Abstract
Articular cartilage is the avascular and aneural tissue which is the primary connective tissue covering the surface of articulating bone. Traumatic damage or degenerative diseases can cause articular cartilage injuries that are common in the population. As a result, the demand for new therapeutic options is continually increasing for older people and traumatic young patients. Many attempts have been made to address these clinical needs to treat articular cartilage injuries, including osteoarthritis (OA); however, regenerating highly qualified cartilage tissue remains a significant obstacle. 3D bioprinting technology combined with tissue engineering principles has been developed to create biological tissue constructs that recapitulate the anatomical, structural, and functional properties of native tissues. In addition, this cutting-edge technology can precisely place multiple cell types in a 3D tissue architecture. Thus, 3D bioprinting has rapidly become the most innovative tool for manufacturing clinically applicable bioengineered tissue constructs. This has led to increased interest in 3D bioprinting in articular cartilage tissue engineering applications. Here, we reviewed current advances in bioprinting for articular cartilage tissue engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue engineering aims to develop biological substitutes to overcome the high shortage of autologous tissues and organs for transplantation. Over the past two decades, tissue engineering applications have progressed rapidly; however, conventional fabrication methods need to be improved in their abilities to create clinically applicable tissue constructs with well-interconnected pores, patient-specific tissue architectures, and heterogeneous material distributions. 3D printing or rapid prototyping was developed in the 1980s, and it included various approaches to create objects from a computer-generated imaging file [1]. This technology quickly became a powerful tool in tissue engineering and biomedical research [2,3,4,5]. Therefore, 3D bioprinting strategy has been applied to address the limitations described above [2, 6,7,8]. It allows for the fabrication of tissue constructs with unique spatial control over the deposition of cells, biomaterials, and bioactive molecules, resulting in higher regenerative capability after implantation [9, 10]. Various types of 3D bioprinting methodologies are now available to meet specific requirements in tissue engineering applications.
Articular cartilage is imperative to reducing friction and absorbing compressive forces in load-bearing joints with little to no capacity for self-regeneration. Current cartilage tissue engineering strategies are insufficient for reproducing tissue equivalent to healthy cartilage [11]. However, recently greater interest has been placed on the zonal differences found in the cartilage matrix and cellular composition [12]. Bioprinting presents an appealing tool for constructing stratified scaffolds, especially in patient-specific size and shape of individual lesions [13]. The next challenge for bioprinting as a means of cartilage regeneration is conducting translational studies. The long-term in vivo stability of bioprinted cartilage constructs has yet to be demonstrated, and no studies have compared these strategies with practices currently used clinically. Here, we review the structural considerations of the normal articular cartilage and current and future directions in cartilage bioprinting for successful clinical translation.
Clinical Needs for Bioengineered Articular Cartilage Constructs
Impact of Cartilage Defects and Osteoarthritis
Cartilage defect injuries cause pain, swelling, and mechanical symptoms in the affected joint, severely hindering the patient’s quality of life. The clinical need for cartilage regenerative therapy is tremendous due to the vast number of patients affected by articular cartilage damage, together with the currently limited therapeutic options. Osteoarthritis (OA) is a prevalent chronic disease within the musculoskeletal system that leads to degeneration and progression, resulting in disability. Globally, this condition affects approximately 250 million people, with over 27 million patients in the USA alone. [14]. A significant factor contributing to the progression of OA is defects in the articular cartilage [15, 16], which are present in 5 to 10% of individuals over the age of 40 [17, 18]. Therefore, OA is a major global healthcare problem, and associated healthcare costs reach up to 2.5% of the gross national product (GNP) in developed nations [19, 20]. The number of patients and related socioeconomic impact will only increase due to aging and obesity.
Problems in Current Therapeutic Approaches
Over the last 60 years, much effort has been made to treat articular cartilage defects and OA. Currently established surgical methods for articular cartilage defects include bone marrow stimulation (BMS), autologous chondrocyte implantation (ACI), matrix-associated autologous chondrocyte implantation (MACI), and autologous osteochondral transplantation (AOTS) [21]. BMS involves the creation of small channels through the subchondral bone, allowing mesenchymal stem cells (MSCs) within the bone marrow to migrate and differentiate within the cartilage defect. ACI involves the use of in vitro-expanded chondrocytes that are derived from the patient’s own cells. The chondrocytes are injected into the defect and covered by a membrane, such as a periosteal patch [22]. MACI is a two-step process that builds upon the ACI method, where healthy cartilage cell-seeded collagen matrix is implanted into the defects [23, 24].
In AOTS, ‘normal’ osteochondral bone plugs are removed from non-weight-bearing areas of articular cartilage and transferred to the defect site. In advanced OA cases, arthroplasty is the treatment of choice, which involves the removal of diseased cartilage and bone and replacing them with metal and polyethylene (PE)-based implants.
While the above-mentioned surgical procedures offer, in part, symptomatic and functional improvement, none of these surgeries have been able to restore native cartilage and halt the natural progression of OA. Specific limitations of current cartilage repair therapies include fibrocartilage formation rather than hyaline cartilage, donor site morbidity resulting from cartilage tissue harvest, and damage to adjacent host cartilage during tailoring of the defect area. While advances in surgical techniques, along with the utilization of stem cell and biomaterial-related technology, have provided some improvements in current surgery, reliable cartilage repair still remains to be achieved [21].
Bioprinting, which allows the production of tissues in desired shapes and micro-architectures, is an attractive future strategy with the potential to overcome existing limitations in current therapeutics. The proposed treatment scheme is to bioengineer a patient-specific cartilage graft using the bioprinting strategy, based on preoperative medical imaging of the cartilage defect, such as computed tomography (CT) or magnetic resonance imaging (MRI). Bioprinting would reproduce specific shapes and dimensions of the graft to fit irregular defects and the microarchitecture of the hyaline cartilage [21]. Figure 1 illustrates the treatment methods for cartilage defects and OA.
