Abstract
3D-printed medical devices and surgical tools are actively used in patients and within our healthcare system. Even as the bioprinting industry has seen significant growth in the past decade, bioprinted cellularized constructs still face substantial translational challenges. Present throughout the body, cartilage is an avascular tissue with limited regenerative capabilities and has been the subject of intensive research in the fields of tissue engineering and 3D bioprinting. In this review, we summarize the different types of cartilage, highlight key injuries or medical conditions within each cartilage type, discuss the use of natural and synthetic materials in cartilage repair, and present the most recent developments in translating 3D bioprinted cartilage constructs from bench to bedside. Emphasizing novel biomaterials and clinical translation, we highlight the current status quo of cartilage treatments, the translational challenges that the industry faces and finally, the opportunities for next-generation cartilage treatment options using 3D bioprinting. While challenges still lie ahead of clinical translation, 3D bioprinting demonstrates great potential as a fabrication technique for cartilage tissue-engineered constructs used in surgical implantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cartilage is a form of connective tissue that plays multiple physiological roles in the body. Of its various roles, it is a vital component in our joints, providing the necessary cushioning and support for our daily movements [1]. However, due to its avascular nature, cartilage has a limited capacity for self-repair and can become damaged for several reasons, including chronic wear and tear and forceful impacts to the joint during physical activity, sports injuries, or a fall. Statistically, millions of people worldwide suffer from cartilage injuries every year, often resulting from sports injuries, accidents, or age-related wear and tear. This poses a significant challenge in the medical field, as cartilage injuries are not only common but can lead to debilitating conditions such as osteoarthritis if left untreated.
Osteoarthritis (OA) is a degenerative joint disease that can affect the many tissues of the joint, degrade cartilage, and can cause inflammation, resulting in pain, stiffness, and loss of mobility. OA is the most common form of arthritis, affecting more than 32.5 million adults in the United States, according to the Centers for Disease Control and Prevention [1]. The current standard of care focuses on treating immediate symptoms through drug or surgical interventions [2]; however, they do not pose a long-term solution to treating osteoarthritis as they are only directed to alleviate the symptoms. A 3D bioprinted scaffold is expected to extend this long-term management by providing a more regenerative approach to treating the symptoms. While cartilage is most often associated with joint health, it is also found in other parts of the body, including intervertebral discs, nose, outer ear and meniscus. In the United States alone, there are over 800,000 meniscus repair surgeries to treat different types of meniscal tears [3]. Cartilage defects in the nose can arise for multiple reasons, including congenital malformation, trauma, and skin cancer removal surgery. One of the most common defects is a deviated septum, which affects 80% of the general population and can greatly impact respiration and aesthetics [4]. Surgeries to repair deviated septum often require either an allograft that may elicit unwanted foreign body responses or the harvesting of autologous tissue, which requires a secondary surgery. For cartilage tissue found within the ear, inherited external ear deformities include anotia (complete absence of the outer ear) and microtia (incompletely formed outer ear), and its incidence varies from 0.8 to 4.53 per 10,000 births globally [5]. Inherited anotia and microtia in children impact their hearing and overall social development and acceptance. Outer ear defects can also be acquired due to trauma, burns, or after skin cancer excision. These issues can severely impact the individual’s quality of life by affecting sound localization and hearing, cosmetic appearance, and the overall psychosocial well-being of the patient.
Tissue engineering emerges as a promising approach to address the high prevalence of these cartilage-specific injuries. By creating biological substitutes that restore, maintain, or improve tissue function, tissue engineering has the potential to provide more regenerative and long-term solutions compared to current repair strategies. However, the success of tissue engineering hinges on the ability to construct complex, three-dimensional (3D) structures that can mimic the native tissue environment. 3D bioprinting is an additive manufacturing technique that enables the precise deposition of biomaterials and bioactive components, such as cells, to create intricately patterned 3D constructs. This technology has shown great promise in the field of cartilage repair, offering control over scaffold design, porosity, and cellular composition. Within 3D bioprinting, biomaterials serve as the “ink”, often termed biomaterial inks (or bioinks when mixed with cells), in the context of bioprinting applications, providing the necessary support for cell attachment, proliferation, and differentiation. The choice of biomaterials is critical, as it must closely mimic the natural extracellular matrix (ECM) of cartilage to ensure optimal cell function and provide sufficient physical and mechanical properties of native cartilage tissue. The development and selection of suitable biomaterials is a key aspect of 3D bioprinting for cartilage repair, which will be the focus of this review paper. We also refer the readers to previous review papers in this field, including but not limited to McGivern et al. (2021) [6] providing a thoughtful overview of the translational applications of 3D bioprinting for cartilage tissue engineering, Szychlinska et al. (2022) [7] focusing on the use of naturally-derived bioinks from land and marine sources for cartilage tissue engineering, Liang et al. (2022) [8] highlighting the polymers used in hydrogel bioinks for articular cartilage repair, Zhou et al. (2023) [9] describing the key elements of 3D bioprinting and bionic strategies, and Turunen et al. (2023) [10] investigating the future solutions for osteoarthritis using 3D bioprinting of articular cartilage. While existing research in 3D bioprinting for cartilage repair is broad, the clinical translation of 3D bioprinted products remains limited. This review paper aims to provide a comprehensive overview of this rapidly evolving field, highlighting the latest advancements, challenges, and future directions, with a specific focus on clinical translation, existing products in the field, and the exciting frontier of 3D bioprinting and regenerative medicine in providing a more effective and long-term solution to cartilage-based injuries.
2 Cartilage tissue architecture and physiology
Cartilage is an avascular, flexible connective tissue found throughout the human body, providing support and cushion to surrounding cells. Cartilage tissue is found in various parts of the human body and is divided into three types: hyaline (or articular), elastic and fibrocartilage (Fig. 1).
2.1 Hyaline/articular cartilage
Hyaline cartilage, often termed articular cartilage, can be found in ball-and-socket joints, such as the hip, knee and shoulder, and plays a vital role in facilitating smooth limb movements [11]. Hyaline cartilage is comprised of specialized cells called chondrocytes, which maintain the cartilaginous matrix via the synthesis of collagen II and other ECM components consisting of glycosaminoglycans (GAGs), proteoglycans and glycoproteins, among other components. ECM composition defines each cartilage type with its unique physical characteristics, including GAGs that improve water retention and thus increase the shock-absorbing properties of cartilage tissue [12]. Specific to articular cartilage, there is a distinct zonation and architecture to the tissue that includes the superficial, middle and deep zones [13]. The outer region of the cartilage is a thin superficial layer that comes into contact with the synovial fluid and is characterized by the presence of a high number of flattened chondrocytes and parallel-oriented collagen fibers to the articular surface, providing resistance to shear stress and compression. Next, we find a transition layer called the middle zone, in which there are fewer spherical-shaped chondrocytes and collagen fibers obliquely organized that form the first line of resistance to forces of compression. The next layer is referred to as the deep zone, which has the lowest water concentration and contains thick collagen fibers arranged perpendicular to the articular surface and in parallel with columnar chondrocytes. Altogether, this zone provides the greatest resistance to compressive forces. After the deep zone, a calcified layer with very low cell density attaches the cartilage to the underlying bone. Because of this organization, articular cartilage is viscoelastic and can resist high, constant and cyclic loads.
2.2 Fibrocartilage
Fibrocartilage is primarily located within the meniscus tissue, intervertebral discs, and where ligaments and tendons attach [14]. The fibrocartilage tissue within the meniscus comprises various cell types: fibroblast-like cells in the outer meniscal region, chondrocyte-like cells in the inner area, and fusiform-like cells arranged parallel to the meniscal surface in the superficial zone. The ECM is abundant in water, glycosaminoglycans, and collagen [15]. Type I collagen, which makes up over 90% of the collagen content, is dispersed throughout the entire meniscus, from the peripheral to the inner area, and is organized into circumferential fibers. Conversely, Type II collagen is primarily found in the inner avascular zone, where it forms an organized network of circumferential and radial fibers. This collagen network is mainly responsible for the high tensile strength and resistance to daily joint movements. In addition to collagen, the matrix proteins include fibronectin, which regulates numerous cellular processes such as tissue repair, blood clotting, and cell migration/adhesion, and elastin, which works in conjunction with collagen fibers to provide tissue resilience.
2.3 Elastic cartilage
Elastic cartilage is a unique type of cartilage tissue only found in the head and neck area. Auricular cartilage is the elastic cartilage found within the ear and epiglottis [16], and it is similar to the other types of cartilage in that it is composed of chondrocytes and ECM rich in collagen II. The distinguishable flexible properties of elastic cartilage are given by the presence of chondrocyte-synthesized elastin fibers in the ECM, which intermingle with collagen II fibers and create a thread-like network that provides the tissue with the ability to bounce back to its original shape, even after a strong force is applied [16].
3 Existing approaches to cartilage repair
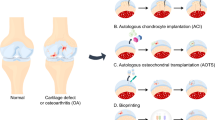
Beyond non-surgical pain management, the current treatment methods to repair cartilage lesions or defects are surgery (repair or removal), allografts or xenografts, autologous tissue grafting, injectable technology (i.e., platelet-rich plasma therapy, autologous stem cell therapy, donor stem cells) or implant technologies (Fig. 2). In many surgical cases, surgeons will either trim or remove the torn or injured cartilage tissue or repair it using suturing techniques. Other surgical techniques developed in an attempt to repair cartilage damage, such as abrasive chondroplasty, microfracture and spongialisation, lead to the formation of transient fibrocartilaginous tissue [17]. As an example, the meniscectomy procedure is currently the gold-standard surgical technique which removes damaged meniscus tissue through arthroscopy. While these routine surgeries can be successful and decrease the patient's discomfort, they are not long-term solutions. A recent study from 2023 evaluated the long-term implications of meniscectomies for patients suffering from OA compared to the patients choosing not to have the surgery, and the number of full knee replacements increased for OA patients who underwent arthroscopic meniscectomy within a ten-year follow-up period compared to the patients who did not have the meniscectomy procedure [18].
