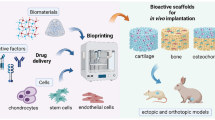
Abstract
Over the recent years, the advent of 3D bioprinting technology has marked a milestone in osteochondral tissue engineering (TE) research. Nowadays, the traditional used techniques for osteochondral regeneration remain to be inefficient since they cannot mimic the complexity of joint anatomy and tissue heterogeneity of articular cartilage. These limitations seem to be solved with the use of 3D bioprinting which can reproduce the anisotropic extracellular matrix (ECM) and heterogeneity of this tissue. In this chapter, we present the most commonly used 3D bioprinting approaches and then discuss the main criteria that biomaterials must meet to be used as suitable bioinks, in terms of mechanical and biological properties. Finally, we highlight some of the challenges that this technology must overcome related to osteochondral bioprinting before its clinical implementation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Bioprinting has emerged over the recent years as a promising technique for osteochondral TE applications. Bioinks (biomaterials and bioactive cues) via 3D bioprinting can be deposited in a spatiotemporally accurate layer-by-layer manner, allowing for high cell seeding density and strong cell–cell interactions [1,2,3,4]. This technology can be classified into two different categories based on whether the bioink contains living cells (cellular 3D bioprinting) or not (acellular 3D bioprinting). Although to date, applications of 3D bioprinting have been focused on cardiovascular, skin regeneration, tracheal splints, cartilaginous structures, and hard tissues like bon, among others. The uniquely capacity of this technique to mimic heterogeneous and anisotropic properties of ECM has attracted much attention to osteochondral tissues [2, 5,6,7,8,9]. In this perspective, 3D fabrication techniques have raised as an alternative for grafting methodologies, which remain as the common gold standard treatment for joint degenerative diseases such as osteoarthritis (OA) or trauma [7]. Osteochondral grafts exhibit low integration at the bone–cartilage interface and poor tissue formation de novo [5, 8]. For its part, 3D bioprinting provides the fabrication of scaffolds with interconnected macroporosity and microporosity which improves nutrient diffusion and removal of waste products, and facilitates the ECM deposition and ingrowth of blood vessels [10, 11]. In the case of osteochondral tissue , considerations regarding to heterogeneity and anisotropy are of special importance due to mechanical and composition requirements, which differ from cartilage to bone tissues. Thus, 3D bioprinting can be advantageous.
To obtain effective and biologically relevant tissue constructions that mimic the native microenvironment, several specifications must be considered (Fig. 10.1). Among these essential aspects, structural and physical specifications such as bulk properties and surface topography play a key role in the development of bioactive tissue constructs. 3D bioprinting is an appropriate fabrication technique with high spatial resolution by which achieves these aspects. However, appropriate biomaterials (bioinks) should be developed with optimal rheological and biological properties , since this is the main limitation of the technique as it will be exposed in this chapter [12,13,14,15]. Material viscosity, gelation method, and speed must be optimized to obtain 3D architectures with enough structural integrity and mechanical properties that allow for not only interactions with the materials but also cell communication [2, 12, 16,17,18,19]. In addition, many manufacturing techniques can also be employed to improve the relatively weak mechanical properties of soft hydrogels , such as ultraviolet (UV) curing [20, 21], pre-cross-linking procedures, or the incorporation of additional elements and materials such as poly (ε-caprolactone) (PCL ) [22] or graphene oxide (GO) elements [23, 24]. Some of these elements can be sacrificial since they will not form part of the final constructs.
Moreover, the 3D bioprinting processes must ensure compliance with some biological specifications . Certainly, biocompatibility and absence of cytotoxicity are essential requisites, but the considerable efforts made over the recent decades on the TE field have demonstrated that bioactive constructs are indispensable. By this way, the need for vascularization remains one of the most daunting challenges in the development of 3D complexes. Molecular diffusion limilations make necessary a minimun distance (≈ 100 μm) between cells and the nearest capillary to facilitate the exchange of nutrients and oxygen, which would be impossible without an adequate vascular network [12, 25, 26]. Finally, for a succeed integration with surrounding environment, degradation and absorption kinetics of the constructs must be fast to avoid side effects. 3D bioprinting provides some advantages regarding to these biological aspects among other biofabrication techniques , since it can facilitate a controlled deposition of cells, maintaining their viability during the process [12, 27].
Finally, economic issues regarding manufacturing requirements, overall cost of materials and fabrication devices, and necessary production time are crucial aspects for successful clinical translation of 3D bioprinting in osteochondral restoration applications. Although the cost of specialized equipment and experienced personnel could be high, there are progressively more affordable 3D fabrication systems with intuitive interfaces for inexperienced users [5, 7, 12].
In this chapter, we discuss how the advent of 3D bioprinting has provided new opportunities for osteochondral TE, and the current advances and challenges that must be addressed by current 3D bioprinting approaches and bioinks for the preparation of polymeric and composited scaffolds.
2 3D Bioprinting Fabrication Strategies for Osteochondral TE
As explained in the introduction, 3D bioprinting techniques have attracted much attention to the treatment of osteochondral degeneration and diseases such as osteoarthritis through osteochondral tissue regeneration. Currently, autografts and allografts are being applied to reduce donor site morbidity and matching mainly in young patients. However, the research is moving towards developing de novo tissue constructions to improve integration with host tissue and nutrient diffusion in larger macroporous scaffolds through cell-based repairs such as autologous chondrocyte implantation [5, 7, 12, 28, 29].
Recently, bioprinting has been subclassified into two categories : scaffold-based and scaffold-free bioprinting. While scaffold-based bioprinting implies the generation of scaffolding materials by 3D printing where cells can be seeded during or post-fabrication, scaffold-free bioprinting is based on the self-assembly of cellular components mimicking embryonic development [30,31,32]. This chapter focuses only on the description of the scaffold-based bioprinting techniques for the development of osteochondral complex tissue. We have made a subdivision between cellular and acellular scaffold-based bioprinting depending on if their bioink formulation contains living cells or not.
2.1 Cellular Bioprinting
In this first paragraph, the most commonly used cellular 3D bioprinting processes will be presented, namely, extrusion-based bioprinting, droplet-based bioprinting, laser-based bioprinting, and stereolithography, [5, 7, 9, 33, 34]. An illustration of these 3D bioprinting processes with its main components is shown in Fig. 10.2.
Schemes of 3D bioprinting process and its main components [35]. Adapted from Biomaterials 83, D. Tang. et al., Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration, pp. 363–382, Copyright 2016, with permission from Elsevier
2.1.1 Extrusion-Based Bioprinting
The extrusion-based bioprinting (EBB ) consists of the dispensation of bioinks using an air-force pump, solenoid or mechanical screw plunger. EBB addresses the challenges of droplet bioprinting process, which cannot deposit very viscous materials or high cell density solutions [1, 12, 33]. As discussed later in the chapter, high viscosity values of the bioinks are desirable to obtain high shape fidelity of the tissue constructs but in some cases, high concentration of the components of the bioinks can result in less cell viability due to cytotoxicity [36]. Nevertheless, EBB is the most used technique for TE applications due to its moderate cost in comparison to the good resolution it provides, as well as, the high cellular concentrated bioinks that can be printed [1]. In addition, good-shaped fidelity can be obtained through a fast phase change from a liquid bioink to a more solid network by different cross-linking procedures, that can be classified into chemical (reversible) and physical (irreversible) cross-linkings. Among all chemical cross-linkings processes photo-initiated free radical polymerization reaction is a commonly used alternative for rapid cross-linking despite its cytotoxicity. This process is widely accepted due to its effectiveness, efficiency, and controllability. Duchi and collaborators developed a coaxial core–shell system for EBB to avoid the cytotoxicity that can trigger UV photocuring due to the generation of free radicals and exposure of the cells to UV. They demonstrated that these problems can be addressed with an accurate control of the deposition parameters [37]. In another work, O’Connell et al. developed an easy-to-handle device for medical surgery, named “biopen,” in an attempt of bringing together 3D printing technology and surgical processes. The tool could print gelatin methacrylamide (GelMA ) and hyaluronic acid methacrylate (HAMA ) hydrogels, which were photocrosslinked. The process was compatible with the deposition of adipose stem cells at chondral wound side protocol [38].