Structural Considerations of Articular Cartilage
In cartilage bioprinting, it is essential to accurately replicate the biochemical composition of cartilage-specific extracellular matrix (ECM) proteins, structural characteristics, and biomechanical parameters found in native cartilage to achieve successful bioengineering of a cartilage construct [25, 26].
Structure of Articular Cartilage
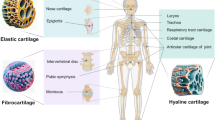
Articular cartilage, a connective tissue covering the surface of articulating bone, is avascular and aneural, with a thickness ranging from 1 to 3 mm depending on the articular joint type and location. It is characterized by separating zones based on calcification level, oxygen tension, and load force, with the superficial zone (10–20% thickness), middle zone (40–60%), and deep zone (30–40%) each distinguished by morphological criteria, such as cell shape, size, and arrangement [27, 28]. The superficial zone contains flat-shaped chondrocytes surrounding parallel microenvironments, the middle zone has a disorganized collagen network with spherical-shaped chondrocytes, and the deep zone contains vertically aligned chondrocytes and collagen matrix [29]. Each zone has a different composition of collagen, proteoglycans expression, and biochemical cues, ultimately affecting the function of each zone [30, 31]. The superficial zone is responsible for lubrication and low frictional properties of the cartilage, while the middle and deep zones offer load-bearing properties and maintenance of the ECM components.
Articular Cartilage Composition
Adult articular cartilage is composed of 65 to 85% water, 20 to 35% organic matter, and 2% chondrocytes. The organic component comprises 60% collagen and 30% proteoglycans [32, 33]. Type 2 collagen is the most common type found in articular cartilage, providing tensile strength to the cartilage surface. Proteoglycans consist of a core protein with numerous glycosaminoglycan (GAG) attachments arranged brush likely. Articular cartilage contains various types of proteoglycans, including aggrecan, biglycan, decorin, and cartilage oligomeric matrix protein (COMP), each with different GAG compositions [34,35,36]. For instance, aggrecan is made up of chondroitin sulfate and keratin sulfate. Proteoglycans are responsible for retaining water within the cartilage matrix, providing compressive strength to the cartilage [37]. GAG composition is known to change in aging and diseased states, such as OA, thereby decreasing the ability to hold water in place [21]. Moreover, articular cartilage tissue has shown a reverse tendency of content between collagen and proteoglycans according to tissue depth; understanding these gradient compositions is necessary for mimicking the cartilage environment [38].
Biomechanical Demands
Articular cartilage is a thin layer of specialized connective tissue with a unique ability to withstand high cyclic loads. The biomechanical demands of articular cartilage are reported to be, on average, a compressive modulus of 0.79 MPa, shear modulus of 0.69 MPa, and tensile modulus of 0.3–10 MPa [39]. As collagen and proteoglycans form a sponge-like structure that traps water, articular cartilage has viscoelastic behavior. The biomechanical demands differ from joint type, even with the location of cartilage within a joint. For instance, in the knee joint, the medial and lateral femoral condylar and tibial cartilages are designed to meet high load-bearing requirements, while the patellofemoral joint cartilage is designed to resist shear forces more effectively [40].
3D Bioprinting Strategies for Articular Cartilage
Bioprinting Techniques
A variety of 3D printing techniques have been developed and tested to bioengineer 3D human tissue/organ constructs for tissue engineering applications [41]. The effectiveness of each printing technique relies heavily on biomaterials choice and targeted applications. In general, bioprinters consist of three main components: the 3-axis stage, printing cartridges, and the dispenser [6]. Stage controllers move the printer head in the X-, Y-, and Z-directions. Printing cartridges, usually in the form of a syringe, store either the polymeric components of the scaffold or the cell-laden hydrogel components. They include a nozzle that determines the amount of material dispensed at set printing parameters. The dispenser system is the final component that causes the deposition of materials, and it varies between the printing techniques. Among the 3D printing techniques, microextrusion, jetting, and stereolithography have been mainly utilized for bioprinting applications (Fig. 2) [42,43,44].
Extrusion-based bioprinting is the most common method for bioprinting applications. Unlike jetting-based printing, extrusion-based printing uses an additive manufacturing machine that relies on fused deposition modeling (FDM). The biomaterial is dispensed directly on the stage in a continuous string form rather than liquid droplets. The pneumatic dispensing system allows accurate control over the air pressure to enable the dispensing of the material with increased pressure needed for more viscous materials [45]. The ultimate force of pneumatic systems is only limited by the air pressure capabilities of the system. On the other hand, mechanical dispensing systems use motor-derived piston or screw to provide more spatial control at the cost of reduced maximum force capabilities. The jetting-based bioprinter dispenses a controlled volume of liquid to a predefined location through non-contact deposition. Selected hydrogel acts as an “ink” in this case, where it can be dispensed in volumes between 10 and 150 pL depending on the dispensing modules used [46]. The two common dispensing methods to generate droplets in inkjet printers are thermal and piezoelectric actuators. The stereolithography-based bioprinting has the ability to fabricate a complex 3D model with high resolution (~ 1.2 μm) [47, 48]. Another advantage of this printing process is precisely controlling the average energy dose to minimize the adverse effect on cells [49]. However, the major downside of stereolithography printing techniques is that there are limited biomaterials for the bioink that can be photo-cured [48].
Cell Sources for Cartilage Bioprinting
As articular cartilage consists of only chondrocytes, it is only logical to utilize them in cartilage bioprinting. Consequently, they are the most extensively studied cell source for cartilage tissue engineering and consistently result in higher expression of cartilage ECM components of GAG and collagen type II compared to chondrogenic-induced MSCs [50,51,52]. The main drawback of utilizing autologous adult knee chondrocytes is the limited number of cells, as the cartilage tissue engineering technique usually requires high cell density compared to other tissue types. Additionally, in vitro expansion of hyaline chondrocytes results in dedifferentiation and loss of chondrogenic phenotype.