Synthetic or donor (allograft) cartilage can lead to infection and rejection, and the use of same-patient engraftment is limited by tissue loss in the donor site. Autologous tissue can be directly harvested from the patient and used in place of damaged or lost cartilage tissue. For example, in outer ear deformities, the current gold-standard treatment to repair external ear deformities involves the use of autologous tissue grafts, mainly extracted from the costochondral cartilage of the ribs [19]. Even though this technique is widely used, it comes with several disadvantages. It is a complex procedure where the surgeon must harvest sufficient amounts of costochondral cartilage and then shape it into something that resembles the outer ear, which extends the time of the procedure and leads to highly variable results depending on the surgeon's ability and experience. Moreover, the patient can experience donor-site morbidity. Other options include the use of synthetic implants made from non-absorbable polymers such as porous polyethylene (for example, Medpor); however, their use can lead to infection and complications, such as protrusion [20]. Another option is the use of xenografts, given the limited availability of donor tissue and the difficulty in matching these grafts to patients within a reasonable time. Osteochondral xenografts have the potential for filling hyaline cartilage defects in an experimental study that are mechanically strong and viable, however, inherent concerns of immunologic xenograft rejections after transplantations are possible [21].
Currently, there are several therapeutic approaches being used clinically for OA treatment. In a process called bone marrow stimulation (BMS), small holes are drilled into the bone underlying the cartilage, which allows mesenchymal stem cells from the bone marrow to migrate towards the cartilage and differentiate into chondrocytes to fill up the defect [22]. In autologous chondrocyte implantation (ACI), the patient’s chondrocytes are extracted from another region, expanded in the lab, and then re-injected in the defect site [23]. Instead of injecting chondrocytes alone, a collagen matrix is added, and the technique becomes known as matrix-associated autologous chondrocyte implantation (MACI) [24]. In severe OA cases, arthroplasty can be the only option where the diseased cartilage and bone are replaced with metal and polyethylene-based implants. Though these techniques are common, none of them have been shown to restore the native cartilage and stop the progression of OA. Additionally, limitations include derivation to fibrocartilage instead of hyaline cartilage, donor site morbidity, and damage to adjacent cartilage.
To overcome the limited regenerative capabilities of damaged cartilage, tissue engineers have directed their efforts to collect and expand autologous chondrocytes to generate cartilage that can be used to implant back into the patient. To be able to grow the cells in a three-dimensional manner, they use artificial scaffolds that serve as a mold for chondrocytes to grow following the specific shape that needs repair [25]. The problem with this approach is that commercially available scaffolds come in a finite range of shapes and sizes and are not personalized to the patient. Moreover, it is common that the cellular and matrix components of these constructs do not reflect the in situ composition of native tissue, thus resulting in different structural and functional properties of the target cartilage tissue.
Injectable methods, like ACI, are minimally invasive, often performed in an outpatient setting, have a quick recovery compared to surgical implantation procedures, and provide targeted delivery that can be precisely administered to the affected area [23]. On the contrary, injectable treatment options have limited durability, provide only temporary relief, do not promote significant cartilage regeneration, and require repeated injections over time, which are both painful and costly as a long-term solution. Implantable methods, like MACI, can provide more long-lasting relief, stimulate cartilage growth and regeneration, and can be tailored to fit the patient’s specific needs [24]. However, implantation requires surgery, which inherently comes with its own set of risks and often requires a longer recovery time for the patient, and these surgeries typically are more expensive than injectable treatment options.
Despite the advances in orthopedic surgeries and implant technology, treatment of cartilage damage remains challenging. Therapeutic strategies based on cell therapy and tissue engineering have emerged as a means to overcome limitations such as graft instability, calcification, and immune responses to foreign bodies.
4 Bioprinting applications in addressing clinical demands
The limited ability of cartilage to self-renew, coupled with the absence of effective drugs to impede OA progression or treat cartilage lesions, has spurred increased research efforts to identify more permanent treatments. An emerging biofabrication approach in cartilage repair involves 3D bioprinting of personalized biomimetic cartilage constructs to replace damaged tissue. This technique enables the integration of various cell types and diverse materials into a unified construct, a critical aspect for replicating the heterogeneous characteristics of cartilage within engineered scaffolds [26]. Bioprinting offers great potential for generating soft scaffolds with mechanics, chemistries, and micro/nanostructures that better mimic the native ECM because biomaterials and bioactive components are deposited into a controlled and pre-defined 3D geometry. This process enables mimicking the complexity found within native human tissues. From anatomy to functional tissue regeneration, this defined organization leads to a proper environment and mechanical support that can guide functional regeneration. The mimicry of macroscopic and microscopic anatomy is imperative for successfully reproducing tissue-specific and physical characteristics.
The process of using 3D bioprinting for cartilage repair involves several key steps, integrating various technologies and materials (Fig. 3). It begins with the design and generation of a digital file readable by the 3D printer. Files used for constructing medical implants are commonly derived from Computer Tomography (CT) or Magnetic Resonance Imaging (MRI) scans or may be created from scratch using Computer-Aided Design (CAD) software. Once the model is crafted, it is converted into a standard 3D printer file format (.STL), representing the outer surface of the modelled object as interconnected triangles. Then, to guide the 3D printer on what to construct within this surface, the object undergoes slicing into printable 2-dimensional planes using specialized slicing software, ultimately saved as a GCODE file.
During the subsequent stage of the pre-processing process, appropriate cell types and encapsulating biomaterials are selected. Chondrocytes or mesenchymal stem cells (MSC) from the patient are isolated and then cultured in vitro, allowing them to proliferate until an adequate cell quantity is achieved. The cells are seeded within the biomaterial to create a cell-laden bioink for printing [27]. In the bioprinting phase, the tissue construct is printed utilizing one of the various available printing techniques. Numerous 3D bioprinting methods have been devised, encompassing extrusion, droplet, and laser-based bioprinting. Notably, extrusion-based 3D bioprinting has gained popularity due to its compatibility with a wide range of hydrogel bioinks. The printing parameters, including nozzle size, printing speed, pressure, and layer thickness, are optimized for precise and accurate deposition [28]. After or during the layer-by-layer deposition, the printed structure needs to be instantly solidified or cross-linked to ensure structural integrity. Methods include physical cross-linking through temperature modulation or chemical cross-linking using cross-linking agents [29, 30].
Following the printing phase, additional processing is typically necessary to prepare the printed construct for use. This often involves in vitro cell differentiation, potential removal of sacrificial supports, and allowing the tissue construct to mature either in a bioreactor or through specialized cell culture techniques like air–liquid interfaces to promote cell differentiation, matrix production, and tissue development [27]. Before implantation, the constructs' appearance, strength, and functionality must be assessed by analyzing cell viability, immunohistochemistry, tissue morphology and mechanical properties of the matured tissue [31].
5 Biomaterials used in 3D bioprinting and cartilage repair
The selection of bioink material is crucial as it must replicate the intricacies of the native ECM while exhibiting appropriate physicochemical properties that are conducive to the printing process. Printability encompasses various material characteristics that contribute to the efficiency and precision of the printing procedure. A crucial factor is the tuning of the bioink's viscosity, achievable either through temperature modulation or shear thinning, enabling the bioink to be dispensed smoothly from the print head nozzles despite higher shear rates within the extrusion process. Conversely, the bioink should promptly solidify post-extrusion, achieved through either physical or chemical cross-linking, guaranteeing the structural integrity of the printed construct [29].
Various natural and synthetic biomaterials have undergone assessment as bioinks for repairing cartilage tissue, whether in vitro and/or in vivo, or used directly in commercial products (Table 1). These materials encompass carbohydrate-based natural polymers like alginate, chitosan, agarose, hyaluronic acid, and dextran, as well as protein-based polymers such as gelatin, fibrin, and collagen. Additionally, fully synthetic polymers like polyglycolic acid (PGA) and polylactic acid (PLA) have been utilized in printing. While natural polymers exhibit good hydrophilicity, biocompatibility, safety, and biodegradability, their mechanical performance is suboptimal for cartilage and bone repair. Efforts have been made to enhance the mechanical properties of hydrogel-based bioinks through physical and chemical cross-linking, involving the addition of nanoparticles, composite materials, or inorganic ingredients to the ink [32, 33].
Synthetic polymers, on the other hand, offer favorable mechanical properties, tunability, and stability, but their hydrophilicity and cell compatibility are inferior to those of natural hydrogels (Table 2) [34, 35]. The ideal bioink material is expected to possess low viscosity, stiffness, and cross-linking degree to facilitate efficient cell proliferation, differentiation, migration, growth factor permeability, nutrient diffusion, and tissue formation. Simultaneously, the same ink should exhibit adequate viscosity, a high cross-linking degree, and adequate stiffness to ensure precise dimensional printing and support its own weight. However, outstanding rheological and mechanical properties may not support cell growth and matrix deposition, leading to a trade-off between biocompatibility and printability [27]. Constant efforts are underway to develop novel bioinks with simultaneous biocompatibility and printability by adjusting the physical, chemical, and biological properties of polymers.
Cartilage bioprinting introduces specific limitations to bioink selection. The chosen bioink must create a suitable environment for stem cell differentiation or the maintenance of printed chondrocyte phenotypes. It should also facilitate the deposition of neo-ECM with a high degree of GAGs and collagen type I/II. The bioink should also exhibit mechanical properties that match or closely mimic those of native cartilage. This includes factors such as stiffness, elasticity, and compressive strength, which are essential for providing mechanical support and functionality to the regenerated cartilage tissue. Depending on the intended application, the bioink should have controllable degradability to allow tissue remodeling and integration with the host tissue over time. Furthermore, bioinks used for cartilage repair must adhere to regulatory standards and guidelines to ensure their safety and efficacy for clinical applications. However, the ideal formulation for cartilage bioprinting remains somewhat elusive, but it is increasingly evident that bioinks may benefit from the addition of biofunctional nanoparticles to enhance performance [32]. The addition of nanoscale materials, such as nanoparticles, to inks has enabled the modification of their rheological, biological, and structural properties during and after printing. Furthermore, the selection of bioink is constrained by the chosen printing strategy, such as extrusion-based, droplet-based, or laser-based bioprinting.