Many authors have used of EBB for the development of multilayered compound scaffolds in the context of cartilage and osteochondral regeneration [10, 37, 39,40,41]. For example, Bartnikowski and collaborators developed a 3D plotted scaffold composed of alginate and hydroxyapatite (HAp), mixed with GelMA , or GelMA with HAMA for the regeneration of a zone of calcified cartilage, concluding that the incorporation of HAMA in these hydrogels improved chondrogenesis [11]. T. Ahlfeld and coworkers used EBB to obtain 3D constructs by printing alginate and methylcellulose with clinically relevant dimensions thanks to the addition of laponite, a nanosilicate clay that improves mechanical properties of the matrices. The cellular viability was maintained for 21 days, making this approach as a promising alternative for 3D bioprinting materials [39].
Another important aspect to consider in EBB is the geometry of the needle, which can play a crucial role in cellular viability, since the shear stress under the extrusion can affect cellular behavior and well-being. Muller et al. developed an interesting study where they used different needle geometries and sizes to print alginate and nanocellulose bioinks for cartilage applications. The computational fluid dynamic analysis of different needle geometries is shown in Fig. 10.3. In conclusion, they demonstrated that the appropriate selection of the needle geometry is as important as bioink optimization for high printing resolution and cell viability [13].
Computational fluid dynamic analysis for a straight and a conical needle, respectively. Regions of high shear stress are indicated in red/orange colors. Clear differences can be observed between the two geometries [13]. Reproduced from “Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications”, Annals of Biomedical Engineering, Vol. 45, No. 1, January 2017, pp. 210–223, Muller et al. with permission of Springer
2.1.2 Laser-Based Bioprinting and Stereolithography
Laser-based bioprinting (LBB ) is implemented by laser-induced forward transfer (LIFT ), which is a method to deposit inorganic materials onto a platform construction through a patterned substrate. Odde and Renn used this technique for the first time in 1999 for the deposition of biological materials and cell patterning into clusters to obtain 2D and 3D structures [12, 34, 42, 43]. LIFT uses very high-powered pulsed laser, and a glass or quartz print ribbon is coated with a thin film of metal or other laser-absorbing material to protect the cells from the laser power. Then, a cell suspension is spread onto the bottom of the ribbon. This suspension is vaporized with a laser pulse focused onto the metal layer, which propels the cell suspension from the ribbon to a receiving platform construction [44]. LBB is very useful for bioinks with very low viscosity, allowing for microscale resolution. However, it is restricted only to very thin structures and presents a high cost and complex manufacturing [12, 45]. Gruene and collaborators demonstrated in their work that LBB is suitable for 3D scaffold-free autologous tissue grafts with high cell density enough to promote chondrogenesis. In addition, the printed mesenchymal stem cells (MSCs) tolerated the complete process maintaining their functionality [46]. Other similar techniques to LIFT are used in LBB approaches. For example, absorbing film-assisted laser-induced forward transfer (AFA-LIFT ) uses a 100 nm sacrificial metal layer to interact with the laser. There is also a version of AFA-LIFT, known as biological laser processing (BioLP), which uses motorized receiving stages and a charge-coupled device (CCD) camera to focus the laser. The sacrificial metallic layer allows having a rapid thermal expansion, to reduce the heating of the small cell suspension volume that is propel from the ribbon to the substrate. Finally, matrix-assisted pulsed laser evaporation direct writing (MAPLE DW ) is similar to AFA-LIFT, but it uses a low powered laser operating in the UV or near-UV region. In addition, the ribbon is coated with a sacrificial biological layer to allow the initial cell attachment [44].
On the other hand, stereolithography (SLA) consists of the irradiation of a photopolymerizable macromer solution with a laser to cross-link patterns with high resolution in the polymerization plane. This technique allows the fabrication of accurate microstructured scaffolds [1]. Thus, this technique is only valid for photopolymerizable materials, exhibiting high microscale resolution and printing speed [12]. X. Zhou et al. used SLA to produce GelMA , poly(ethylene glycol) diacrylate (PEGDA) and GO scaffolds that induced chondrogenic differentiation of human MSCs (hMSCs) by promotion of glycosaminoglycan (GAG) and collagen levels. A scheme of the scaffold fabrication is showed in Fig. 10.4 [24].
Illustration of 3D printed GO scaffolds for enhancing chondrogenesis of hMSCs through SLA approach [24]. Reprinted from 3D bioprinted graphene oxide-incorporated matrix for promoting chondrogenic differentiation of human bone marrow MSCs, Zhou et al., Carbon, volume 116, pp. 615–624, Copyright 2017, with permission from Elsevier
2.1.3 Droplet-Based Bioprinting
Droplet-based bioprinting (DBB ) is a deposition method were prepolymer solution droplets are jetted onto a platform in a predefined pattern. It could be performed by the aid of piezoelectric or thermal actuators (Fig. 10.2). The polymerization takes place after deposition by UV light, ionic, thermal or chemical cross-linking processes. The main advantages of this bioprinting technique are its low cost and the wide range of polymers that can be used. However, the viscosity range of this solution is very limited and cell density cannot be very high [12].
In addition, the bioprinting process can make a negative impact on cellular viability. Regarding to this and in order to understand better the process that can affect them, Hendriks et al. have developed an analytical model with which they can relate the cell survival to the cell membrane elongation and this last one, with the size and speed of the droplet, as well as, substrates characteristics [47]. Another interesting work is the one carried out by Graham et al., where they developed high-resolution 3D geometries by DBB, which consisted of the 3D printing of aqueous droplets containing Human Embryonic Kidney (HEK) cells and ovine MSCs (oMSCs ). These platforms included arborized cell junctions and osteochondral interfaces, exhibiting high viability. In addition, oMSCs showed a chondrogenic differentiation to cartilage-like structures after 5 weeks of culture [48].
2.2 Acellular Bioprinting Techniques
Acellular bioprinting covers the generation of nonliving material constructs based on the pattern and assembly of materials and the successively cell post-printing seeding [3]. This strategy offers several advantages over printed cellular constructs such as higher resolution and greater shape complexity due to the manufacturing conditions in which is avoided the printing of either cells or heat-sensitive biological cues [49]. Acellular tissue scaffolds, alone or in combination with cellular techniques, have shown promising results for bone (BTE) and cartilage (CTE) TE.
2.2.1 Fused Deposition Modeling (FDM)
FDM , also known as fused filament fabrication (FFF ), is based on extrusion, through a computer-guided nozzle, of melting or semimolten thermoplastic filaments which are finally deposited onto a platform where its solidification takes place in a layer-by-layer fashion [23]. Thus, this printing technique, which later helps in the development of other bioprinting techniques, concretely extrusion based bioprinting [3], has been widely applied in the synthesis of acellular porous scaffolds for osteochondral TE due to the fact that the final construct provides a mechanical properties in a closer magnitude to articular cartilage and cancellous bone [50,51,52]. Strengths such as its rapid printing capability, the ability to obtain large construct with good mechanical integrity, easy scalability, and the no need of solvent and support structure has made this technique widely explored, especially for bone tissue. However several disadvantages should be mentioned such as the reduce number of filament materials that can be used, or the high temperature required to melt the filament which limits the printability with cells or temperature sensitive biological cues. In addition, it is very complicated the fabrication of constructs with small pore size while maintaining the porosity (100 μm) [53,54,55]. Thermoplastic polymers such as PCL , poly-lactic acid (PLA) and poly(lactic-co-glycolic acid) (PLGA ) [52, 56, 57], which are the most common biodegradable synthetic polymers used in this manufacturing process, are the main responsible for this mechanical properties, especially in the case of PCL [55].