Emerging cell sources for articular cartilage tissue engineering include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). ESCs can propagate infinitely while retaining the differentiation potential toward all lineage cell types. iPSCs are obtained by adult somatic cells by manipulating certain transcription factors associated with pluripotency, differentiation, and proliferation. Like ESCs, they possess infinite self-renewal capacity and differentiation potential toward all three germ layers. Both ESCs and iPSCs are attractive cell sources for cartilage bioprinting due to the possibility of obtaining a vast number of homogenous cells, as well as the theoretical differentiation ability toward chondrogenic cells, including hyaline chondrocytes. While a few studies have investigated the potential of ESCs and iPSCs in cartilage tissue engineering and bioprinting, it is still early to decide the clinical value of these cells [53, 54]. ESCs carry ethical concerns in the process of obtaining the cells from embryos, as well as the risk of tumorigenicity and immune rejection. iPSCs, while relatively free from ethical concerns, still carry the risk of tumorigenicity due to the genetic and epigenetic changes the cells experience during cellular reprogramming. Tissue engineering wise, another big hurdle facing these cells is the need for more differentiation protocols toward cartilage tissue. Up to now, protocols for chondrogenic differentiation of iPSCs differ substantially among institutions, ranging from utilization of fluorescence-activated cell sorting (FACS) to co-culturing iPSCs with chondrocytes mitotically inactivated by irradiation [54, 55]. Overall, collagen type I laden fibrocartilage rather than hyaline cartilage is often observed during the differentiation of ESCs and iPSCs [56, 57].
Autologous MSCs are usually harvested from adult tissue and show high self-renewal capacity, as well as multipotency, especially toward the chondrogenic differentiation [58, 59]. The advantages over primary chondrocytes include ease of harvest, high proliferation capacity, and potential utilization as an allogeneic cell source. In addition, the trophic effects of MSCs, which include immunomodulatory and chemoattractant functions, make MSCs an attractive cell source for cartilage tissue engineering applications [31, 60]. When utilizing MSCs, several issues should be considered. First, the differentiation potential of MSCs differs, in part, by the donor tissue from which they are harvested. MSCs can be isolated from various tissues, including bone marrow, fat, synovium, and umbilical cord matrix [61,62,63]. Bone marrow and adipose tissue-derived MSCs are extensively used, primarily because of their widespread availability and minimal donor site morbidity [64]. Unlike bone marrow-derived MSCs, synovium-derived MSCs show the highest chondrogenic differentiation capacity, which is not affected by donor age [65]. Secondly, the mode of action of MSCs is not yet fully understood. The regeneration mechanism involving stem cell-based approaches is still elusive [66]. Numerous in vivo studies reported that the transplanted MSCs could secrete trophic factors that accelerate the regeneration process [67]. Thirdly, ‘cartilage-like tissue’ derived from chondrogenically differentiated MSCs differ from mature hyaline cartilage tissue in many aspects, including biochemical composition, biomechanical properties, and microarchitecture. The resulting cartilage tissue is usually hypertrophic cartilage, often expressing hypertrophic markers of type X collagen, MMP13, and alkaline phosphatase [68].
Interestingly, nasal chondrocytes have been studied as a potential cell source for articular cartilage regeneration due to their availability and ability to form functional hyaline cartilage tissue. Several studies have investigated using nasal chondrocytes in tissue engineering approaches for cartilage repair, and some have shown promising outcomes [69, 70]. A clinical trial investigated the safety and effectiveness of using nasal chondrocytes to repair articular cartilage defects in humans [71]. The study enrolled 10 patients with knee cartilage defects who underwent a procedure to implant the engineered autologous cartilage tissue from their own nasal chondrocytes. The results showed that the nasal chondrocyte-based engineered cartilage tissue was safe and effective, with significant improvements in pain, function, and imaging outcomes indicating successful cartilage repair. The study suggests that nasal chondrocytes can be a viable cell source for repairing human articular cartilage defects.
Bioinks for Cartilage Bioprinting
Bioprinting requires a printing medium, often termed ‘bioink’ that allows printing the desired biomaterials or cells. An optimal bioink should possess rheological properties, such as shear thinning, suitable for the printing process, along with tissue-specific biological properties that promote cell support in the printed constructs as synthetic ECMs [72, 73]. In cell-based cartilage bioprinting, hydrogels have been commonly used as bioinks to deliver chondrocytes, due to the structural similarities with cartilage ECMs, together with features of good biological properties and high water content capability. Common hydrogels used for cartilage bioprinting include collagen, silk fibroin, hyaluronic acid (HA), agarose, chitosan, alginate, gelatin methacryloyl (GelMA), and poly(ethylene glycol)-diacrylate (PEGDA)-based hydrogels (Fig. 3) [74,75,76,77,78].
The hydrogel-based bioinks are essential for the cell-based printing process; however, they often lack the proper biomechanical properties compared with those of the native cartilage. To improve the mechanical properties, hydrogel bioinks have been reinforced by co-printing with solid polymers [e.g., poly(ε-caprolactone) (PCL)]. In such hybrid constructs, the polymeric framework provides the structural and biomechanical properties necessary for load bearing, while cell-laden hydrogel structures provide the biological microenvironment suitable for the chondrogenesis [78]. A combination of hydrogels, such as alginate and GelMA printed together with a PCL backbone, has significantly enhanced the structural integrity of the printed cell-laded constructs [79,80,81]. Another strategy is to utilize nanofibril cellulose in combination with hydrogels. This nanocellulose could improve the printability of hydrogel-based bioinks. For example, an alginate-based bioink combined with nanocellulose improved shape fidelity and printing resolution when printing cartilaginous tissue structures [54, 82]. While various synthetic and natural materials have been used to reinforce the biomechanical properties of the printed hydrogel-based constructs to match those with native cartilage closely, such strategies should consider the effects of these materials on the remodeling process after transplantation. Since PCL and nanocellulose hardly biodegrade, how the by-products during the degradation process will affect the host responses remain unknown.