5.1 Natural polymers
Natural polymers are widely employed as bioinks for cell-friendly 3D construct printing due to their biocompatibility, biodegradability, and similarity to the ECM of living tissues [36, 37]. Some of the commonly used natural polymers in bioprinting include collagen, agarose, gelatin, alginate, chitosan, cellulose, fibrinogen, and hyaluronic acid.
5.1.1 Collagen
Collagen is a fundamental protein found abundantly in the ECM of various tissues, making it a vital component for tissue engineering and regenerative medicine applications [38]. In the context of bioprinting, collagen-based materials have attracted significant attention due to their biocompatibility, bioactivity, and ability to mimic the native tissue environment [39]. Collagen possesses excellent biodegradability and promotes cell adhesion, proliferation, and differentiation, making it an ideal material for bioprinting scaffolds [40]. These scaffolds provide structural support to the printed cells, allowing them to organize and grow in a 3D manner that closely resembles the native tissue architecture [41]. Furthermore, collagen-based bioinks can be tailored to mimic the mechanical properties of different tissues, enhancing their functionality in bioprinting applications, but are often limited in the mechanical properties required for cartilage-based repair. Researchers have explored various techniques to improve the printability and mechanical properties of collagen-based bioinks. For instance, the addition of cross-linking agents such as genipin or glutaraldehyde enhances the stability and mechanical strength of printed constructs [42]. Moreover, blending collagen with other biopolymers, such as alginate or gelatin, can improve the structural integrity and control the degradation rate of the printed scaffolds [43]. Besides the favorable characteristics of collagen, it can be extracted from several sources, making it one of the most abundant ECM-derived materials. Animal skin is one of the common sources of collagen [44]. Marine collagen from fish has gripped the research attention because of its excellent absorption properties, low molecular weight, biocompatibility, little risk of disease transmission from animals to humans, and easy extraction [45]. Several companies, like Jellagen, have pushed the boundaries of the capabilities of marine-derived collagen for tissue engineering applications. From a structural perspective, collagen is classified into more than 28 types according to the arrangement of their polypeptide chains and variations in their terminal groups, besides variations in the lengths of the helical regions and distributions of non-helical segments [46, 47]. Of all the extracellular matrix proteins in vertebrates, fibrillar collagens are the most plentiful. They impart stability, connectivity, and structure to tissues and organs [48, 49]. The fibrillar collagen found in greatest abundance in most tissues is type I collagen. It is located predominantly on fibril surfaces and in the connective tissues of skin and bone [50, 51]. Biocompatibility, biodegradability, and low antigenicity of collagen make it an attractive material for various applications, including cartilage repair and regeneration.
5.1.2 Alginate
Alginate is a natural polymer derived from seaweed, specifically brown algae. It is widely used in bioprinting due to its biocompatibility, biodegradability, and ability to form hydrogels by simply mixing it with divalent cations such as calcium ions to form stable hydrogels that provide structural support to printed cells [52]. The gelation process can be controlled to achieve desired mechanical properties, such as stiffness or elasticity, by adjusting the concentration of alginate and the cross-linking parameters [53]. One of the key advantages of alginate is its ability to encapsulate cells within the bioink due to its cell-friendly cross-linking/gelation process. This enables the creation of complex 3D structures with embedded cells, which is crucial for fabricating functional tissues [54]. Alginate-based bioinks can support cell viability, proliferation, and differentiation, making them suitable for a wide range of tissue engineering applications, including cartilage, bone, and vascular tissue regeneration [52]. For example, O'Connell's group studied the use of agarose-alginate composites as bioinks for the 3D printing of cartilage constructs and found that the ink composition with the best rheological properties for bioprinting was a 5.0% wt/vol bioink composed of alginate and agarose in a 2:3 ratio [55, 56]. It was reported that the hydrogel nature of alginate provides a biomimetic environment for chondrocytes and mimics the proteoglycan-rich extracellular matrix of native cartilage [57]. To improve the printability, mechanical properties, and long-term stability of alginate bioinks, recent research has formulated composite systems by blending alginate with other natural biopolymers, i.e., gelatin [58], hyaluronic acid [59], and nanocellulose [60]. Chemical modifications like oxidation of alginate to form aldehyde groups have also been done to enable covalent cross-linking with gelatin through Schiff base reaction [57]. Composite bioinks containing laponite nanoclay [61], graphene oxide [62], or hydroxyapatite nanoparticles [63] have been developed to increase mechanical strength. Overall, the research demonstrates that the modulus and stability of alginate bioinks can be tailored to match the properties of articular cartilage or intervertebral discs through careful formulation design. Encapsulated chondrocytes and MSCs show viability, proliferation and deposition of cartilage-specific extracellular matrix in these optimized alginate-based bioinks [64].
5.1.3 Cellulose
Cellulose is a biopolymer composed of glucose units and is the main structural component of plant cell walls. It has gained significant interest in the field of bioprinting due to its abundance, biocompatibility, and potential as a sustainable biomaterial [65]. Cellulose-based materials offer several advantages for bioprinting applications. They possess excellent mechanical properties, such as high stiffness and tensile strength, which make them suitable for fabricating scaffolds with structural integrity [66]. Cellulose can be processed into various forms, including hydrogels, nanofibers, and microfibers, enabling the creation of bioinks with different viscosities and rheological properties. In bioprinting, cellulose-based bioinks can be used to create complex tissue structures. Cellulose fibers can act as a structural framework, providing support and guiding cell organization and tissue formation [67]. The bioink formulation can be optimized by incorporating cells, growth factors, or other bioactive agents to enhance cellular activities, such as proliferation and differentiation [68]. One of the challenges in working with cellulose-based bioinks is their limited printability, as they often exhibit high viscosity and poor shape fidelity during the printing process. Researchers have addressed this by modifying cellulose materials or combining them with other polymers to improve their printability and shape retention [69]. For example, blending cellulose with alginate or gelatin can enhance the mechanical properties and printability of the bioinks [70].
5.1.4 Agarose
Agarose is a linear polysaccharide derived from seaweed that has been widely used as a biomaterial for tissue engineering applications. Its ability to form thermally reversible gels has made it a popular material for bioprinting techniques that employ thermal gelation mechanisms [71]. Agarose hydrogels have been used as bioinks in extrusion-based bioprinting to fabricate scaffolds containing living cells. For example, a recent study by Yu et al. utilized agarose/alginate bioinks to bioprint tubular constructs containing cartilage-derived stem cells [72]. The agarose provided structural support for the printed constructs while allowing for cell viability and proliferation. Additionally, agarose hydrogels have been used to encapsulate cells during laser-induced forward transfer techniques [73]. Agarose has also been combined with nanocellulose to create bioinks optimal for cardiac tissue engineering. The agarose–nanocellulose bioink was used to bioprint heart valve conduits containing human coronary artery smooth muscle cells [67]. In another study, Oliver-Ferrándiz reported the use of agarose in combination with alginate to form alginate-agarose hydrogel mixed with human dental pulp stem cells (hDPSCs), suitable for cartilage regeneration [74]. Overall, the ability to tailor and tune the physical and biological properties of agarose hydrogels makes them a versatile bioink choice for extrusion and laser-based bioprinting approaches toward engineering various tissues.
5.1.5 Fibrinogen
Fibrinogen is a protein derived from blood plasma that has emerged as a promising biomaterial ink for bioprinting approaches [75]. Fibrinogen can form hydrogels when mixed with thrombin, which cleaves fibrinogen into self-assembling fibrin fibers. The ability to tune the structural and biofunctional properties of fibrinogen hydrogels makes it an interesting bioink material. However, limitations remain regarding the long-term stability and late-stage gelation [76]. Further modification of fibrinogen-based bioinks could enable more advanced bioprinting of vascularized tissue constructs [77]. For example, one study performed methacrylation on fibrinogen to design a new biomedical hydrogel for 3D cell culture or as a biodegradable delivery matrix for in vivo implantation [76]. The methacrylation did not alter important biological attributes of the fibrinogen, including the ability to support cell adhesion and 3D cell culture, as well as undergo proteolysis. Animal experiments confirmed the biodegradability of the methacrylated fibrinogen hydrogel for potential use in tissue engineering, 3D bioprinting, or as a biodegradable matrix for in vivo sustained delivery. Another recent study prepared degradable, tunable, and biocompatible fibrinogen-keratin hydrogels for controllable protein delivery [77]. The hydrogels presented promising biological performance, indicating suitability as a controlled protein delivery carrier. To overcome mechanical limitations, researchers have blended fibrinogen with other biomaterials to create bioinks with improved mechanical properties and shape fidelity [78, 79]. For example, one study developed a fibrinogen-alginate bioink for skin bioprinting [80], while another reported the printability of fibrinogen-based bioinks through mixing and in situ cross-linking [79]. These demonstrate the potential of fibrinogen as a suitable biomaterial ink, especially when combined with other biomaterials, like alginate.
5.2 Synthetic polymers
5.2.1 Polycaprolactone
Polycaprolactone (PCL) is a biodegradable, biocompatible, and printable polymer generated by cationic and anionic ring-opening polymerization of ε-caprolactone and in the presence of a suitable catalyst [81]. Because of its properties, the Food and Drug Administration (FDA) has approved this material for medical devices [82]. It displays considerably high but adjustable mechanical strength and a long degradation time, making it an appropriate material to use as a supporting device, especially for hard tissue, tissue engineering, surgical sutures, and drug delivery vesicles [83]. The degradation time can be tuned by modifying its molecular weight, thus expanding its potential for use in engineering soft tissues. PCL is semicrystalline at room and human body temperatures, with its amorphous chains in a random arrangement. This allows the free movement of PCL chains, increasing its metabolite permeability once implanted into the body. Some disadvantages of this polymer are its hydrophobicity, the lack of support for cell adhesion, and the inability to directly bioprint due to the high melting temperature (> 60 °C) [84].