The replacement of the hot rollers system of FDM by a pressurized syringe with a thermostatically controlled heating jacket, defined as extrusion printing, has increased the number of synthetic materials used for 3D biofabrication [58]. For example, Woodfield et al. have shown the success bioprinting of an amphiphilic biodegradable poly(ether ester) multiblock copolymers as carrier materials for articular cartilage repair based on hydrophilic poly(ethylene glycol)-terephthalate (PEGT ) and hydrophobic poly(butylene terephthalate) (PBT) (PEGT/PBT). Furthermore, constructs with a gradient in pore-size trying to mimic the complex zonal structure of cartilage were designed showing an efficiency inhomogeneous chondrocyte distribution but no differences in cartilage-like tissue formation related to cell density were observed [58]. More recently, Schuurman et al. have demonstrated the production of highly cartilage-like tissue abundance by improving the efficiency of cell seeding by distributing the cells along the PEGT/PBT scaffolds in form of pellets. However, additional options should be explored in order to generate de novo cartilage zonally organized [59].
The presence of nanoscale features in the constructs plays an important role in the generation of TE by affecting cell attachment, proliferation and cytoskeletal assembly. However, FDM, as well as other AM techniques, have not fulfilled this biomimetic nano-resolution. In this sense and in order to overcome this limitation, recent strategies have been proposed for the post-fabrication functionalization with techniques such as layer-by-layer deposition (LbL) [60], plasma deposition [61], and the attachment based on mussel-inspired materials [62]. These strategies include not only the change of topography surface, also the incorporation of some thermal labile biological cues which should be incorporated afterwards. Regarding to this, dexamethasone which is an osteoinductive drug has been incorporated in 3D PCL /poloxamer scaffold during FDM without affecting its properties [63]. However, some labile compounds require their incorporation using the post-fabrication treatment mentioned before or be printed by other bioprinting techniques. Examples of the last one are described in Sect. 10.3.1.
2.2.2 Melt Electrospinning Writing
Melt-electrospinning writing (MEW ) is an emerging manufacturing approach wherein major principles of melt extrusion-based additive manufacturing (AM) and electrospinning are combined. A melt polymer is extruded through a nozzle and beginning electrically charged due to the application of a high voltage between the nozzle tip and the collected platform where fiber are deposited upon each other [64]. The main different in comparison with electrospinning is the lack of organic solvent as in MEW the polymer is melted. This fact allows to improve cell viability and to obtain 3D structures with well-orientated fibers by avoiding both their mechanical and electrical coiling [65]. On the other hand, this fibrous construct can be based on fibers with diameters down to 1 μm [66, 67], far away from the >200 μm provided by FDM manufacture technique [65]. All these aspects provide a really well organized network construct that can be built to millimeters thickness with a convenience pore size for allowing the cell invasion and vascularization of de novo tissue [65, 68]. The potential of this technique for the reinforcement of soft hydrogel matrices has been recently published because it is well known that the actual TE scaffolds based on hydrogels are unable to reach the stiffness and therefore the biological requirements to promote the neotissue. Concretely, electrospun PCL fibers obtained by MEW are infused with GelMA , providing a scaffold with mechanical properties in the range of articular cartilage [66]. More recently, and following the same strategy, constructs based on highly negatively charged star-shaped poly(ethylene glycol)–heparin hydrogel (sPEG/Hep) reinforced with medical grade PCL (mPCL) fibers by MEW were also obtained for articulate CTE. Despite the fact that the fibers provide an outstanding increase in mechanical properties such as anisotropy and viscoelasticity, the system does not meet the expectation under simulated dynamic load-bearing conditions, the necessity to explore different composite material soft fiber-reinforced hydrogels [69]. In this sense, it is interesting to mention the importance of trying to mimic the natural fiber structure in natural soft tissue which is mainly based on collagen. Thus, Bas et al. have compared the behavior of soft network composites reinforced with either stretchable curvy or straight mPCL fibers presenting the curvy fibers the more similar behavior to natural soft tissue [70].
2.2.3 Selective Lase Sintering (SLS)
SLS , which was developed at the University of Texas [71], is an AM technique where a construct is obtained by sequential deposition of biopolymers, bioceramics, or biocomposites powders which are spread in the bed with a roller following by their fusion via the increase in temperature coming from a computer controlled high-power carbon dioxide laser. Thus, a first thing layer (100–200 nm) is formed and the process is repeated layer-by-layer. Features of the powder such as particle size and shape can affect the SLS process [72]. In comparison with FDM, it might be easy to incorporate composite materials such as polymers-bioceramics as there is no requirement of the materials to be in filament form [73]. Other advantages are the high precision, nonrequirement of solvent or porogens, and the manufacturing of mimetic scaffolds with complicated geometries [74]. Therefore, SLS has found its potential application for BTE and more concretely in bone complex structure and intricate shapes such as maxillofacial and craniofacial [75]. Materials that do not decompose under the laser beam [73, 74] can be used for SLS. Thereby, apart from the metallic devices which are the most common one fabricated by SLS, it has also been explored for BTE using biodegradable polymers such as PCL [74], PLA [76], and polyvinyl alcohol (PVA) [77], polymer–ceramics composites such as nano-HAp-PCL [78], aliphatic polycarbonate-HAp [79], PLA-HAp [80], PLA-carbonated HAp microsphere [24],calcium phosphate (Ca-P)/poly(hydroxybutyrate-co-hydroxyvalerate (PHBV) [75], polyamide-HAp [81], GO reinforced PVA [82], PLA-(Ca-P) [83], and PLGA /HAp and Beta tricalcium phosphate (β-TCP) [84]. Nevertheless, this technique has hardly been applied for CTE but it is worth to mention the modification of SLS defined as microsphere-based SLS technique [85] where the powder used has a spherical shape in the microscale. This version has led to the subsequent application in the manufacturing of scaffolds that mimic the complex multiple tissue structure of osteochondral defects (subchondral bone, intermediate calcified cartilage and the superficial cartilage region) [28, 86]. Pointedly, an approach trying to obtain HAp gradient scaffolds has been built by sintering PCL and PCL-HAp microparticles by SLS. The potential of SLS in the regeneration of osteochondral tissue was showed in vivo” experiments in a rabbit model by forming new tissue with both, articular cartilage and subchondral bone regions [87].
2.2.4 Cellular/Acellular Bioprinting Techniques
Cellular/acellular bioprinting techniques arise from the need to overcome the actual limitation of both main types of bioinks, natural and synthetic polymers. Hydrogel-based bioprinting constructs are restricted in term of mechanical strength especially when their applications rely on the treatment of load-bearing tissue such as osteochondral tissue. On the other hand, synthetic polymers present limited cell affinity due to the lack of surface cell recognition sites [88, 89]. Furthermore, a common disadvantage for both of them is the inefficiency of in vivo hydrolytic and enzymatic degradation which should match the speed of tissue in-growth. For example, PCL presents a very low degradation rate (1.5–2 years) [90] while natural polymers such as Chitosan shown a variable enzymatic degradation depending of the host response [91].
At this point, both the concept of substrate support and sacrificial templates are introduced due to the important role that they play in these hybrid bioprinting strategies. Sacrificial templates are usually synthetic polymers that are used during the manufacturing process of hydrogel-based bioink to provide to each layer with the requirement mechanical properties during the layer by layer deposition and they are removed in a second step [1]. Alternatively, they have found a great application when trying to obtain vascularized tissue such as BTE because the vascular channels in the scaffolds are printing with these sacrificial materials and subsequently removed [1, 3]. On the other hand, substrate support includes the pre-printing of templates that are not removed after hydrogel addition. Thus, the hybrid system can encompass the advantages of both systems, the good mechanical properties of the thermoplastic polymers and the good cells adhesion of natural polymers [92]. Examples of hybrid techniques for the development of these systems are described below.