The microenvironment generated by bioinks directly affects the chondrogenic differentiation of the printed stem cells by providing biochemical and biomechanical cues [83]. Recent work demonstrated that different bioinks could influence chondrogenic cell types after stem cell differentiation [84]. For example, alginate- and agarose-based hydrogels are more likely to induce hyaline chondrogenic differentiation, whereas GelMA- and PEGDA-based hydrogels result in more fibrocartilaginous differentiation [78]. In addition, cartilage-derived decellularized ECM hydrogels may promote constructive remodeling at the implantation site and encourage cartilage tissue formation rather than scar tissue [85, 86]. All in all, bioinks for cartilage bioprinting are currently being developed to enhance shape fidelity with high printing resolution and the biochemical and biomechanical characteristics like native cartilage.
Tissue-derived ECM components could provide tissue-specific structural and biochemical signals to promote cellular activities and function, as well as tissue maturation and formation [87,88,89]. Hence, the cartilage-derived decellularized ECM is a promising option for articular cartilage bioprinting applications due to the preserved collagens, GAGs, and growth factors that could mimic the cartilage-specific microenvironment. For example, a study showed a hybrid bioink combined with a cartilage-derived ECM powder and a high concentration collagen solution (ECM-c) for printing irregular cartilage defects [90]. This approach utilizing the cartilage ECM powder enhanced the stability of the 3D-printed tissue construct and accelerated chondrogenesis overall of the printed ECM-c constructs. Similarly, the ECM powder was combined with a silk fibroin (SF) solution for the cartilage bioprinting [91]. Interestingly, one study developed a hybrid bioink using SF powder and decellularized cartilage-derived ECM (SF-dECM) [92]. The SF-dECM bioink-based construct showed improved printability and mechanical strength compared with only SF used to construct and accelerated chondrogenesis-related gene expression.
Cartilage-Specific Stimulating Factors
Biochemical stimulating factors have been commonly used to accelerate articular cartilage differentiation and maturation, especially in stem cell-based approaches. Growth factors, including transforming growth factor-β (TGF-β) 1 and 3, bone morphogenetic protein (BMP) family, and basic fibroblast growth factor (bFGF), are known to regulate chondrogenesis [93,94,95]. Therefore, the printed cell-laden construct is often cultured in chondrogenic media containing these factors to induce chondrogenic differentiation. Even though this strategy often results in increased cartilage ECM synthesis, heterogeneous chondrogenic differentiation within the construct has often occurred. To overcome this limitation, these factors have been incorporated into the bioink system either by direct mixing or polymeric microencapsulation [79, 96, 97].
To accelerate chondrogenesis, biomechanical stimulation has been utilized. For instance, cyclic loading mechanical stress could improve cell migration, chondrogenic differentiation, and zonal stratification in engineered cartilage [98,99,100]. Biomechanical stress in the form of shear, compression, tension, and pressure, all of which are present in the typical joint biomechanical environment, can be given in vitro using various types of bioreactor-based preconditioning processes [101, 102]. This biomechanical stimulation also enhances the nutrient exchange within the 3D constructs [61]. It is valuable as cartilage tissue is avascular.
Clinical Bioprinting Workflow for Articular Cartilage Reconstruction
Medical Imaging and 3D CAD Modeling
3D bioprinting strategy aims to achieve reproducible, complex tissue structures that are well vascularized and suitable for future clinical use. Since human tissues and organs have arbitrary 3D shapes composed of multiple cell types and ECMs with functional organization, this strategy can be the most effective way to achieve this goal [6]. The CAD/CAM processes are critical technologies needed for the future clinical applications of 3D bioprinting because these processes provide an automated way to replicate a 3D shape of a targeted tissue structure [103]. The process generally starts by scanning the patient to produce 3D volumetric information of a target object using medical imaging modalities. These imaging tools acquire information from cross-sectional slices of the body. The data are stored in the Digital Imaging and Communications in Medicine (DICOM) format, a standard format for digital imaging in medicine. This information is transformed into a CAD model by the reverse engineering process.
The process starts with interpolating points within and between image slices to improve resolution and generate voxels from the measured data. Next, a CAD model is created by extracting localized volumetric data from a targeted tissue structure to develop a surface model. In this step, the sophisticated reconstruction of the CAD model is required for the bioprinting process due to the complexity of the tissue or organ. Finally, a motion program, instructional computer code for the printer to follow designed paths, is generated with a CAM system. This CAM process is divided into three steps: slicing, tool path, and motion program generation. Slicing is to obtain information on sliced 2D shapes of an object for the layer-by-layer process. Then, tool path generation creates a path for the tool to follow to fill the cross-sectional space of each layer. The printed tissue-specific architecture has the proper inner functional structure constructed with multiple cellular components for efficient tissue regeneration. Therefore, a well-organized strategy for tool path generation is required.
Bioprinting Process and Operation
Based on a 3D CAD model, a patient-specific cartilage tissue construct can be fabricated by the clinical bioprinting workflow in good manufacturing practice (GMP) facility or operating room. Figure 4 shows the 3D bioprinting workflow strategy from the medical image to the printed tissue constructs developed by the CAD/CAM process and automated printing of 3D shapes imitating target cartilage tissue [6, 103]. After product validation, the printed construct is moved to the operating room for reconstructive surgery.
Future Directions and Challenges
Over the last decade, significant advancements have been made in the field of articular cartilage bioprinting. Increased knowledge in biomaterials has allowed the development of cartilage-specific bioink systems that recapitulate the biochemical and biomechanical properties of native cartilage ECMs. Hybrid printing and incorporating nanomaterials have increased the printing outcomes and structural integrity of the printed cell-laden constructs. Articular cartilage defects are present in diverse shapes and sizes from partial defects to large-sized, full-thickness defects. Current chondral or osteochondral reconstruction requests destroying significant amounts of host cartilage tissue. 3D bioprinting could be an innovative tool to overcome this limitation. An in situ printing method over cartilage defects has been developed to minimize cartilage damage during surgery (Fig. 5) [104,105,106]. To translate this strategy into the clinic, the complex equipment and multiple steps need to be simplified [107].