5.2.2 Polyurethane
Polyurethanes (PUs) are a group of polymers with a urethane moiety as their repeating unit. PUs are created by the reaction of diisocyanate, oligodiol (i.e., macrodiol or polyol), and a chain extender (i.e., diol or diamine [85]). PUs are widely used in biomedical materials due to their biocompatibility, easily adjustable chemical and mechanical properties, and biodegrading ability [86]. They show high load-bearing capacity, tear resistance, and flexibility [87]. They also exhibit moderate compatibility with blood. All these features can be synthetically tailored to fit specific tissues [88], which makes them suitable for a wide range of biomedical applications. However, one drawback is that PUs are not biostable [89], which makes them less appropriate for the long-term replacement of cartilage tissues.
5.2.3 Polylactic acid
Polylactic acid (PLA) is a biopolymer made from fermented carbohydrates that is biodegradable, biocompatible, and nontoxic [90]. PLA is non-toxic and breaks down slowly into non-toxic and non-tissue-reactive compounds that can be fully metabolized, paving the way for biomedical uses. PLA's properties and bio-functionality can be enhanced by copolymerizing PLA with other polymers. For instance, scaffolds from composites of PLA and poly(glycolic acid) (PGA) were reported to have adjustable degradation rates ideal for regenerating musculoskeletal tissues [91]. Also, adding inorganic hydroxyapatite makes PLA mimic bone's extracellular matrix better, improving bone cell adhesion and conductivity to overcome PLA's low cell adhesion potential [92, 93]. A study by Moran et al. [94] coated PGA meshes with varying PLA content (0–68%) to examine PLA/PGA composite scaffold physical characteristics and chondrocyte interactions for cartilage engineering. Increasing PLA fraction linearly augmented scaffold compressive modulus (up to 20 kPa) and degradation time (up to 45 days) while reducing cell seeding efficiency from 48 to 27%. Furthermore, a moderate 27% PLA content balanced enhanced mechanical stability with maintained chondrocyte expansion over 4 weeks.
On the other hand, PLA lacks sufficient cell adhesion properties, which can prevent its use for cell growth applications. However, this shortcoming can be addressed by blending PLA with biopolymers, like hyaluronic acid. Hyaluronic acid enables robust cell attachment and proliferation through electrostatic interactions with cell surface proteins [95]. The improved cellular properties allow the composite to support bone cell proliferation for enhanced healing [92, 93]. Another example of copolymers is PLA/lignin. Lignin is a biopolymer that possesses several favorable properties for biomedical uses, including antimicrobial, antioxidant, anti-ultraviolet, biocompatible, and non-toxic activities [96]. Due to these attributes, lignin has great potential for integration with PLA to generate composite biomaterials [97]. Specifically, PLA/lignin composite nanofiber scaffolds prepared by electrospinning have emerged as promising candidates for cartilage and bone tissue engineering applications. The lignin component enhances the mechanical strength, thermal stability, UV radiation resistance, and oxidative stress resilience of PLA-based materials [98, 99]. Beyond electrospun scaffolds, PLA/lignin composite films have also been fabricated through physical mixing methods. These composites displayed compatibility with stem cells, indicating their potential for diverse regenerative medicine applications [100].
5.2.4 Silicone elastomers
Silicone elastomers are versatile polymeric materials made by cross-linking silicone polymer chains along with reinforcing agents, catalysts, and curing processes to yield materials with differing properties like heat-cured rubber, liquid silicone rubber, and room-temperature vulcanized rubber [101, 102]. Silicone polymers can form various materials, including elastomers, gels, and adhesives, depending on chemical formulation and processing conditions. Key advantages are thermal stability from -40 to 185 °C and retention of mechanical properties across this wide temperature range. Silicone elastomers exhibit high resistance to UV, heat, and chemicals, good flame resistance, electrical properties, and steam sterilizability [103].
Silicones are optically transparent, permeable to gases and moisture, and simple to fabricate into different configurations like tubing and seals. Silicone adhesives demonstrate strong skin adhesion without irritation along with high gas permeability suited for biomedical device applications [104]. Silicone has demonstrated utility in wound healing and bone defect treatments. Specific concentrations of silicon ions increased bone marrow stem cell proliferation and mineralized matrix deposition [105]. Other studies found silicone compounds stimulated osteoblast proliferation and expression of bone-related proteins, highlighting silicone's role in bone growth [106]. Additional in vitro and animal research has shown silicone works synergistically with calcium for bone health and metabolism [107, 108]. Hybrid composites made from biodegradable PCL and silica aerogel have promise as optimized bone engineering scaffolds [101, 102]. Silicone-containing PCL hybrids displayed robust cell viability and tissue integration, indicating potential for bone regeneration applications [109]. Silica aerogel scaffolds were also synthesized using tetraethoxysilane (TEOS) or methyltrimethoxysilane (MTMS) precursors prior to PCL incorporation. The resulting composites exhibited hydrophobicity, minimal swelling and mass loss, and stability under physiological conditions [110, 111]. They were able to maintain human osteoblast culture viability for a long time, confirming excellent cytocompatibility and potential for articular cartilage regeneration, especially at the cartilage-bone interface [112].
5.2.5 Polyethylene glycol
Polyethylene glycol (PEG) polymers have gained considerable attention for biomedical uses due to their favorable properties, including injectability, lack of cell adhesion, biocompatibility, and low immunogenicity [113, 114]. PEG hydrogels are formed by cross-linking PEG polymer chains into fluid-filled 3D networks with excellent swelling capacity [115]. One advantage of PEG is its easy modification, as it can be functionalized with bioactive molecules to enable drug delivery applications. For instance, the reactive hydroxyl terminal group readily conjugates with drug compounds, yielding PEG-drug conjugates suitable for controlled release [116, 117].
Although PEG possesses cell-friendly characteristics that encourage its use in tissue engineering, some limitations exist, such as low degradability under physiological conditions and susceptibility to rapid aerobic degradation [118, 119]. Researchers have developed several modifications to PEG polymers to enhance degradation and tailor them for specific biomedical uses. PEG-based hydrogels have been shown to exhibit exceptional ability to induce bone growth when combined with osteogenic materials [120]. Hydroxyapatite, an inorganic component of normal bone, has great compatibility with bone tissue and outstanding bone conductivity [121]. Incorporating HA directly into PEG hydrogels imparted osteoconductive properties to the composite material. Nejadnik et al. demonstrated that incorporating HA nanoparticles substantially increased the mineralization of PEG gels [122]. Similarly, calcium phosphate nanoparticles, composed of varying proportions of calcium and phosphate ions, were also found to boost the bone growth potential of crosslinked PEG hydrogels. An injectable composite gel was synthesized by reacting four-arm sulfhydryl-PEG with calcium phosphate nanoparticles. This gel increased extracellular matrix mineralization and alkaline phosphatase activity in mouse osteoblast precursors. Furthermore, it markedly improved the healing of critical skull defects in rat models [123].
PEG hydrogels have shown promise for both bone and cartilage regeneration. Composite PEG-hydroxyapatite or PEG-calcium phosphate hydrogels exhibit osteoconductivity and promote mineralization and bone growth [124]. Additionally, swelling and mechanical properties of PEG hydrogels can be tuned through molecular weight and cross-linking density changes to successfully mimic the behavior of cartilage tissue [125]. The introduction of biodegradable oligolactic acid segments into PEG networks produced hydrogels that slowly degrade to favorably provide space for chondrocyte expansion and cartilaginous matrix deposition [126, 127].
5.2.6 Ceramics
Ceramics, particularly bioglass and lithium calcium silicate, are promising materials in the field of cartilage repair. In 2016, researchers from Imperial College London and the University of Milano-Bicocca developed a new bioglass material composed of inorganic silica and PCL [128]. This new formulation mimics the shock-absorbing and load-bearing qualities of native cartilage and can be 3D printed into the exact size and shape needed. When implanted, the structure, stiffness, and chemistry of the bioglass encourage cartilage cells to grow through microscopic pores. Similarly, lithium calcium silicate (LCS) has been used to prepare scaffolds that promote cartilage maturation. While LCS is known for its stability and bioactivity, it has been found to promote chondrocyte maturation by immunomodulating M2 macrophage polarization [129], shifting to anti-inflammatory M2 phenotypes and promoting the proliferation, migration, and maturation of chondrocytes. Moreover, LCS can be 3D printed for osteochondral regeneration. In a study by Chen et al., LCS scaffolds were prepared using a sol–gel method and further processed using 3D printing. The compressive strength of the 3D printed scaffold could be controlled in the range of 15–40 MPa when the pore size was varied from 170 to 400 µm [130]. These scaffolds showed controllable biodegradability and good apatite-mineralization ability.