MHDS is a solid free-form fabrication which allows for obtaining hybrid constructs with more than one bioinks. Concretely, those bioinks (thermoplastic polymers, natural polymers) are loaded in different thermostatically controlled syringes and parameters such as temperature, pneumatic pressure and motion are stabilized independently for each syringe. Thus, alternant layers of different bioinks either loaded or unloaded with cells, some reinforce additives and biological cues can be co-printed [93, 94]. Several works have been developed based on MHDS for bone and cartilage tissue regeneration based on the hybrid system PCL –alginate [92, 94, 95]. Although, initial thought about MHDS techniques point to a possible reduction of cell viability when alternating thermoplastic polymer-natural polymer loaded with cells layer are deposited. Recent study based on the system PCL-Alginate loaded with primary chondrocytes isolated from chick embryos have demonstrated the high cell vitality after deposition (higher than 80%). The melting PCL cool down faster enough to minimize the effect on cell viability [95]. Furthermore, the mechanical stability conferred by the thermoplastic polymer allows for the printing of hydrogels with lower cross-linking density which could be beneficial for cell viability [92].
Template-Fused Deposition Modelling (t-FDM ) has been used to create sacrificial templates. The template is printed by FDM and a cross-linkable material is poured onto the template where the polymerization takes places [96]. The template whether is removed or not should have biocompatible properties. An example of sacrificial template can be found in Guo et al. where a polyurethane construct was obtained with well-defined topological properties (in the range of trabecular bone) after its cross-linking polymerization on a PLA template [97]. An example where the template is not removed is described by Dong et al. where hybrid chitosan–PCL scaffolds have been described for their application in BTE. In vitro results of these systems when encapsulating rabbit bone marrow mesenchymal stem cells (BMMSCs ) in the chitosan matrix have shown an improvement of osteogenesis differentiation compared to PCL control scaffolds alone [98]. However, this hybrid system can fail under mechanical stresses due to an inefficient thermoplastic–natural hydrogel interface adhesion. In this sense, a covalent attachment between both materials has been proposed for the hybrid system based on GelMa and poly(hydroxymethylglycolide-co-ε-caprolactone)/poly(e-caprolactone) (pHMGCL/PCL) , showing an increase of mechanical integrity while also keeping their ability to promote ECM formation [99]. Additional strategies to increase the mechanical properties are described in Sect. 10.3.1.
3 3D Printing Polymeric and Composited Materials for Osteochondral Tissue Engineering
Biomaterials used for 3D printing fabrication must meet different criteria to successful development of the scaffolds. The first requisite is having good rheological properties, which means that bioinks must be mechanically suitable for printing depending on the used bioprinting technique, and provide an appropriate environment to the cells after bioprinting to promote adhesion, proliferation and differentiation. Secondly, it is essential that the material maintains its structural integrity, in other words, high shape fidelity after the deposition process. This is directly related to printability, which refers to the relationship between the substrates and the bioinks. The bioprinting solutions should maintain vertical tension having a high contact angle with the substrate surface, and it normally depends on how fast is the cross-linking process. Finally, the bioinks must provide a biocompatible and not cytotoxic environment for cell encapsulation and deposition. However, many materials usually meet one or two requisites, being necessary the development of bioinks which present all these criteria. Usually, materials that are printable and maintain their structure after bioprinting through a rapid cross-linking, make necessary the use of high temperature for thermal curing or UV light for photopolymerization, which compromise the encapsulation of cells in the bioinks. In addition, the most biocompatible materials do not exhibit good rheological properties for extrusion or bioprinting deposition, like for example hydrogels [1, 21, 100]. Hydrogels are highly appropriate biomaterials for 3D bioprinted scaffolds for osteochondral TE due to its high biocompatibility, which make them suitable for cell encapsulation, and biodegradability properties [101]. Hydrogels are networks of 3D cross-linked polymers that able to uptake huge amount of water due to their inherent hydrophilic properties. This capability can be modulate depending on the biological tissue of interest. In addition, hydrogels pose injectability properties for minimally invasive therapies of cartilaginous-like tissues [8, 11, 101, 102]. One approach to improve mechanical properties of hydrogel bioinks is to increase the concentration of the components, obtaining highly viscous solutions with suitable printability. However, cell viability is usually decreased in high concentrated bioinks due to higher stress must be applied to the solution [6, 13, 36, 103].
Among all biomaterials explored for 3D bioprinting technology, we can distinguish between those derived from natural polymers, such as collagen, gelatin, alginate, chitosan, and hyaluronic acid (HA), or synthetic-derived polymers, such as PCL , PLGA , PLA, PEG, and PEGDA. As it has been explained, synthetic materials exhibit robust mechanical properties, but poor biocompatibility and toxic degradation products. For these reasons, the use of composites is more widespread. Composites are a combination of two or more than three individual materials. They are used for enhancing mechanical strength and fabrication of more intricately designed constructs, as well as improving their long-term stability. Thanks to this combination, the suitable strength and mechanical properties of the scaffold can be suitably modulated depending on the properties of the native tissue [1, 8, 101]. Nanoclays and PEGDA, for example, have been incorporated into some hydrogels solutions to control their viscosity [101]. One interesting example is the work developed by Yang et al., who synthesized a biphasic graft consisting of cartilage and subchondral bone, using synthetic (PLGA ) and natural (alginate) polymers and a multi-nozzle deposition system [14]. Over the recent years the use of decellularized extracellular matrix (dECM ) has been investigated for osteochondral regeneration. dECM consists of a complex of GAGs, collagen, and elastin that mimics the native tissue environment. In addition, the ECM can lead and mediate the differentiation of stem cells [101].
HA is a naturally derived polysaccharide that has been amply used in osteochondral tissue regenerative therapies. It is an anionic, GAG distributed widely throughout connective, epithelial, and neural tissues. As it is also one of the main components of the ECM , contributing significantly to cell proliferation and migration. All these properties make to HA a suitable material for 3D bioprinting application [11, 21, 38, 40, 104,105,106]. For example, Shaoquan et al. developed a semi-interpenetrating polymer network (semi-IPN) based on HA and hydroxyethylmethacrylate-derivatized dextran (dex-HEMA), which showed shear thinning rheology and mechanical strength. The scaffolds exhibited high porous structure, supporting the viability of encapsulated chondrocytes [107]. Ju Young and coworkers used HA with alginate and chondrocytes in Dulbecco’s Modified Eagle Medium (DMEM) for chondral section, while collagen-I in DMEM constituted the osteo-section. Thus, they fabricated a two-compartment scaffold for osteochondral tissue mimetic structures [105].
Gelatin is a naturally derived polysaccharide widely used in bioprinting techniques due to its thermosensitive properties which eases the development of shaped fidelity structures [20, 21, 38, 104]. Gelatin is the denatured form of collagen, which resembles the ECM environments providing key biological motifs for cell adhesion and proliferation [102]. An example within numerous studies developed with gelatin or its methacrylate form, is the one carried out by Levato et al., who developed novel constructs consisting of GelMA and gellan gum for osteogenic and chondrogenic differentiation of MSCs [100, 108]. Gelatin has been also found to participate in some regulation ways for chondrogenesis. For example, Chameettachal et al. developed tyrosinase cross-linked silk–gelatin bioinks and demonstrated that these bioinks could upregulate the expression of hypoxia markers such as hypoxia inducible factor 1 alpha (HIF1A) which positively regulated also the expression of chondrogenic markers such as aggrecan or cartilage oligomeric matrix protein 1 (COMP1). The gelatin, particularly, showed the induction of matrix metalloproteinase 2 (MMP2) activity , which is known to promote the creation of a pericellular zone for the accumulation of growth factors and de novo matrix [109]. Costantini and collaborators also used GelMA for the development of 3D bioprinted constructs through a coaxial needle system. The bioinks, composed of GelMA, chondroitin sulfate amino ethyl methacrylate (CS-AEMA) , and HAMA , showed the upregulated expression of chondrogenic markers, like COL2A1 and aggrecan, as well as osteogenic markers like COL1A1. Thus, the presented approach demonstrated to be a suitable candidate for 3D bioprinted applications for cartilage TE field [40]. In addition, gelatin is usually combined with HA since it is known not only for promoting chondrogenesis in the 3D constructs but also for improving mechanical properties of the constructs during the bioprinting process [59, 106, 110].