In situ cartilage printing: a Direct printing of human chondrocyte-laden bioink. a Schematic illustration of in situ printing using photo-curable PEGDA-based hydrogel. b Osteochondral plug, c printed cell-laden hydrogel in the defect of the osteochondral plug. d, e printed cells in the constructs. b Robotic-assisted in situ printing: a in situ bioprinting on the knee joint of rabbit and b histological examination of the printed constructs after 12 weeks of implantation. c In situ printing process on chondral defect; a in situ printing using HA-based hydrogel, b UV exposure, and c–f repair of the chondral defect
Various novel bioink systems have been tested for improving printing outcomes with high-resolution capability and structural integrity. Advanced cartilage-specific bioink systems, including hydrogels and polymers, that can serve as cell-laden bioinks and supporting structures but also provide biological properties and biomechanical support, are necessitated for 3D bioprinting process. In addition, advances in bioinks depending on 3D bioprinting methods are essential for long-term success in clinical cartilage tissue engineering applications. Several research groups attempted to develop photo-curing bioinks using acrylated decellularized cartilage ECMs [108, 109], showing cartilage-specific biological properties that accelerated cartilage tissue formation.
Strategies to control post-transplantation maturation need to be developed. Articular cartilage is a dynamic tissue and various factors after transplantation, including the degree of injury, inflammation, and subchondral bone manipulation, affect the maturation and outcome of the neo-tissue formation. There is limited information regarding the in vivo outcomes after bioprinted cartilage tissue transplantation. The addition of immune-modulating cells and factors, zonal stratification, and controlling the degradation rate of bioinks may affect the remodeling process and improve long-term preclinical and clinical results after transplantation.
Here, we overviewed bioprinting strategies for articular cartilage tissue reconstruction. 3D bioprinting technologies offer the opportunity for the reconstruction of the structural and functional complexity of human articular cartilage that incorporates cells, biomaterials, and bioactive molecules, resulting in sophisticated tissue constructs that repair cartilage defects or OA injuries. Although there is much work to be accomplished to advance these technologies toward successful clinical translation, our efforts will constantly contribute to producing clinically applicable tissue constructs. We envision that the bioprinted articular cartilage constructs have great potential to be translated into clinical applications within a short period of time.
References
Xue, W., B. V. Krishna, A. Bandyopadhyay, and S. Bose. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 3:1007–1018, 2007.
Kim, J. H., J. J. Yoo, and S. J. Lee. Three-dimensional cell-based bioprinting for soft tissue regeneration. Tissue Eng. Regen. Med. 13:647–662, 2016.
Derby, B. Printing and prototyping of tissues and scaffolds. Science. 338:921–926, 2012.
Hockaday, L. A., K. H. Kang, N. W. Colangelo, P. Y. Cheung, B. Duan, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication.4:035005, 2012.
Ozbolat, I. T., and M. Hospodiuk. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 76:321–343, 2016.
Kang, H. W., S. J. Lee, I. K. Ko, C. Kengla, J. J. Yoo, and A. Atala. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34:312–319, 2016.
Moroni, L., J. A. Burdick, C. Highley, S. J. Lee, Y. Morimoto, et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 3:21–37, 2018.
Murphy, S. V., and A. Atala. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32:773–785, 2014.
Lee, M., B. M. Wu, and J. C. Dunn. Effect of scaffold architecture and pore size on smooth muscle cell growth. J. Biomed. Mater. Res. A. 87:1010–1016, 2008.
Tsang, V. L., and S. N. Bhatia. Three-dimensional tissue fabrication. Adv. Drug Deliv. Rev. 56:1635–1647, 2004.
Makris, E. A., A. H. Gomoll, K. N. Malizos, J. C. Hu, and K. A. Athanasiou. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 11:21–34, 2015.
Tatman, P. D., W. Gerull, S. Sweeney-Easter, J. I. Davis, A. O. Gee, and D. H. Kim. Multiscale biofabrication of articular cartilage: bioinspired and biomimetic approaches. Tissue Eng. Part B. 21:543–559, 2015.
Di Bella, C., A. Fosang, D. M. Donati, G. G. Wallace, and P. F. Choong. 3D bioprinting of cartilage for orthopedic surgeons: reading between the lines. Front Surg. 2:39, 2015.
Hjelle, K., E. Solheim, T. Strand, R. Muri, and M. Brittberg. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy. 18:730–734, 2002.
Curl, W. W., J. Krome, E. S. Gordon, J. Rushing, B. P. Smith, and G. G. Poehling. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 13:456–460, 1997.
Cibere, J., E. Sayre, A. Guermazi, S. Nicolaou, J. Kopec, et al. Natural history of cartilage damage and osteoarthritis progression on magnetic resonance imaging in a population-based cohort with knee pain. Osteoarthr. Cartil. 19:683–688, 2011.
Shelbourne, K. D., S. Jari, and T. Gray. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. JBJS. 85:8–16, 2003.
Lawrence, R. C., D. T. Felson, C. G. Helmick, L. M. Arnold, H. Choi, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States Part II. Arthritis Rheum. 58:26–35, 2008.
March, L. M., and C. J. Bachmeier. Economics of osteoarthritis: a global perspective. Baillieres Clin. Rheumatol. 11:817–834, 1997.
Hunziker, E. B., K. Lippuner, M. J. Keel, and N. Shintani. An educational review of cartilage repair: precepts & practice–myths & misconceptions–progress & prospects. Osteoarthr. Cartil. 23:334–350, 2015.