5.2.7 Composite materials
Composite materials are a strong focus area in cartilage repair due to the complex nature of cartilage itself. Composites, often a combination of synthetic and natural materials, can replicate the structural, mechanical and biological properties of cartilage, providing a conducive environment for cell attachment and nutrient exchange. While hydrogels are advantageous in mimicking the ECM of native cartilage, they often suffer from low mechanical properties, which can limit their applications in cartilage repair due to the inherent high compressive and tensile force requirements. Increasing efforts have been made to improve the mechanics of natural hydrogel bioinks (Fig. 4), including applying multi-material scaffolds, chain entanglements, interpenetrating networks, latent cross-linking, printing optimization and nanoparticle incorporation. For example, a study published in 2020 demonstrated the first hydrogel with the strength and modulus of native cartilage, with cartilage-equivalent tensile fatigue strength at 100,000 cyles [131]. This hydrogel is composed of a bacterial cellulose (BC) nanofiber network combined with a poly(vinyl alcohol) (PVA)-poly(2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt) (PAMPS) double network hydrogel. The nanofiber network mimics the native collagen fiber network in cartilage, while the double network hydrogel provides a fixed negative charge with an osmotic restoring force similar to aggrecan in cartilage. Engineering hydrogels with similar stiffness becomes essential for cartilage stem and progenitor cell differentiation. A recent study published in 2023 reports the use of chain entanglements to significantly stiffen protein-based hydrogels without compromising their mechanical strength [132]. Polyprotein (FL)8 consists of eight tandem repeats of a ferredoxin-like protein, which is introduced for chain entanglement into an otherwise soft protein hydrogel to significantly increase its stiffness. The layered architecture of hyaline cartilage can be more precisely tuned with multi-material scaffolds (i.e., varying ratios of methacrylated gelatin and nanohydroxyapatite [133]), where three compositions reflect the three distinct layers based on water absorption, biodegradation and mechanical properties. Interpenetrating networks of different photocrosslinkable inks (i.e., the mixture of polyethylene glycol diacrylate, gelatin methacryloyl, and chondroitin sulfate methacrylate [134]) can balance the mechanical properties and suitable 3D microenvironment for cartilage repair. Manufacturing plays an important role in shaping the chosen biomaterials into porous 3D structures that mimic the cartilage’s macro and microarchitecture.
6 From the bench to the clinic
The clinical translation of 3D bioprinting in cartilage repair faces several challenges. The limited spatial complexity of tissue-engineering implants in terms of cells, materials, and active factors has hindered the success of engineered cartilage [135, 136]. Despite numerous breakthroughs and the use of cell seeding techniques post-printing of constructs, there are still no commercial products using 3D bioprinted cartilage tissue where cells or other biologically active components are directly co-printed during the manufacturing process (Table 3). Regulatory concerns include the need for information regarding the use of additives in materials or material composition for the intended use, the verification of the software for the bioprinting design, the method of sterilizing the process or final product, and the bioprinting process to provide accurate and high-quality products that will not harm the patient [137]. Additionally, current process challenges include the difficulty of fabricating customizable implants in situ using 3D bioprinting directly onto the defect site (without the need for transplantation or external fabrication), the quality control behind ex vivo constructs, and the overall need for optimization and standardization of the process for faster translation into the clinic. Material considerations involve the determination of the optimal bioink composition to maintain the physical properties of native cartilage tissue while ensuring improved regeneration over time, ultimately providing a more long-term solution to current treatment options for cartilage-based injuries or degeneration due to the regenerative potential. By precisely layering cells, growth factors, and biocompatible materials, 3D bioprinting creates functional constructs that mimic cartilage anatomy, promoting tissue regeneration and durability. The combination of regulatory, process, and material considerations is the current roadblock in translating 3D bioprinting as a manufacturing technique into the clinic for cartilage repair [138].
As seen in Table 3, several products are commercially used for cartilage repair, but only one company, 3D BioTherapeutics, stands out for using 3D bioprinting as the manufacturing technique for fabricating personalized cell-laden cartilage constructs in clinical trials. The New York-based company is a clinical-stage biotechnology company specializing in using their 3D bioprinting and material technologies to fabricate safe, functional, and personalized living tissues and organs for patients. Their first product, called AuriNovo™, is a patient-specific, living tissue implant created using 3D bioprinting technology for surgical reconstruction of the external ear in people born with microtia Grades II-IV. The implant is a collagen hydrogel scaffold encapsulating the patient’s own auricular cartilage cells that are bioprinted in the exact size and shape to match the patient’s opposite ear, aiming to provide a viable treatment alternative to rib cartilage grafts and traditionally used synthetic materials. The clinical trial for AuriNovo™ started on August 9, 2021, however, it was terminated due to a company decision unrelated to safety [139]. In general, clinical trials may need to end early for several reasons. First, if a trial demonstrates clear benefit in one arm of the study, it may be terminated to avoid exposing participants to an inferior treatment. Second, funding issues, low patient recruitment, and emerging safety or efficacy signals could also prompt early termination of a clinical trial [140].
7 Conclusions and future perspectives
The clinical translation of 3D bioprinting for cartilage repair is primarily influenced by the material and regulatory challenges that present themselves in mimicking the complexity of native cartilage tissues. The choice of biomaterials that integrate strength and stability with excellent biocompatibility is highly desirable for cartilage tissue engineering. Natural polymers, like collagen and chitosan, among others, provide an excellent platform for cells. However, these polymers and representative scaffolds lack the mechanical strength and durability required for native cartilage tissue. On the other hand, synthetic polymers, like polycaprolactone or silicone-based materials, have excellent mechanical properties but limited cellular adhesion and cellular integration capabilities. The explorations of composite materials and blended biomaterials emerge as a necessity to achieve the delicate balance between mechanics and biocompatibility required for successful cartilage repair. These biomaterial choices are imperative for mimicking the physical and cellular properties of the scaffold, but they do not recapitulate the architectural complexities within the native tissue. Additive manufacturing techniques, like 3D bioprinting, have emerged with their precise positional capabilities as a tool for fabricating pre-defined macro and micro-architectures. 3D bioprinting enables not only the layer-by-layer printing of both the chosen biomaterials and cells using pre-defined outer dimensions, but also provides a means for structuring the internal geometries. This manufacturing approach allows for more accurate reproducibility and has the opportunity to capture the internal dimensional control that is inherent to native cartilage tissue. In fact, the three types of cartilage tissue have distinct differences in the ECM composition and must be taken into account when providing advanced cartilage tissue engineering strategies.
Transitioning 3D bioprinting for cartilage repair from pre-clinical promise to clinical reality involves overcoming multifaceted challenges. Process and quality control mechanisms are important to ensure the reproducibility and reliability of bioprinted cartilage constructs. Regulatory hurdles, although formidable, underscore the need for standards that guarantee both safety and efficacy for both the manufacturing process (3D bioprinting) and the chosen bioink formulation. With no 3D bioprinted products currently available on the market, the regulatory burden presents a significant roadblock in the clinical translation. Traditional regulatory frameworks are designed for mass-manufactured therapies, not personalized solutions that are patient-specific. This presents a challenge in classifying these products and establishing standardization. The inclusion of living cells in the fabrication process adds another layer of complexity, making risk assessments more challenging. Emerging bioprinted products fall under the Class III (highest risk) category within the FDA and require extensive clinical trials to ensure the safety of the products over time. With the requirements of the premarket approval (PMA), this regulatory route is longer and more expensive compared to the Class II, 510(k) pathway, with additional hurdles such as patient recruitment. Specific frameworks for the regulation and testing of 3D bioprinted treatments are still limited, but provide an exciting opportunity for regulatory bodies to adapt and evolve to accommodate the unique aspects of 3D bioprinting in medicine.
In conclusion, the clinical translation of 3D bioprinting for cartilage repair holds immense potential, provided we navigate the complex landscape with a clear understanding of the challenges at hand. The need for collaboration among researchers, clinicians, regulatory bodies, and industry partners is necessary to enable these emerging products into the clinic. With attention to material intricacies, stability, and the demanding clinical pathway, the promise of 3D bioprinting for cartilage repair can transition from hopeful anticipation to next-generation treatment options.