Apart from bioink design, cross-linking mechanisms are another aspect to be optimized in order to obtain more complex constructs reducing undesirable side effects. Cross-linking procedures in bioprinting need to be secure for cell encapsulation and fast, promoting a state change from liquid (viscous) to almost solid network. The cross-linking can be physical, chemical, or a combination of both, but it must maintain native cell adhesion properties of the biomaterial [8]. Chemical cross-linking processes are the most accepted due to its effectiveness, efficiency and controllability, being able to synthetize handle scaffolds with good mechanical properties and stiffness. Photopolymerization is one of the most commonly approach used for the development of 3D bioprinted scaffolds. The chemical reaction can be triggered by the irradiation of a photoinitiator (PI) containing-hydrogel at a specific wavelength. However, photocuring also shows some drawbacks due mainly to the cytotoxicity and inflammation reactions that are provoked by the generation of free radicals by UV exposure that can damage DNA and cellular components [20, 111, 112]. For this reason, activated PIs under visible or A-UV light are being extensively used during the last years [20, 113]. However, many authors have demonstrated in their studies that a proper adjustment of the UV irradiation time, intensity, and wavelength could ensure cell viability [37, 38, 104, 106, 110, 114, 115].
3.1 Incorporation of Additives for Enhancing Mechanical Properties
In order to achieve 3D bioprinted scaffolds with clinical relevant dimensions, there are two main strategies without using a bath as supporting medium. The first one consisted of the improvement of mechanical properties of the solution through a rapid cross-linking process, as it has been discussed in the previous section. The second one is the incorporation of support materials, such as PCL , which can confer space and structural integrity to low viscous bioinks [5, 39, 116, 117]. Daly and coworkers developed a study to compare the printability of different bioinks for 3D bioprinting of hyaline and fibrocartilage, using the most common hydrogels: agarose, GelMA , alginate and BioINK™ , which consists of a poly(ethylene glycol) methacrylate (PEGMA) based hydrogel. The tissue staining for type II collagen revealed that alginate and agarose based bioinks supported properly the development of hyaline-like cartilage, while GelMA and BioINK™ supported the growth of fibrocartilage. They used PCL filaments to reinforce the mechanical properties of the hydrogels, being able to synthesized constructs with a compressive moduli similar to articular cartilage [22].
In another interesting work, Ahlfeld et al. used Laponite, a synthetic nanosilicate clay which is known for its drug delivery properties. They combined alginate and methylcellulose with Laponite to develop constructs with high printing fidelity as well as, controlled released of active compounds [39]. Co-printing approaches with PLGA or nanocellulose rather than PCL as supporting materials have also been boarded [118]. Nanocellulose, for example, is able to increase the viscosity of an alginate solution bioink up to sevenfold, improving therefore the bioprintability [5]. In a work by Markstedt and collaborators, a bioink composed of nanofibrillate cellulose and alginate was developed to a patterned meniscus cartilage in a single-step bioprinting process [119, 120]. Müller et al. also developed a sulfate alginate-based bioink in combination with nanocellulose to make it printable. This mix was photocurable, arising as a good alternative for cartilage tissue regeneration applications [13]. In addition, to avoid the limitations of PCL as a reinforcing material, the increasing of porosity of the reinforcing phase can be also an alternative approach [9, 14].
3.2 Incorporation of Bioactive Compounds
The incorporation of active compounds such as growth factors and inorganic compounds is a very common approach to enhance cell adhesion and proliferation, as well as, differentiation to a specific tissue. Bartnikowski et al. incorporated a paste of HAp to a GelMA , and GelMA-HAMA bioink for the development of a zone of calcified cartilage, as well as improvement of bioinks printability. They concluded that the incorporation of HAMA enhanced chondrogenesis and the bioprinted scaffolds showed good cell culture viability for 28 days [11]. In another interesting work, Wang et al. studied the effect of HAp in an HA-based bioink. They demonstrated that a small amount of HAp enhanced chondrogenesis and hypertrophic differentiation of adipose derived MSCs. In addition, they were able to develop stratified scaffolds with mineralized and nonmineralized layers (HA-HAp based and HA-based) [121]. In another work, Zhou and coworkers incorporated GO to their gelatin-based 3D bioprinted scaffolds to promote chondrogenic differentiation, demonstrating that multifunctional carbon-based nanomaterials can be a suitable additive for osteochondral TE approaches [24].
Traditionally, several growth factors including transforming growth factors (TGFs ), insulin-like growth factors (IGFs ) and bone morphogenetic protein (BMPs ) have been incorporated to osteochondral TE scaffolds to promote chondrogenic or osteogenic stem cells differentiation as it has been reviewed recently [122]. Similar approach has been also used in AM scaffolds. Until now, TGF-β has been incorporated either directly to the cell culture media [41, 123, 124] or by physical encapsulation in the hydrogel [94, 125, 126]. An example of TGF-β physical encapsulation has been reported by Kundu et al. where an alginate–TGF-β–BMMSCs printed scaffold reinforced with PCL has been manufactured by MHDS. Scaffolds loaded with TGF-β produced higher GAGs content after 4 weeks compared to the unloaded ones [94]. However, recent studies focusing on silk–gelatin constructs incubated with TGF-β1 have shown hypertrophy instead of articular cartilage MSC differentiation. This evidence has led to an increasing need to find new strategies which could avoid the hypertrophic differentiation. In this sense, the overexpression of nuclear receptor subfamily 2 group F member 2 (NR2F2) in MSCs was promoted previous to scaffold cell implantation. This overexpression has provided the generation of abundant cartilage matrix [127]. Another strategy focused on the 3D bioprinting encapsulation of bioactive drug Y27632 [(+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide dihydrochloride] which has been shown to reduce the hypertrophic market collagen X (Col X) in comparison with TGF-β when MSCs were seeded on polyurethane (PU)–HA constructs [126]. BMPs are another group of growth factors widely applied for promoting osteogenic differentiation. In a recent work the surface of PLA constructs has been modified by the assembly of multilayer nanocoating based on gelatin (Gel) and poly-lysine (PLL) finally cross-linked with genipin (GnP). An increase cell adhesion of both human umbilical vein endothelial cells (HUVECs) and hMSCs respect to the control (unmodified PLA construct was reported. More interesting, this approach allowed for the smart release of growth factors such as recombinant human bone morphogenetic protein (rhBMP-2) and recombinant human vascular endothelial (rhVEGF) by promoting osteogenic differentiation of hMSCs and .proliferation and differentiation of HUVECs Thus, it has been possible the generation of vascularized bone grafts [60]. In order to avoid undesirable growth factors degradation when adding directly to the cell culture media, Dong et al. have developed hybrid chitosan–PCL scaffolds loaded with BMP-2. A sustained in vitro release of BMP-2 promoted BMMSC osteogenic differentiation [98].
4 Conclusion and Future Perspectives
In general, the arrival and development of 3D bioprinting has made a huge impact on tissue regeneration field. Its implementation in osteochondral TE field is highly appropriate owing to the particular heterogeneity and anisotropy that the osteochondral tissue exhibits. 3D bioprinting allows for fabricating very intricate heterogeneous 3D constructs by an accurate spatiotemporal positioning of cells and biomolecules, controlling the structure, size, shape, pore, and orientation of each component with micrometer precision. In addition, the porosity and gradient created in the scaffolds by 3D bioprinting ensures a good cell–cell communication and vascularization of the construct, which is essential for an appropriate distribution of oxygen and nutrients, and thus for long-term stability.
However, despite all the advantages that this technology holds in the field, some challenging aspects have to be solved before translation of the technology to the clinic occurs. It cannot be denied that 3D bioprinting will be responsible for a new generation of personalized therapeutic approach, but the materials and technology should be meticulously chosen when aiming for translation to the clinic. Currently, the most daunting challenges that restrict the clinical translation of this technology are the capacity for large-scale fabrication, sterilization process, stringent quality control for 3D scaffolds for human trials, and the affordability of the medical expenditure. Although numerous preclinical studies are being developed, clinical trials are very limited due to regulatory issues, differences in patient responses, as well as implantation constraints. In addition, the necessity of skilled experts and cost efficacy of the fabrication devices are still bottlenecks for the clinical translation of the technology.