Van Blitterswijk, C., and J. De Boer. Tissue Engineering. Cambridge: Academic Press, 2014.
Niethammer, T. R., A. Loitzsch, A. Horng, A. Baur-Melnyk, M. Bendiks, et al. Graft hypertrophy after third-generation autologous chondrocyte implantation has no correlation with reduced cartilage quality: matched-pair analysis using T2-weighted mapping. Am. J. Sports Med. 46:2414–2421, 2018.
Martín, A. R., J. M. Patel, H. M. Zlotnick, J. L. Carey, and R. L. Mauck. Emerging therapies for cartilage regeneration in currently excluded “red knee” populations. NPJ Regen. Med. 4:12, 2019.
Jeznach, O., D. Kołbuk, and P. Sajkiewicz. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. J. Biomed Mater. Res. A. 106:2762–2776, 2018.
Gadjanski, I. Recent advances on gradient hydrogels in biomimetic cartilage tissue engineering. F1000Res. 6:18, 2017.
Marjorie, D., S. Lilian, M. Marie, S. Matthieu, P. G. Emeline, S. Gilles, J. Christian, and Danièlenoël. 3D bioprinting of articular cartilage: recent advances and perspectives. Bioprinting. 28:15, 2022.
Pattappa, G., B. Johnstone, J. Zellner, D. Docheva, and P. Angele. The Importance of physioxia in mesenchymal stem cell chondrogenesis and the mechanisms controlling its response. Int. J. Mol. Sci. 20:18, 2019.
Ansari, S., S. Khorshidi, and A. Karkhaneh. Engineering of gradient osteochondral tissue: from nature to lab. Acta Biomater. 87:41–54, 2019.
Medvedeva, E. V., E. A. Grebenik, S. N. Gornostaeva, V. I. Telpuhov, A. V. Lychagin, et al. Repair of damaged articular cartilage: current approaches and future directions. Int J Mol Sci. 19:16, 2018.
Klein, T. J., J. Malda, R. L. Sah, and D. W. Hutmacher. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng. Part B. 15:143–157, 2009.
Armstrong, C., and V. C. Mow. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J. Bone Joint Surgery Am. 64:88–94, 1982.
Bhattarai, A., J. T. A. Mäkelä, B. Pouran, H. Kröger, H. Weinans, et al. Effects of human articular cartilage constituents on simultaneous diffusion of cationic and nonionic contrast agents. J. Orthop. Res. 39:771–779, 2021.
Alcaide-Ruggiero, L., V. Molina-Hernández, M. M. Granados, and J. M. Domínguez. Main and minor types of collagens in the articular cartilage: the role of collagens in repair tissue evaluation in chondral defects. Int. J. Mol. Sci. 22:26, 2021.
Kadler, K. E., C. Baldock, J. Bella, and R. P. Boot-Handford. Collagens at a glance. J. Cell Sci. 120:1955–1958, 2007.
Chung, C., and J. A. Burdick. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 60:243–262, 2008.
Posey, K. L., F. Coustry, and J. T. Hecht. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 71–72:161–173, 2018.
Knudson, C. B., and W. Knudson. Cartilage proteoglycans. Semin Cell. Dev. Biol. 12:69–78, 2001.
Fulcher, G. R., D. W. Hukins, and D. E. Shepherd. Viscoelastic properties of bovine articular cartilage attached to subchondral bone at high frequencies. BMC Musculoskelet. Disord. 10:1–7, 2009.
Jakob, M., O. Démarteau, R. Suetterlin, M. Heberer, and I. Martin. Chondrogenesis of expanded adult human articular chondrocytes is enhanced by specific prostaglandins. Rheumatology (Oxford). 43:852–857, 2004.
Walker, P. S., and J. V. Hajek. The load-bearing area in the knee joint. J. Biomech. 5:581–589, 1972.
Qian, Y., D. Hanhua, S. Jin, H. Jianhua, S. Bo, W. Qingsong, and S. Yusheng. A Review of 3D printing technology for medical applications. Engineering. 4:729–742, 2018.
Daniela, F. D. C., A. P. Midhun, G. Stefanie, M. Christoph, Y. L. Ying, S. Jan, B. Andreas, T. Benjamin, F. Horst, and B. Marcel. Synchronized dual bioprinting of bioinks and biomaterials inks as a translational strategy for cartilage tissue engineering. Print. Addit. Manuf. 6:63, 2019.
Aisenbrey, E. A., A. Tomaschke, E. Kleinjan, A. Muralidharan, C. Pascual-Garrido, et al. A stereolithography-based 3D printed hybrid scaffold for in situ cartilage defect repair. Macromol. Biosci. 18:29, 2018.
Liang, Q., Y. Ma, X. Yao, and W. Wei. Advanced 3D-printing bioinks for articular cartilage repair. Int. J. Bioprint. 8:511, 2022.
Sears, N. A., D. R. Seshadri, P. S. Dhavalikar, and E. Cosgriff-Hernandez. A review of three-dimensional printing in tissue engineering. Tissue Eng. Part B. 22:298–310, 2016.
Cui, X., T. Boland, D. D. D’Lima, and M. K. Lotz. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv. Formul. 6:149–155, 2012.
Zhang, X., X. Jiang, and C. Sun. Micro-stereolithography of polymeric and ceramic microstructures. Sens. Actuators A. 77:149–156, 1999.
Kim, S. H., Y. K. Yeon, J. M. Lee, J. R. Chao, Y. J. Lee, et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 9:1620, 2018.
Chan, V., P. Zorlutuna, J. H. Jeong, H. Kong, and R. Bashir. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab. Chip. 10:2062–2070, 2010.
Mauck, R., X. Yuan, and R. S. Tuan. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 14:179–189, 2006.
da Silva, M. L., A. M. Fontes, D. T. Covas, and A. I. Caplan. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 20:419–427, 2009.
Caplan, A. I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 213:341–347, 2007.