References
Arthritis Foundation, Osteoarthritis, https://www.arthritis.org/diseases/osteoarthritis. Accessed 11 Apr 2024
M. Maqbool, G. Fekadu, X. Jiang, F. Bekele, T. Tolossa, E. Turi, G. Fetensa, K. Fanta, An up to date on clinical prospects and management of osteoarthritis. Ann. Med. Surg. 72, 103077 (2021)
Mullaney, T, The most common knee surgery for seniors is costly, and usually a waste. CNBC: 2018, https://www.cnbc.com/2018/04/05/knee-surgery-for-seniors-is-costly-and-usually-a-waste.html. Accessed 11 Apr 2024
Stanford Medicine, Deviated Septum Stanford Medicine, Otolaryngology - Head and Neck Surgery, https://med.stanford.edu/ohns/OHNS-healthcare/sinuscenter/resources/patient_guides/deviated-septum.html. Accessed 11 Apr 2024
D.V. Luquetti, E. Leoncini, P. Mastroiacovo, Microtia-anotia: A global review of prevalence rates. Birth Defects Res. A 91(9), 813–822 (2011)
S. McGivern, H. Boutouil, G. Al-Kharusi, S. Little, N.J. Dunne, T.J. Levingstone, Translational application of 3D bioprinting for cartilage tissue engineering. Bioengineering 8(10), 144 (2021)
M.A. Szychlinska, F. Bucchieri, A. Fucarino, A. Ronca, U. D’Amora, Three-dimensional bioprinting for cartilage tissue engineering: insights into naturally-derived bioinks from land and marine sources. J. Function. Biomater. 13(3), 118 (2022)
Q. Liang, Y. Ma, X. Yao, W. Wei, Advanced 3D-printing bioinks for articular cartilage repair. Inter. J. Bioprinting 8(3), 511 (2022)
J. Zhou, Q. Li, Z. Tian, Q. Yao, M. Zhang, Recent advances in 3D bioprinted cartilage-mimicking constructs for applications in tissue engineering. Mater. Today Bio 23, 100870 (2023)
S. Turunen, T. Kalpio, C. Lindahl, C.J.M. Shanthinathan, T. Akhter, S. Concaro, S. Simonsson. Future solutions for osteoarthritis using 3D bioprinting of articular cartilage. In Handbook of Surgical Planning and 3D Printing, Elsevier, 335–369 (2023)
C.B. Carballo, Y. Nakagawa, I. Sekiya, S.A. Rodeo, Basic science of articular cartilage. Clin. Sports Med. 36(3), 413–425 (2017)
Y. Liu, K.M. Shah, J. Luo, Strategies for articular cartilage repair and regeneration. Front. Bioeng. Biotech. 9, 770655 (2021)
A.J. Sophia Fox, A. Bedi, S.A. Rodeo, The basic science of articular cartilage: structure, composition, and function. Sports Health 1(6), 461–468 (2009)
M. Benjamin, E. Evans, Fibrocartilage. J. Anat. 171, 1–15 (1990)
G.-K. Tan, J.J. Cooper-White, Interactions of meniscal cells with extracellular matrix molecules: towards the generation of tissue engineered menisci. Cell Adhesion Migration 5(3), 220–226 (2011)
C. Chung, J.A. Burdick, Engineering cartilage tissue. Adv. Drug Deliv. Rev. 60(2), 243–262 (2008)
C. Vinatier, J. Guicheux, Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Annal. Phys. Rehab. Med. 59(3), 139–144 (2016)
C.M. Park, S. Ryoo, M. Choi, S.J. Lee, J.J. Yoo, H.A. Kim, Total Knee Replacement After Arthroscopic Meniscectomy in Knee Osteoarthritis: A Nationwide Population-Based Cohort Study. J. Korean Med. Sci. 38(1), e6 (2023)
B.P. Cohen, J.L. Bernstein, K.A. Morrison, J.A. Spector, L.J. Bonassar, Tissue engineering the human auricle by auricular chondrocyte-mesenchymal stem cell co-implantation. PLoS ONE 13(10), e0202356 (2018)
K. Ali, J.G. Trost, T.A. Truong, R.J. Harshbarger III. Ear Reconstruction: Total Ear Reconstruction Using Porous Polyethylene. In Seminars in plastic surgery, Thieme Medical Publishers 31:161 (2017)
K.E. Birdwhistell, S.P. Franklin, D.J. Hurley, B.D. Heins, J.F. Peroni, Osteochondral allograft and xenograft immunogenicity decrease following ex vivo tissue culture. J. Cart. Joint Preser. 3(4), 100115 (2023)
L. Zhang, Y. Zhu, T. Xu, W. Fu, Bone marrow stimulation in arthroscopic rotator cuff repair is a cost-effective and straightforward technique to reduce retear rates: A systematic review and meta-analysis. Front. Surg. 10, 1047483 (2023)
R.L. Davies, N.J. Kuiper, Regenerative medicine: a review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioeng. 6(1), 22 (2019)
P. Behrens, T. Bitter, B. Kurz, M. Russlies, Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 13(3), 194–202 (2006)
S. Janjanin, W.-J. Li, M.T. Morgan, R.M. Shanti, R.S. Tuan, Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J. Surg. Res. 149(1), 47–56 (2008)
S. Shen, M. Chen, W. Guo, H. Li, X. Li, S. Huang, X. Luo, Z. Wang, Y. Wen, Z. Yuan, Three dimensional printing-based strategies for functional cartilage regeneration. Tiss. Eng. Part B: Rev. 25(3), 187–201 (2019)
C. Mandrycky, Z. Wang, K. Kim, D.-H. Kim, 3D bioprinting for engineering complex tissues. Biotech. Adv. 34(4), 422–434 (2016)
J. Groll, J.A. Burdick, D.-W. Cho, B. Derby, M. Gelinsky, S.C. Heilshorn, T. Juengst, J. Malda, V.A. Mironov, K. Nakayama, A definition of bioinks and their distinction from biomaterial inks. Biofabrication 11(1), 013001 (2018)
S. Turunen, S. Kaisto, I. Skovorodkin, V. Mironov, T. Kalpio, S. Vainio, A. Rak-Raszewska, 3D bioprinting of the kidney—hype or hope? AIMS Cell Tiss. Eng. 2(3), 119–162 (2018)
E. Mueller, I. Poulin, W.J. Bodnaryk, T. Hoare, Click chemistry hydrogels for extrusion bioprinting: progress, challenges, and opportunities. Biomacromol 23(3), 619–640 (2022)
S. Loai, B.R. Kingston, Z. Wang, D.N. Philpott, M. Tao, H.-L.M. Cheng, Clinical perspectives on 3D bioprinting paradigms for regenerative medicine. Regen. Med. Front. 1(1), e190004 (2019)
T. Agarwal, I. Chiesa, D. Presutti, V. Irawan, K.Y. Vajanthri, M. Costantini, Y. Nakagawa, S.-A. Tan, P. Makvandi, E.N. Zare, Recent advances in bioprinting technologies for engineering different cartilage-based tissues. Mater. Sci. Eng. 123, 112005 (2021)
X. Han, S. Chang, M. Zhang, X. Bian, C. Li, D. Li, Advances of hydrogel-based bioprinting for cartilage tissue engineering. Front. Bioeng. Biotech. 9, 746564 (2021)
M. Brovold, J.I. Almeida, I. Pla-Palacín, P. Sainz-Arnal, N. Sánchez-Romero, J.J. Rivas, H. Almeida, P.R. Dachary, T. Serrano-Aulló, S. Soker. Naturally-derived biomaterials for tissue engineering applications. Novel Biomater. Regen. Med. 1077, 421–449 (2018)
P. Phutane, D. Telange, S. Agrawal, M. Gunde, K. Kotkar, A. Pethe, Biofunctionalization and applications of polymeric nanofibers in tissue engineering and regenerative medicine. Polym. 15(5), 1202 (2023)
S. Vanaei, M. Parizi, F. Salemizadehparizi, H. Vanaei, An overview on materials and techniques in 3D bioprinting toward biomedical application. Eng. Regen 2, 1–18 (2021)
F. Liu, Q. Chen, C. Liu, Q. Ao, X. Tian, J. Fan, H. Tong, X. Wang, Natural polymers for organ 3D bioprinting. Polym. 10(11), 1278 (2018)
A. Lee, A. Hudson, D. Shiwarski, J. Tashman, T. Hinton, S. Yerneni, J. Bliley, P. Campbell, A. Feinberg, 3D bioprinting of collagen to rebuild components of the human heart. Science 365(6452), 482–487 (2019)
X. Liu, C. Zheng, X. Luo, X. Wang, H. Jiang, Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. 99, 1509–1522 (2019)
C. Somaiah, A. Kumar, D. Mawrie, A. Sharma, S.D. Patil, J. Bhattacharyya, R. Swaminathan, B.G. Jaganathan, Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE 10(12), e0145068 (2015)
M. Maher, M. Castilho, Z. Yue, V. Glattauer, T.C. Hughes, J.A. Ramshaw, G.G. Wallace, Shaping collagen for engineering hard tissues: Towards a printomics approach. Acta Biomater. 131, 41–61 (2021)
G. Montalbano, G. Borciani, G. Cerqueni, C. Licini, F. Banche-Niclot, D. Janner, S. Sola, S. Fiorilli, M. Mattioli-Belmonte, G. Ciapetti, Collagen hybrid formulations for the 3d printing of nanostructured bone scaffolds: An optimized genipin-crosslinking strategy. Nanomater. 10(9), 1681 (2020)
T. Hu, A.C. Lo, Collagen–alginate composite hydrogel: Application in tissue engineering and biomedical sciences. Polym. 13(11), 1852 (2021)
A.M.E. Matinong, Y. Chisti, K.L. Pickering, R.G. Haverkamp, Collagen extraction from animal skin. Biology 11(6), 905 (2022)
M. Furtado, L. Chen, Z. Chen, A. Chen, W. Cui, Development of fish collagen in tissue regeneration and drug delivery. Eng. Regen. 3(3), 217–231 (2022)
A. Sorushanova, I. Skoufos, A. Tzora, A.M. Mullen, D.I. Zeugolis, The influence of animal species, gender and tissue on the structural, biophysical, biochemical and biological properties of collagen sponges. J. Mater. Sci. 32, 1–12 (2021)
V.R. Sherman, W. Yang, M.A. Meyers, The materials science of collagen. J. Mech. Behav. Biomed. Mater. 52, 22–50 (2015)
A. Terzi, N. Gallo, S. Bettini, T. Sibillano, D. Altamura, M. Madaghiele, L. De Caro, L. Valli, L. Salvatore, A. Sannino, Sub-and supramolecular X-ray characterization of engineered tissues from equine tendon, bovine dermis, and fish skin type-I collagen. Macromol. Biosci. 20(5), 2000017 (2020)