In conclusion, the emergence of printing technologies for the construction of mimetic scaffolds for the regeneration of osteochondral tissue seems to be a significant milestone. As all novel technologies, 3D bioprinting should face regulatory hurdles for clinical translation that must be solved in the following years, as these technologies provide real benefits and advantages to really complicated osteochondral diseases and lesions.
References
Mandrycky C, Wang Z, Kim K, Kim DH (2016) 3D bioprinting for engineering complex tissues. Biotechnol Adv 34(4):422–434. https://doi.org/10.1016/j.biotechadv.2015.12.011
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785. https://doi.org/10.1038/nbt.2958
Cui H, Nowicki M, Fisher JP, Zhang LG (2017) 3D bioprinting for organ regeneration. Adv Healthc Mater 6(1):1601118. https://doi.org/10.1002/adhm.201601118
Arslan-Yildiz A, Assal RE, Chen P, Guven S, Inci F, Demirci U (2016) Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 8(1):014103. https://doi.org/10.1088/1758-5090/8/1/014103
O’Connell G, Garcia J, Amir J (2017) 3D bioprinting: new directions in articular cartilage tissue engineering. ACS Biomater Sci Eng 3:2657. https://doi.org/10.1021/acsbiomaterials.6b00587
Muller M, Becher J, Schnabelrauch M, Zenobi-Wong M (2015) Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 7(3):035006. https://doi.org/10.1088/1758-5090/7/3/035006
Ozbolat IT (2017) Bioprinting of osteochondral tissues: a perspective on current gaps and future trends. Int J Bioprint. 3(2). doi:https://doi.org/10.18063/ijb.2017.02.007
Radhakrishnan J, Subramanian A, Krishnan UM, Sethuraman S (2017) Injectable and 3D bioprinted polysaccharide hydrogels: from cartilage to osteochondral tissue engineering. Biomacromolecules 18(1):1–26. https://doi.org/10.1021/acs.biomac.6b01619
Daly AC, Freeman FE, Gonzalez-Fernandez T, Critchley SE, Nulty J, Kelly DJ (2017) 3D bioprinting for cartilage and osteochondral tissue engineering. Adv Healthc Mater 6. https://doi.org/10.1002/adhm.201700298
Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, Alblas J, Dhert WJ (2012) Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods 18(1):33–44. https://doi.org/10.1089/ten.TEC.2011.0060
Bartnikowski M, Akkineni AR, Gelinsky M, Woodruff MA, Klein TJ (2016) A hydrogel model incorporating 3D-plotted hydroxyapatite for osteochondral tissue engineering. Materials (Basel) 9(4). https://doi.org/10.3390/ma9040285
Pedde RD, Mirani B, Navaei A, Styan T, Wong S, Mehrali M, Thakur A, Mohtaram NK, Bayati A, Dolatshahi-Pirouz A, Nikkhah M, Willerth SM, Akbari M (2017) Emerging biofabrication strategies for engineering complex tissue constructs. Adv Mater 29(19). https://doi.org/10.1002/adma.201606061
Muller M, Ozturk E, Arlov O, Gatenholm P, Zenobi-Wong M (2017) Alginate sulfate-Nanocellulose bioinks for cartilage bioprinting applications. Ann Biomed Eng 45(1):210–223. https://doi.org/10.1007/s10439-016-1704-5
Yang SS, Choi WH, Song BR, Jin H, Lee SJ, Lee SH, Lee J, Kim YJ, Park SR, Park S-H, Min B-H (2015) Fabrication of an osteochondral graft with using a solid freeform fabrication system. Tiss Eng Regen Med 12(4):239–248. https://doi.org/10.1007/s13770-015-0001-y
Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312–319. https://doi.org/10.1038/nbt.3413
Ahadian S, Yamada S, Ramon-Azcon J, Estili M, Liang X, Nakajima K, Shiku H, Khademhosseini A, Matsue T (2016) Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater 31:134–143. https://doi.org/10.1016/j.actbio.2015.11.047
Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A (2016) Bioink properties before, during and after 3D bioprinting. Biofabrication 8(3):032002. https://doi.org/10.1088/1758-5090/8/3/032002
Ji S, Guvendiren M (2017) Recent advances in bioink design for 3D bioprinting of tissues and organs. Front Bioeng Biotechnol 5. https://doi.org/10.3389/fbioe.2017.00023
Donderwinkel I, van Hest JCM, Cameron NR (2017) Bio-inks for 3D bioprinting: recent advances and future prospects. Polym Chem 8(31):4451–4471. https://doi.org/10.1039/c7py00826k
Klotz BJ, Gawlitta D, Rosenberg AJ, Malda J, Melchels FP (2016) Gelatin-Methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol 34(5):394–407. https://doi.org/10.1016/j.tibtech.2016.01.002
Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD (2010) Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A 16(8):2675–2685. https://doi.org/10.1089/ten.TEA.2009.0798
Daly AC, Critchley SE, Rencsok EM, Kelly DJ (2016) A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 8(4):045002. https://doi.org/10.1088/1758-5090/8/4/045002
Brunello G, Sivolella S, Meneghello R, Ferroni L, Gardin C, Piattelli A, Zavan B, Bressan E (2016) Powder-based 3D printing for bone tissue engineering. Biotechnol Adv 34(5):740–753. https://doi.org/10.1016/j.biotechadv.2016.03.009
Zhou X, Nowicki M, Cui H, Zhu W, Fang X, Miao S, Lee S-J, Keidar M, Zhang LG (2017) 3D bioprinted graphene oxide-incorporated matrix for promoting chondrogenic differentiation of human bone marrow mesenchymal stem cells. Carbon 116:615–624. https://doi.org/10.1016/j.carbon.2017.02.049
Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA (2016) Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 113(12):3179–3184. https://doi.org/10.1073/pnas.1521342113
Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO (2007) The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6(12):997–1003. https://doi.org/10.1038/nmat2013
Ikada Y (2006) Challenges in tissue engineering. J R Soc Interface 3(10):589–601. https://doi.org/10.1098/rsif.2006.0124
Boushell MK, Hung CT, Hunziker EB, Strauss EJ, Lu HH (2016) Current strategies for integrative cartilage repair. Connect Tissue Res 58(5):393–406. https://doi.org/10.1080/03008207.2016.1231180
Ahmed TAE, Hincke MT (2009) Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev 16(3):305–329. https://doi.org/10.1089/ten.teb.2009.0590
Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT (2017) The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv 35(2):217–239. https://doi.org/10.1016/j.biotechadv.2016.12.006
Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, Ozbolat IT (2016) Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci Rep 6:28714. https://doi.org/10.1038/srep28714. https://www.nature.com/articles/srep28714#supplementary-information
Ozbolat IT (2015) Scaffold-based or scaffold-free bioprinting: competing or complementing approaches? J Nanotechnol Eng Med 6(2):024701–024706. https://doi.org/10.1115/1.4030414
Ozbolat IT, Hospodiuk M (2016) Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76:321–343. https://doi.org/10.1016/j.biomaterials.2015.10.076
Pereira RF, Bártolo PJ (2015) 3D bioprinting of photocrosslinkable hydrogel constructs. J Appl Polym Sci 132(48):n/a-n/a. doi:https://doi.org/10.1002/app.42458
Tang D, Tare RS, Yang LY, Williams DF, Ou KL, Oreffo RO (2016) Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials 83:363–382. https://doi.org/10.1016/j.biomaterials.2016.01.024
Ahn G, Min KH, Kim C, Lee JS, Kang D, Won JY, Cho DW, Kim JY, Jin S, Yun WS, Shim JH (2017) Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Sci Rep 7(1):8624. https://doi.org/10.1038/s41598-017-09201-5
Duchi S, Onofrillo C, O'Connell CD, Blanchard R, Augustine C, Quigley AF, Kapsa RMI, Pivonka P, Wallace G, Di Bella C, Choong PFM (2017) Handheld co-axial bioprinting: application to in situ surgical cartilage repair. Sci Rep 7(1):5837. https://doi.org/10.1038/s41598-017-05699-x