Gibson, J. D., M. B. O’Sullivan, F. Alaee, D. N. Paglia, R. Yoshida, et al. Regeneration of articular cartilage by human ESC-derived mesenchymal progenitors treated sequentially with BMP-2 and Wnt5a. Stem Cells Transl. Med. 6:40–50, 2017.
Nguyen, D., D. A. Hägg, A. Forsman, J. Ekholm, P. Nimkingratana, et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep. 7:658, 2017.
Yi, Y., K. B. Choi, C.-L. Lim, J.-P. Hyun, H.-Y. Lee, et al. Irradiated human chondrocytes expressing bone morphogenetic protein 2 promote healing of osteoporotic bone fracture in rats. Tissue Eng. Part A. 15:2853–2863, 2009.
Hwang, N. S., S. Varghese, and J. Elisseeff. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS ONE.3:e2498, 2008.
Qu, C., K. A. Puttonen, H. Lindeberg, M. Ruponen, O. Hovatta, et al. Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. Int. J. Biochem. Cell Biol. 45:1802–1812, 2013.
Chen, J., C. Wang, S. Lü, J. Wu, X. Guo, et al. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 319:429–438, 2005.
Boeuf, S., and W. Richter. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Res. Ther. 1:31, 2010.
Pleumeekers, M. M., L. Nimeskern, J. L. M. Koevoet, M. Karperien, K. S. Stok, and G. J. V. M. van Osch. Trophic effects of adipose-tissue-derived and bone-marrow-derived mesenchymal stem cells enhance cartilage generation by chondrocytes in co-culture. PLoS ONE.13:e0190744, 2018.
Mahmoudifar, N., and P. M. Doran. Chondrogenic differentiation of human adipose-derived stem cells in polyglycolic acid mesh scaffolds under dynamic culture conditions. Biomaterials. 31:3858–3867, 2010.
Chang, C. H., C. C. Chen, C. H. Liao, F. H. Lin, Y. M. Hsu, and H. W. Fang. Human acellular cartilage matrix powders as a biological scaffold for cartilage tissue engineering with synovium-derived mesenchymal stem cells. J. Biomed. Mater. Res. Part A. 102:2248–2257, 2014.
Baksh, D., R. Yao, and R. S. Tuan. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 25:1384–1392, 2007.
Nejadnik, H., J. H. Hui, E. P. Feng Choong, B. C. Tai, and E. H. Lee. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am. J. Sports Med. 38:1110–1116, 2010.
De Bari, C., F. Dell’Accio, P. Tylzanowski, and F. P. Luyten. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheumatism. 44:1928–1942, 2001.
De Bari, C., and A. J. Roelofs. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 40:74–80, 2018.
Park, Y., C. Ha, J. Kim, W. Han, J. Rhim, et al. Single-stage cell-based cartilage repair in a rabbit model: cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthr. Cartil. 25:570–580, 2017.
Somoza, R. A., J. F. Welter, D. Correa, and A. I. Caplan. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng. Part B. 20:596–608, 2014.
Chen, W., C. Li, M. Peng, B. Xie, L. Zhang, and X. Tang. Autologous nasal chondrocytes delivered by injectable hydrogel for in vivo articular cartilage regeneration. Cell Tissue Bank. 19:35–46, 2018.
Vinatier, C., O. Gauthier, M. Masson, O. Malard, A. Moreau, et al. Nasal chondrocytes and fibrin sealant for cartilage tissue engineering. J. Biomed. Mater. Re.s A. 89:176–185, 2009.
Mumme, M., A. Barbero, S. Miot, A. Wixmerten, S. Feliciano, et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 388:1985–1994, 2016.
Raul Sanchez-Sanchez, J.M.R.-R., Antoni Macias-Garcia, Laura Mendoza-Cerezo, and Antonio Diaz-Parralejo. Relationship between shear-thinning rheological properties of bioinks and bioprinting parameters. Int. J. Bioprint. 9:422, 2023.
Habib, M. A., and B. Khoda. Rheological analysis of bio-ink for 3D bio-printing processes. J. Manuf. Process. 76:708–718, 2022.
Lee, V., G. Singh, J. P. Trasatti, C. Bjornsson, X. Xu, et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C. 20:473–484, 2014.
Song, S. J., J. Choi, Y. D. Park, J. J. Lee, S. Y. Hong, and K. Sun. A three-dimensional bioprinting system for use with a hydrogel-based biomaterial and printing parameter characterization. Artif. Organs. 34:1044–1048, 2010.
Almeida, C. R., T. Serra, M. I. Oliveira, J. A. Planell, M. A. Barbosa, and M. Navarro. Impact of 3-D printed PLA-and chitosan-based scaffolds on human monocyte/macrophage responses: unraveling the effect of 3-D structures on inflammation. Acta Biomater. 10:613–622, 2014.
Khalil, S., and W. Sun. Bioprinting endothelial cells with alginate for 3D tissue constructs. J. Biomech. Eng. 131:15, 2009.
Daly, A. C., S. E. Critchley, E. M. Rencsok, and D. J. Kelly. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication.8:045002, 2016.
Kundu, J., J. H. Shim, J. Jang, S. W. Kim, and D. W. Cho. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 9:1286–1297, 2015.
Visser, J., F. P. Melchels, J. E. Jeon, E. M. Van Bussel, L. S. Kimpton, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 6:1–10, 2015.
Xu, T., K. W. Binder, M. Z. Albanna, D. Dice, W. Zhao, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication.5:015001, 2013.
Müller, M., E. Öztürk, Ø. Arlov, P. Gatenholm, and M. Zenobi-Wong. Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 45:210–223, 2017.
Skardal, A., M. Devarasetty, H. W. Kang, I. Mead, C. Bishop, et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 25:24–34, 2015.