M. Yamauchi, Y. Taga, S. Hattori, M. Shiiba, M. Terajima. Analysis of collagen and elastin cross-links. In Methods in Cell Biology, 143; Elsevier, 115–132 (2018)
D.R. Eyre, M. Weis, J. Rai, Analyses of lysine aldehyde cross-linking in collagen reveal that the mature cross-link histidinohydroxylysinonorleucine is an artifact. J. Biol. Chem. 294(16), 6578–6590 (2019)
A.R. Zahrani. Master’s Thesis. The University of Otago; Dunedin, New Zealand. Extraction and Isolation of Collagen Type I from Fish Skin. (2011)
K.Y. Lee, D.J. Mooney, Alginate: properties and biomedical applications. Progr. Polym. Sci. 37(1), 106–126 (2012)
S. Liparoti, V. Speranza, F. Marra, Alginate hydrogel: The influence of the hardening on the rheological behaviour. J. Mech. Behav. Biomed. Mater. 116, 104341 (2021)
K. Hölzl, S. Lin, L. Tytgat, S. Van Vlierberghe, L. Gu, A. Ovsianikov, Bioink properties before, during and after 3D bioprinting. Biofabrication 8(3), 032002 (2016)
C.C. Piras, D.K. Smith, Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem. 8(36), 8171–8188 (2020)
G.R. López-Marcial, A.Y. Zeng, C. Osuna, J. Dennis, J.M. García, G.D. O’Connell, Agarose-based hydrogels as suitable bioprinting materials for tissue engineering. ACS Biomater. Sci. Eng. 4(10), 3610–3616 (2018)
T. Kreller, T. Distler, S. Heid, S. Gerth, R. Detsch, A. Boccaccini, Physico-chemical modification of gelatine for the improvement of 3D printability of oxidized alginate-gelatine hydrogels towards cartilage tissue engineering. Mater. Design 208, 109877 (2021)
S. Schwarz, S. Kuth, T. Distler, C. Gögele, K. Stölzel, R. Detsch, A.R. Boccaccini, G. Schulze-Tanzil, 3D printing and characterization of human nasoseptal chondrocytes laden dual crosslinked oxidized alginate-gelatin hydrogels for cartilage repair approaches. Mater. Sci. Eng. 116, 111189 (2020)
C. Antich, J. de Vicente, G. Jiménez, C. Chocarro, E. Carrillo, E. Montañez, P. Gálvez-Martín, J.A. Marchal, Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 106, 114–123 (2020)
M. Lafuente-Merchan, S. Ruiz-Alonso, A. Espona-Noguera, P. Galvez-Martin, E. López-Ruiz, J. Marchal, M. López-Donaire, A. Zabala, J. Ciriza, L. Saenz-del-Burgo, Development, characterization and sterilisation of Nanocellulose-alginate-(hyaluronic acid)-bioinks and 3D bioprinted scaffolds for tissue engineering. Mater. Sci. Eng. 126, 112160 (2021)
J. Wei, B. Wang, Z. Li, Z. Wu, M. Zhang, N. Sheng, Q. Liang, H. Wang, S. Chen, A 3D-printable TEMPO-oxidized bacterial cellulose/alginate hydrogel with enhanced stability via nanoclay incorporation. Carbohyd. Polym. 238, 116207 (2020)
M. Ghanbari, M. Salavati-Niasari, F. Mohandes, Thermosensitive alginate–gelatin–nitrogen-doped carbon dots scaffolds as potential injectable hydrogels for cartilage tissue engineering applications. RSC Adv. 11(30), 18423–18431 (2021)
H. Yuan, X. Zheng, W. Liu, H. Zhang, J. Shao, J. Yao, C. Mao, J. Hui, D. Fan, A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Coll. Surf. Biointerf. 192, 111041 (2020)
X. Yang, Z. Lu, H. Wu, W. Li, L. Zheng, J. Zhao, Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. 83, 195–201 (2018)
T.H. Jovic, G. Kungwengwe, A.C. Mills, I.S. Whitaker, Plant-derived biomaterials: a review of 3D bioprinting and biomedical applications. Front. Mech. Eng. 5, 19 (2019)
M.L. Chinta, A. Velidandi, N.P.P. Pabbathi, S. Dahariya, S.R. Parcha, Assessment of properties, applications and limitations of scaffolds based on cellulose and its derivatives for cartilage tissue engineering: A review. Int. J. Bio. Macromol. 175, 495–515 (2021)
K. Markstedt, A. Mantas, I. Tournier, H. Martínez Ávila, D. Hagg, P. Gatenholm, 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromol 16(5), 1489–1496 (2015)
S.J. Eichhorn, A. Dufresne, M. Aranguren, N. Marcovich, J. Capadona, S.J. Rowan, C. Weder, W. Thielemans, M. Roman, S. Renneckar, Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 45, 1–33 (2010)
W.N.L. Wan Jusoh, M.S. Sajab, P. Mohamed Abdul, H. Kaco, Recent Advances in 3D Bioprinting: A Review of Cellulose-Based Biomaterials Ink. Polym. 14(11), 2260 (2022)
C. Chen, Y. Xi, Y. Weng, Recent advances in cellulose-based hydrogels for tissue engineering applications. Polym. 14(16), 3335 (2022)
S.V. Murphy, A. Atala, 3D bioprinting of tissues and organs. Nat. Biotech. 32(8), 773–785 (2014)
Y. Yu, Y. Zhang, J.A. Martin, I.T. Ozbolat, Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 135(9), 091011 (2013)
K. Elkhoury, M. Morsink, L. Sanchez-Gonzalez, C. Kahn, A. Tamayol, E. Arab-Tehrany, Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 6(11), 3904–3923 (2021)
M. Oliver-Ferrándiz, L. Milián, M. Sancho-Tello, J.J. Martín de Llano, F. Gisbert Roca, C. Martínez-Ramos, C. Carda, M. Mata, Alginate-agarose hydrogels improve the in vitro differentiation of human dental pulp stem cells in chondrocytes. A histological study. Biomed 9(7), 834 (2021)
P.A. Janmey, J.P. Winer, J.W. Weisel, Fibrin gels and their clinical and bioengineering applications. J. Roy. Soc. Interf. 6(30), 1–10 (2009)
H. Simaan-Yameen, O. Bar-Am, G. Saar, D. Seliktar, Methacrylated fibrinogen hydrogels for 3D cell culture and delivery. Acta Biomater. 164, 94–110 (2023)
S.J. Min, J.S. Lee, H. Nah, H.-J. Moon, S.J. Lee, H.J. Kang, Y.-S. Hwang, I.K. Kwon, D.N. Heo, Degradable and Tunable Keratin-fibrinogen Hydrogel as Controlled Release System for Skin Tissue Regeneration. J. Bion. Eng. 20(3), 1049–1059 (2023)
A. Shpichka, D. Osipova, Y. Efremov, P. Bikmulina, N. Kosheleva, M. Lipina, E.A. Bezrukov, R.B. Sukhanov, A.B. Solovieva, M. Vosough, Fibrin-based bioinks: New tricks from an old dog. Inter. J. Bioprinting 6(3), 269 (2020)
B.A. de Melo, Y.A. Jodat, E.M. Cruz, J.C. Benincasa, S.R. Shin, M.A. Porcionatto, Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 117, 60–76 (2020)
A. Cavallo, T. Al Kayal, A. Mero, A. Mezzetta, L. Guazzelli, G. Soldani, P. Losi, Fibrinogen-Based Bioink for Application in Skin Equivalent 3D Bioprinting. J. Funct. Biomater. 14(9), 459 (2023)
J.C. Salamone. Concise polymeric materials encyclopedia; CRC press (1998). https://doi.org/10.1201/9780367811686
F. Ghorbani, A. Zamanian, M. Sahranavard, Mussel-inspired polydopamine-mediated surface modification of freeze-cast poly (ε-caprolactone) scaffolds for bone tissue engineering applications. Biomed. Eng. 65(3), 273–287 (2020)
E. Malikmammadov, T.E. Tanir, A. Kiziltay, V. Hasirci, N. Hasirci, PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. 29(7–9), 863–893 (2018)
F. Ghorbani, M. Sahranavard, Z. Mousavi Nejad, D. Li, A. Zamanian, B. Yu, Surface functionalization of three dimensional-printed polycaprolactone-bioactive glass scaffolds by grafting GelMA under UV irradiation. Front. Mater. 7, 528590 (2020)
M. Griffin, N. Castro, O. Bas, S. Saifzadeh, P. Butler, D.W. Hutmacher, The current versatility of polyurethane three-dimensional printing for biomedical applications. Tiss. Eng. Part B: Rev. 26(3), 272–283 (2020)
W. Wang, C. Wang. Polyurethane for biomedical applications: A review of recent developments. Design Manuf. Med. Dev. 115–151 (2012)
R. Ferrari, J. Sinner, J. Bill, W. Brucksch, Compounding Polyurethanes. Ind. Eng. Chem. 50(7), 1041–1044 (1958)
V. Melnig, N. Apetroaei, N. Dumitrascu, Y. Suzuki, V. Tura, Improvement of polyurethane surface biocompatibility by plasma and ion beam techniques. J Optoelectron Adv Mater 7(5), 2521À2528 (2005)
H. Janik, M. Marzec, A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. 48, 586–591 (2015)
L. Sin, A. Rahmat, W. Rahman, Degradation and stability of poly (lactic acid). Polylactic Acid. 247–299 (2013)
S. Castañeda-Rodríguez, M. González-Torres, R.M. Ribas-Aparicio, M.L. Del Prado-Audelo, G. Leyva-Gómez, E.S. Gürer, J. Sharifi-Rad, Recent advances in modified poly (lactic acid) as tissue engineering materials. J. Bio. Eng. 17(1), 21 (2023)
H. Ma, W. Su, Z. Tai, D. Sun, X. Yan, B. Liu, Q. Xue, Preparation and cytocompatibility of polylactic acid/hydroxyapatite/graphene oxide nanocomposite fibrous membrane. Chinese Sci. Bull. 57, 3051–3058 (2012)
M.G. Flores-Sánchez, N.C. Islas-Arteaga, A.M. Raya-Rivera, D.R. Esquiliano-Rendon, J. Morales-Corona, O.E. Uribe-Juarez, F.I. Vivar-Velázquez, G.P. Ortiz-Vázquez, R. Olayo, Effect of a plasma synthesized polypyrrole coverage on polylactic acid/hydroxyapatite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. 109(11), 2199–2211 (2021)
J.M. Moran, D. Pazzano, L.J. Bonassar, Characterization of polylactic acid–polyglycolic acid composites for cartilage tissue engineering. Tiss. Eng. 9(1), 63–70 (2003)
C.-S. Wu, H.-T. Liao, A new biodegradable blends prepared from polylactide and hyaluronic acid. Polym. 46(23), 10017–10026 (2005)