O'Connell CD, Di Bella C, Thompson F, Augustine C, Beirne S, Cornock R, Richards CJ, Chung J, Gambhir S, Yue Z, Bourke J, Zhang B, Taylor A, Quigley A, Kapsa R, Choong P, Wallace GG (2016) Development of the biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 8(1):015019. https://doi.org/10.1088/1758-5090/8/1/015019
Ahlfeld T, Cidonio G, Kilian D, Duin S, Akkineni AR, Dawson JI, Yang S, Lode A, Oreffo ROC, Gelinsky M (2017) Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 9(3):034103. https://doi.org/10.1088/1758-5090/aa7e96
Costantini M, Idaszek J, Szoke K, Jaroszewicz J, Dentini M, Barbetta A, Brinchmann JE, Swieszkowski W (2016) 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 8(3):035002. https://doi.org/10.1088/1758-5090/8/3/035002
Chawla S, Kumar A, Admane P, Bandyopadhyay A, Ghosh S (2017) Elucidating role of silk-gelatin bioink to recapitulate articular cartilage differentiation in 3D bioprinted constructs. Bioprinting 7:1–13. https://doi.org/10.1016/j.bprint.2017.05.001
Odde DJ, Renn MJ (1999) Laser-guided direct writing for applications in biotechnology. Trends Biotechnol 17(10):385–389. https://doi.org/10.1016/S0167-7799(99)01355-4
Odde DJ, Renn MJ (2000) Laser-guided direct writing of living cells. Biotechnol Bioeng 67(3):312–318. https://doi.org/10.1002/(sici)1097-0290(20000205)67:3<312::aid-bit7>3.0.co;2-f
Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB (2010) Laser-based direct-write techniques for cell printing. Biofabrication 2(3):032001. https://doi.org/10.1088/1758-5082/2/3/032001
Kingsley DM, Dias AD, Chrisey DB, Corr DT (2013) Single-step laser-based fabrication and patterning of cell-encapsulated alginate microbeads. Biofabrication 5(4):045006. https://doi.org/10.1088/1758-5082/5/4/045006
Gruene M, Deiwick A, Koch L, Schlie S, Unger C, Hofmann N, Bernemann I, Glasmacher B, Chichkov B (2011) Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C Methods 17(1):79–87. https://doi.org/10.1089/ten.TEC.2010.0359
Hendriks J, Willem Visser C, Henke S, Leijten J, Saris DB, Sun C, Lohse D, Karperien M (2015) Optimizing cell viability in droplet-based cell deposition. Sci Rep 5:11304. https://doi.org/10.1038/srep11304
Graham AD, Olof SN, Burke MJ, Armstrong JPK, Mikhailova EA, Nicholson JG, Box SJ, Szele FG, Perriman AW, Bayley H (2017) High-resolution patterned cellular constructs by droplet-based 3D printing. Sci Rep 7(1):7004. https://doi.org/10.1038/s41598-017-06358-x
O'Brien CM, Holmes B, Faucett S, Zhang LG (2015) Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B Rev 21(1):103–114. https://doi.org/10.1089/ten.teb.2014.0168
Hutmacher DW, Schantz JT, Lam CXF, Tan KC, Lim TC (2007) State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med 1(4):245–260. https://doi.org/10.1002/term.24
Chen SS, Falcovitz YH, Schneiderman R, Maroudas A, Sah RL (2001) Depth-dependent compressive properties of normal aged human femoral head articular cartilage: relationship to fixed charge density. Osteoarthr Cartil 9(6):561–569. https://doi.org/10.1053/joca.2001.0424
Goldstein SA (1987) The mechanical properties of trabecular bone: dependence on anatomic location and function. J Biomech 20(11):1055–1061. https://doi.org/10.1016/0021-9290(87)90023-6
Adepu S, Dhiman N, Laha A, Sharma CS, Ramakrishna S, Khandelwal M (2017) Three-dimensional bioprinting for bone tissue regeneration. Cur Opin Biomed Eng? 2(supplement C):22–28. doi:https://doi.org/10.1016/j.cobme.2017.03.005
Mota C, Puppi D, Chiellini F, Chiellini E (2015) Additive manufacturing techniques for the production of tissue engineering constructs. J Tissue Eng Regen Med 9(3):174–190. https://doi.org/10.1002/term.1635
Woodruff MA, Hutmacher DW (2010) The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci 35(10):1217–1256. https://doi.org/10.1016/j.progpolymsci.2010.04.002
Huang Q, JCHG, Hutmacher DW, Lee EH (2004) In Vivo Mesenchymal Cell Recruitment by a Scaffold Loaded with Transforming Growth Factor β1 and the Potential for in Situ Chondrogenesis. Tiss Eng 8(3):469–482. doi:https://doi.org/10.1089/107632702760184727
Zein I, Hutmacher DW, Tan KC, Teoh SH (2002) Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23(4):1169–1185. https://doi.org/10.1016/S0142-9612(01)00232-0
Woodfield TBF, Malda J, de Wijn J, Péters F, Riesle J, van Blitterswijk CA (2004) Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 25(18):4149–4161. https://doi.org/10.1016/j.biomaterials.2003.10.056
Schuurman W, Levett PA, Pot MW, van Weeren PR, Dhert WJA, Hutmacher DW, Melchels FPW, Klein TJ, Malda J (2013) Gelatin-Methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci 13(5):551–561. https://doi.org/10.1002/mabi.201200471
Cui H, Zhu W, Holmes B, Zhang LG (2016) Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration. Adv Sci 3(8):1600058. doi:10.1002/advs.201600058
Domingos M, Intranuovo F, Gloria A, Gristina R, Ambrosio L, Bártolo PJ, Favia P (2013) Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater 9(4):5997–6005. https://doi.org/10.1016/j.actbio.2012.12.031
Lee SJ, Lee D, Yoon TR, Kim HK, Jo HH, Park JS, Lee JH, Kim WD, Kwon IK, Park SA (2016) Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomaterialia 40(supplement C):182–191. doi:https://doi.org/10.1016/j.actbio.2016.02.006
Costa PF, Puga AM, Díaz-Gomez L, Concheiro A, Busch DH, Alvarez-Lorenzo C (2015) Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int J Pharm 496(2):541–550. https://doi.org/10.1016/j.ijpharm.2015.10.055
Brown TD, Dalton PD, Hutmacher DW (2011) Direct writing by way of melt electrospinning. Adv Mater 23(47):5651–5657. https://doi.org/10.1002/adma.201103482
Bas O, De-Juan-Pardo EM, Chhaya MP, Wunner FM, Jeon JE, Klein TJ, Hutmacher DW (2015) Enhancing structural integrity of hydrogels by using highly organised melt electrospun fibre constructs. Eur Polym J 72(supplement C):451–463. doi:https://doi.org/10.1016/j.eurpolymj.2015.07.034
Visser J, Melchels FPW, Jeon JE, van Bussel EM, Kimpton LS, Byrne HM, Dhert WJA, Dalton PD, Hutmacher DW, Malda J (2015) Reinforcement of hydrogels using three-dimensionally printed microfibres. 6:6933. https://doi.org/10.1038/ncomms7933. https://www.nature.com/articles/ncomms7933#supplementary-information
Gernot Hochleitner TJ, Brown TD, Hahn K, Moseke C, Jakob F, Dalton PD, Groll J (2015) Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 7(3)
Brown TD, Edin F, Detta N, Skelton AD, Hutmacher DW, Dalton PD (2014) Melt electrospinning of poly(ε-caprolactone) scaffolds: phenomenological observations associated with collection and direct writing. Mater Sci Eng C 45(supplement C):698-708. doi:https://doi.org/10.1016/j.msec.2014.07.034
Onur B, Elena MD-J-P, Christoph M, Davide DA, Jeremy GB, Laura JB, Wellard RM, Stefan K, Ernst R, Carsten W, Travis JK, Isabelle C, Dietmar WH (2017) Biofabricated soft network composites for cartilage tissue engineering. Biofabrication 9(2):025014
Bas O, D’Angella D, Baldwin JG, Castro NJ, Wunner FM, Saidy NT, Kollmannsberger S, Reali A, Rank E, De-Juan-Pardo EM, Hutmacher DW (2017) An integrated design, material, and fabrication platform for engineering biomechanically and biologically functional soft tissues. ACS Appl Mater Interfaces 9(35):29430–29437. https://doi.org/10.1021/acsami.7b08617
Deckard CR (1997) Apparatus for producing parts by selective sintering. Google Patents.