Roseti, L., C. Cavallo, G. Desando, V. Parisi, M. Petretta, et al. Three-dimensional bioprinting of cartilage by the use of stem cells: a strategy to improve regeneration. Materials (Basel). 11:25, 2018.
Pati, F., J. Jang, D. H. Ha, S. Won Kim, J. W. Rhie, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5:3935, 2014.
Yang, S. S., W. H. Choi, B. R. Song, H. Jin, S. J. Lee, et al. Fabrication of an osteochondral graft with using a solid freeform fabrication system. Tissue Eng. Regenerat. Med. 12:239–248, 2015.
Ali, M., A. K. Pr, J. J. Yoo, F. Zahran, A. Atala, and S. J. Lee. A photo-crosslinkable kidney ECM-derived bioink accelerates renal tissue formation. Adv. Healthc. Mater.8:e1800992, 2019.
Visscher, D. O., H. Lee, P. P. M. van Zuijlen, M. N. Helder, A. Atala, et al. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 121:193–203, 2021.
Lee, H., W. Kim, J. Lee, K. S. Park, J. J. Yoo, et al. Self-aligned myofibers in 3D bioprinted extracellular matrix-based construct accelerate skeletal muscle function restoration. Appl. Phys. Rev.8:021405, 2021.
Song, B. R., S. S. Yang, H. Jin, S. H. Lee, D. Y. Park, et al. Three dimensional plotted extracellular matrix scaffolds using a rapid prototyping for tissue engineering application. Tissue Eng. Regenerat. Med. 12:172–180, 2015.
Jung, C. S., B. K. Kim, J. Lee, B.-H. Min, and S.-H. Park. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng. Regenerat. Med. 15:155–162, 2018.
Zhang, X., Y. Liu, C. Luo, C. Zhai, Z. Li, et al. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C.118:111388, 2021.
Mwale, F., D. Stachura, P. Roughley, and J. Antoniou. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J. Orthop. Res. 24:1791–1798, 2006.
Yoon, B. S., and K. M. Lyons. Multiple functions of BMPs in chondrogenesis. J. Cell Biochem. 93:93–103, 2004.
Goldring, M. B., K. Tsuchimochi, and K. Ijiri. The control of chondrogenesis. J. Cell Biochem. 97:33–44, 2006.
Bouffi, C., O. Thomas, C. Bony, A. Giteau, M. C. Venier-Julienne, et al. The role of pharmacologically active microcarriers releasing TGF-beta3 in cartilage formation in vivo by mesenchymal stem cells. Biomaterials. 31:6485–6493, 2010.
Lee, C. H., S. A. Rodeo, L. A. Fortier, C. Lu, C. Erisken, and J. J. Mao. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 6:266ra171, 2014.
van der Kraan, P. M., J. de Lange, E. L. Vitters, H. M. van Beuningen, G. J. van Osch, et al. Analysis of changes in proteoglycan content in murine articular cartilage using image analysis. Osteoarthr. Cartil. 2:207–214, 1994.
Li KW, Klein TJ, Chawla K, Nugent GE, Bae WC, Sah RL. 2004. In vitro physical stimulation of tissue-engineered and native cartilage. In Cartilage and osteoarthritis:325–51: Springer. Number of 325–51 pp.
Lima, E. G., R. L. Mauck, S. H. Han, S. Park, K. W. Ng, et al. Functional tissue engineering of chondral and osteochondral constructs. Biorheology. 41:577–590, 2004.
Grad, S., D. Eglin, M. Alini, and M. J. Stoddart. Physical stimulation of chondrogenic cells in vitro: a review. Clin. Orthop. Relat. Res. 469:2764–2772, 2011.
Fu, L., P. Li, H. Li, C. Gao, Z. Yang, et al. The application of bioreactors for cartilage tissue engineering: advances, limitations, and future perspectives. Stem Cells Int. 2021:6621806, 2021.
Kengla, C., E. Renteria, C. Wivell, A. Atala, J. J. Yoo, and S. J. Lee. Clinical relevant bioprinting workflow and imaging process for tissue construct design and validation. 3D Printing Manufac. 4:239–247, 2017.
Cui, X., K. Breitenkamp, M. Finn, M. Lotz, and D. D. D’Lima. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A. 18:1304–1312, 2012.
Ma, K., T. Zhao, L. Yang, P. Wang, J. Jin, et al. Application of robotic-assisted in situ 3D printing in cartilage regeneration with HAMA hydrogel: an in vivo study. J. Adv. Res. 23:123–132, 2020.
Li, L., F. Yu, J. Shi, S. Shen, H. Teng, et al. In situ repair of bone and cartilage defects using 3D scanning and 3D printing. Sci. Rep. 7:9416, 2017.
Samandari, M., A. Mostafavi, J. Quint, A. Memic, and A. Tamayol. In situ bioprinting: intraoperative implementation of regenerative medicine. Trends Biotechnol. 40:1229–1247, 2022.
Behan, K., A. Dufour, O. Garcia, and D. Kelly. Methacrylated cartilage ecm-based hydrogels as injectables and bioinks for cartilage tissue engineering. Biomolecules. 12:15, 2022.
Zhu, S., P. Chen, Y. Chen, M. Li, C. Chen, and H. Lu. 3D-Printed Extracellular matrix/polyethylene glycol diacrylate hydrogel incorporating the anti-inflammatory phytomolecule honokiol for regeneration of osteochondral defects. Am. J. Sports Med. 48:2808–2818, 2020.
Acknowledgements
This review was supported by the National Institutes of Health (1P41EB023833-01), the National Science Foundation (2100739), and the National Research Foundation of Korea (NRF-2022R1A2C1091873).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that any no conflict of interest
Additional information
Associate Editor Emmanuel Opara oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, D.Y., Kim, SH., Park, SH. et al. 3D Bioprinting Strategies for Articular Cartilage Tissue Engineering. Ann Biomed Eng 52, 1883–1893 (2024). https://doi.org/10.1007/s10439-023-03236-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-023-03236-8