S. Sugiarto, Y. Leow, C.L. Tan, G. Wang, D. Kai, How far is Lignin from being a biomedical material? Bioact. Mater. 8, 71–94 (2022)
C.E. Byrne, C.E. Astete, M. Vaithiyanathan, A.T. Melvin, M. Moradipour, S.E. Rankin, B.L. Knutson, C.M. Sabliov, E.C. Martin, Lignin-graft-PLGA drug-delivery system improves efficacy of MEK1/2 inhibitors in triple-negative breast cancer cell line. Nanomed. 15(10), 981–1000 (2020)
F. Luzi, I. Tortorella, A. Di Michele, F. Dominici, C. Argentati, F. Morena, L. Torre, D. Puglia, S. Martino, Novel nanocomposite PLA films with lignin/zinc oxide hybrids: Design, characterization, interaction with mesenchymal stem cells. Nanomater. 10(11), 2176 (2020)
R. Boni, A. Ali, A. Shavandi, A.N. Clarkson, Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 25, 1–21 (2018)
N. Tamai, A. Myoui, M. Hirao, T. Kaito, T. Ochi, J. Tanaka, K. Takaoka, H. Yoshikawa, A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2). Osteoarthr. Cartilage 13(5), 405–417 (2005)
L.A. Osório, E. Silva, R.E. Mackay, A review of biomaterials and scaffold fabrication for organ-on-a-chip (OOAC) systems. Bioeng. 8(8), 113 (2021)
N. Siddiqui, S. Asawa, B. Birru, R. Baadhe, S. Rao, PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotech. 60, 506–532 (2018)
M. Zare, E.R. Ghomi, P.D. Venkatraman, S. Ramakrishna, Silicone-based biomaterials for biomedical applications: antimicrobial strategies and 3D printing technologies. J. App. Polym. Sci. 138(38), 50969 (2021)
I. Zulkiflee, S. Masri, M. Zawani, A. Salleh, I.N. Amirrah, M.F.M.R. Wee, S.M. Yusop, M.B. Fauzi, Silicon-Based Scaffold for Wound Healing Skin Regeneration Applications: A Concise Review. Polym. 14(19), 4219 (2022)
P. Han, C. Wu, Y. Xiao, The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomater. Sci. 1(4), 379–392 (2013)
M.-Y. Shie, S.-J. Ding, H.-C. Chang, The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 7(6), 2604–2614 (2011)
R. Jugdaohsingh, Silicon and bone health. J. Nutr. Health Aging 11(2), 99 (2007)
J.R. Henstock, L.T. Canham, S.I. Anderson, Silicon: the evolution of its use in biomaterials. Acta Biomater. 11, 17–26 (2015)
Y.-H. Lin, Y.-C. Chiu, Y.-F. Shen, Y.-H.A. Wu, M.-Y. Shie, Bioactive calcium silicate/poly-ε-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J. Mater. Sci. Mater. Med. 29, 1–13 (2018)
R. Izquierdo, N. Garcia-Giralt, M.T. Rodriguez, E. Cáceres, S.J. García, J.L. Gómez Ribelles, M. Monleón, J.C. Monllau, J. Suay, Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J. Biomed. Mater. Res. Part A 85(1), 25–35 (2008)
H.-W. Kim, J.C. Knowles, H.-E. Kim, Hydroxyapatite/poly (ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomater. 25(7–8), 1279–1287 (2004)
A.D.R. Pontinha, B.B. Moreira, B.L. Melo, D.D. Melo-Diogo, I.J. Correia, P. Alves, Silica Aerogel-Polycaprolactone Scaffolds for Bone Tissue Engineering. Inter. J. Mol. Sci. 24(12), 10128 (2023)
N.A. Peppas, K.B. Keys, M. Torres-Lugo, A.M. Lowman, Poly (ethylene glycol)-containing hydrogels in drug delivery. J. Controll. Rel. 62(1–2), 81–87 (1999)
S.J. Bryant, K.S. Anseth, Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly (ethylene glycol) hydrogels. J. Biomed. Mater. Res. 59(1), 63–72 (2002)
C.P. Pathak, A.S. Sawhney, J.A. Hubbell, In situ photopolymerization and gelation of water-soluble monomers: A new approach for local administration of peptide drugs. Polym. Chem. 33, 65–66 (1992)
L. Liu, Y. Xiang, Z. Wang, X. Yang, X. Yu, Y. Lu, L. Deng, W. Cui, Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Mater. 11(1), 81 (2019)
M. Gan, Q. Zhou, J. Ge, J. Zhao, Y. Wang, Q. Yan, C. Wu, H. Yu, Q. Xiao, W. Wang, Precise in-situ release of microRNA from an injectable hydrogel induces bone regeneration. Acta Biomater. 135, 289–303 (2021)
M. Herten, R.E. Jung, D. Ferrari, D. Rothamel, V. Golubovic, A. Molenberg, C.H.F. Hämmerle, J. Becker, F. Schwarz, Biodegradation of different synthetic hydrogels made of polyethylene glycol hydrogel/RGD-peptide modifications: an immunohistochemical study in rats. Clin. Oral Impl. Res. 20(2), 116–125 (2009)
H. Pohlit, I. Bellinghausen, M. Schömer, B.R. Heydenreich, J. Saloga, H. Frey, Biodegradable pH-sensitive poly (ethylene glycol) nanocarriers for allergen encapsulation and controlled release. Biomacromolecules 16(10), 3103–3111 (2015)
W. Liu, X. Jing, Z. Xu, C. Teng, PEGDA/HA mineralized hydrogel loaded with Exendin4 promotes bone regeneration in rat models with bone defects by inducing osteogenesis. J. Biomater. Appl. 35(10), 1337–1346 (2021)
Q. Li, Z. Liu, W. Chen, B. Yuan, X. Liu, W. Chen, A novel bio-inspired bone-mimic self-healing cement paste based on hydroxyapatite formation. Cement Concr. Compos. 104, 103357 (2019)
M.R. Nejadnik, A.G. Mikos, J.A. Jansen, S.C.G. Leeuwenburgh, Facilitating the mineralization of oligo (poly (ethylene glycol) fumarate) hydrogel by incorporation of hydroxyapatite nanoparticles. J. Biomed. Mater. Res. Part A 100(5), 1316–1323 (2012)
R. Cheng, T. Xin, L. Liu, F. Wang, T. Ye, L. Deng, W. Cui, A “three-in-one” injectable hydrogel platform with osteogenesis, angiogenesis and antibacterial for guiding bone regeneration. Appl. Mater. Today 20, 100763 (2020)
S. Sun, Y. Cui, B. Yuan, M. Dou, G. Wang, H. Xu, J. Wang, W. Yin, D. Wu, C. Peng, Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotech. 11, 1117647 (2023)
Q.T. Nguyen, Y. Hwang, A.C. Chen, S. Varghese, R.L. Sah, Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomater. 33(28), 6682–6690 (2012)
S.J. Bryant, K.S. Anseth, Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J. Biomed. Mater. Res. Part A 64(1), 70–79 (2003)
C.-Y. Ko, C.-Y. Yang, S.-R. Yang, K.-L. Ku, C.-K. Tsao, D.C.-C. Chuang, I.M. Chu, M.-H. Cheng, Cartilage formation through alterations of amphiphilicity of poly (ethylene glycol)–poly (caprolactone) copolymer hydrogels. RSC Adv. 3(48), 25769–25779 (2013)
F. Tallia, H.-K. Ting, S.J. Page, J.P. Clark, S. Li, T. Sang, L. Russo, M.M. Stevens, J.V. Hanna, J.R. Jones, Bioactive, degradable and tough hybrids through calcium and phosphate incorporation. Front. Mater. 9, 901196 (2022)
D. Zhai, L. Chen, Y. Chen, Y. Zhu, Y. Xiao, C. Wu, Lithium silicate-based bioceramics promoting chondrocyte maturation by immunomodulating M2 macrophage polarization. Biomater. Sci. 8(16), 4521–4534 (2020)
L. Chen, C. Deng, J. Li, Q. Yao, J. Chang, L. Wang, C. Wu, 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomater. 196, 138–150 (2019)
F. Yang, J. Zhao, W.J. Koshut, J. Watt, J.C. Riboh, K. Gall, B.J. Wiley, A synthetic hydrogel composite with the mechanical behavior and durability of cartilage. Adv. Funct. Mater. 30(36), 2003451 (2020)
L. Fu, L. Li, Q. Bian, B. Xue, J. Jin, J. Li, Y. Cao, Q. Jiang, H. Li, Cartilage-like protein hydrogels engineered via entanglement. Nat. 618(7966), 740–747 (2023)
J. Liu, L. Li, H. Suo, M. Yan, J. Yin, J. Fu, 3D printing of biomimetic multi-layered GelMA/nHA scaffold for osteochondral defect repair. Mater. Design 171, 107708 (2019)
J. Guan, F.-Z. Yuan, Z.-M. Mao, H.-L. Zhu, L. Lin, H.H. Chen, J.-K. Yu, Fabrication of 3D-printed interpenetrating hydrogel scaffolds for promoting chondrogenic differentiation. Polym. 13(13), 2146 (2021)
S. Vijayavenkataraman. 3D bioprinting: Challenges in commercialization and clinical translation. J. 3D Print. Med. 7(2), 2059–4755 (2023)
A. Elemoso, G. Shalunov, Y.M. Balakhovsky, A.Y. Ostrovskiy, Y.D. Khesuani, 3D Bioprinting: The roller coaster ride to commercialization. Int. J. Bioprint. 6(3), 301 (2020)
T. Mladenovska, P.F. Choong, G.G. Wallace, C.D. O’connell, The regulatory challenge of 3D bioprinting. Regen. Med. 18(8), 659–674 (2023)
K. Pushparaj, B. Balasubramanian, M. Pappuswamy, V. Anand Arumugam, K. Durairaj, W.-C. Liu, A. Meyyazhagan, S. Park, Out of box thinking to tangible science: A benchmark history of 3D bio-printing in regenerative medicine and tissues engineering. Life 13(4), 954 (2023)
Clinical Trials, AuriNovo for Auricular Reconstruction, https://clinicaltrials.gov/study/NCT04399239. Accessed 11 April 2024
R.E. Deichmann, M. Krousel-Wood, J. Breault, Bioethics in practice: considerations for stopping a clinical trial early. Ochsner J. 16(3), 197–198 (2016)
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript's conception. Material preparation, data collection and analysis were performed by EM, AA, GN and ST. The manuscript was edited and revised by MB. The first draft of the manuscript was written by EM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mueller, E., Nomdedeu-Sancho, G., El-Derby, A. et al. Clinical translation of 3D bioprinting for cartilage repair: a biomaterial perspective. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00730-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00730-0