Ecosystems 6:274–288
Vander Zanden J, Essington T, Vadeboncoeur Y (2005) Is pelagic top-down control in lakes augmented by benthic energy pathways? Can J Fish Aquat Sci 62:1422–1431
Walker I (2001) Midges: Chironomidae and related Diptera. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments: zoological indicators, vol 4. Kluwer Academic Publisher, Berlin, pp 43–66
Wiederholm T (1983) Chironomidae of the Holarctic region. Keys and diagnoses. Part 1. Larvae. Entomol Scand (Suppl) 19:1–457
Woodward CA, Shulmeister J (2006) New Zealand chironomids as proxies for human-induced and natural environmental change: transfer functions for temperature and lake production (chlorophyll a). J Paleolimnol 36:407–429
Acknowledgments
We thank two anonymous reviewers for comments that greatly improve the initial version of this manuscript. This research was developed as part of the program Impact des PERturbations sur les REseaux TROphiques en lacs (IPER-RETRO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frossard, V., Millet, L., Verneaux, V. et al. Chironomid assemblages in cores from multiple water depths reflect oxygen-driven changes in a deep French lake over the last 150 years. J Paleolimnol 50, 257–273 (2013). https://doi.org/10.1007/s10933-013-9722-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-013-9722-x