Schmid M, Amado A, Wegener K (2015) Polymer powders for selective laser sintering (SLS). AIP Conf Proc 1664(1):160009. https://doi.org/10.1063/1.4918516
Almoatazbellah Y, Scott JH, Paul DD (2017) Additive manufacturing of polymer melts for implantable medical devices and scaffolds. Biofabrication 9(1):012002
Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S (2005) Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 26(23):4817–4827. https://doi.org/10.1016/j.biomaterials.2004.11.057
Duan B, Wang M, Zhou WY, Cheung WL, Li ZY, Lu WW (2010) Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater 6(12):4495–4505. https://doi.org/10.1016/j.actbio.2010.06.024
Kanczler JM, Mirmalek-Sani S-H, Hanley NA, Ivanov AL, Barry JJA, Upton C, Shakesheff KM, Howdle SM, Antonov EN, Bagratashvili VN, Popov VK, Oreffo ROC (2009) Biocompatibility and osteogenic potential of human fetal femur-derived cells on surface selective laser sintered scaffolds. Acta Biomater 5(6):2063–2071. https://doi.org/10.1016/j.actbio.2009.03.010
Cijun S, Zhongzheng M, Haibo L, Yi N, Huanlong H, Shuping P (2013) Fabrication of porous polyvinyl alcohol scaffold for bone tissue engineering via selective laser sintering. Biofabrication 5(1):015014
Xia Y, Zhou P, Cheng X, Xie Y, Liang C, Li C, Xu S (2013) Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue engineering applications. Int J Nanomedicine 8:4197–4213. https://doi.org/10.2147/ijn.s50685
XiaoHui S, Wei L, PingHui S, QingYong S, QingSong W, YuSheng S, Kai L, WenGuang L (2015) Selective laser sintering of aliphatic-polycarbonate/hydroxyapatite composite scaffolds for medical applications. Int J Adv Manuf Technol 81(1):15–25. https://doi.org/10.1007/s00170-015-7135-x
Kuznetsova D, Prodanets N, Rodimova S, Antonov E, Meleshina A, Timashev P, Zagaynova E (2017) Study of the involvement of allogeneic MSCs in bone formation using the model of transgenic mice. Cell Adhes Migr 11(3):233–244. https://doi.org/10.1080/19336918.2016.1202386
Savalani MM, Hao L, Dickens PM, Zhang Y, Tanner KE, Harris RA (2012) The effects and interactions of fabrication parameters on the properties of selective laser sintered hydroxyapatite polyamide composite biomaterials. Rapid Prototyp J 18(1):16–27. https://doi.org/10.1108/13552541211193467
Shuai C, Feng P, Gao C, Shuai X, Xiao T, Peng S (2015) Graphene oxide reinforced poly(vinyl alcohol): nanocomposite scaffolds for tissue engineering applications. RSC Adv 5(32):25416–25423. https://doi.org/10.1039/c4ra16702c
Chong W, Qilong Z, Min W (2017) Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication 9(2):025031
Zhou WY, Lee SH, Wang M, Cheung WL, Ip WY (2008) Selective laser sintering of porous tissue engineering scaffolds from poly(l-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J Mater Sci Mater Med 19(7):2535–2540. https://doi.org/10.1007/s10856-007-3089-3
Du Y, Liu H, Shuang J, Wang J, Ma J, Zhang S (2015) Microsphere-based selective laser sintering for building macroporous bone scaffolds with controlled microstructure and excellent biocompatibility. Colloids and Surf B Biointerf 135(supplement C):81–89. doi:https://doi.org/10.1016/j.colsurfb.2015.06.074
Di Bella C, Fosang A, Donati DM, Wallace GG, Choong PFM (2015) 3D bioprinting of cartilage for orthopedic surgeons: reading between the lines. Front Surg 2:39. https://doi.org/10.3389/fsurg.2015.00039
Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J, Noh I, Mikos AG, Zhang S (2017) Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 137 (Supplement C):37–48. doi:https://doi.org/10.1016/j.biomaterials.2017.05.021
Cai Q, Wan Y, Bei J, Wang S (2003) Synthesis and characterization of biodegradable polylactide-grafted dextran and its application as compatilizer. Biomaterials 24(20):3555–3562. https://doi.org/10.1016/S0142-9612(03)00199-6
Ciardelli G, Chiono V, Vozzi G, Pracella M, Ahluwalia A, Barbani N, Cristallini C, Giusti P (2005) Blends of poly-(ε-caprolactone) and polysaccharides in tissue engineering applications. Biomacromolecules 6(4):1961–1976. https://doi.org/10.1021/bm0500805
Sun H, Mei L, Song C, Cui X, Wang P (2006) The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 27(9):1735–1740. https://doi.org/10.1016/j.biomaterials.2005.09.019
Chung C, Burdick JA (2008) Engineering cartilage tissue. Adv Drug Deliv Rev 60(2):243–262. https://doi.org/10.1016/j.addr.2007.08.027
Schuurman W, Khristov V, Pot MW, PRv W, Dhert WJA, Malda J (2011) Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 3(2):021001
Shim J-H, Huh J-B, Park JY, Jeon Y-C, Kang SS, Kim JY, Rhie J-W, Cho D-W (2012) Fabrication of blended Polycaprolactone/poly (lactic-co-glycolic acid)/β-Tricalcium phosphate thin membrane using solid freeform fabrication Technology for Guided Bone Regeneration. Tissue Eng A 19(3–4):317–328. https://doi.org/10.1089/ten.tea.2011.0730
Kundu J, Shim J-H, Jang J, Kim S-W, Cho D-W (2015) An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med 9(11):1286–1297. https://doi.org/10.1002/term.1682
Izadifar Z, Chang T, Kulyk W, Chen X, Eames BF (2015) Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng Part C Methods 22(3):173–188. https://doi.org/10.1089/ten.tec.2015.0307
Margaret AN, Nathan JC, Michael WP, Lijie Grace Z (2016) 3D printing of novel osteochondral scaffolds with graded microstructure. Nanotechnology 27(41):414001
Guo R, Lu S, Page JM, Merkel AR, Basu S, Sterling JA, Guelcher SA (2015) Fabrication of 3D scaffolds with precisely controlled substrate modulus and pore size by templated-fused deposition modeling to direct osteogenic differentiation. Adv Healthc Mater 4(12):1826–1832. https://doi.org/10.1002/adhm.201500099
Dong L, Wang S-J, Zhao X-R, Zhu Y-F, Yu J-K (2017) 3D- printed poly(ε-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering. Sci Rep 7(1):13412. https://doi.org/10.1038/s41598-017-13838-7
Boere KWM, Visser J, Seyednejad H, Rahimian S, Gawlitta D, van Steenbergen MJ, Dhert WJA, Hennink WE, Vermonden T, Malda J (2014) Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater 10(6):2602–2611. https://doi.org/10.1016/j.actbio.2014.02.041
Levato R, Webb WR, Otto IA, Mensinga A, Zhang Y, van Rijen M, van Weeren R, Khan IM, Malda J (2017) The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater 61:41–53. https://doi.org/10.1016/j.actbio.2017.08.005
Kim JE, Kim SH, Jung Y (2016) Current status of three-dimensional printing inks for soft tissue regeneration. Tiss Eng Regen Med 13(6):636–646. https://doi.org/10.1007/s13770-016-0125-8
Chuah YJ, Peck Y, Lau JE, Hee HT, Wang DA (2017) Hydrogel based cartilaginous tissue regeneration: recent insights and technologies. Biomater Sci 5(4):613–631. https://doi.org/10.1039/c6bm00863a
Zhai X, Ma Y, Hou C, Gao F, Zhang Y, Ruan C, Pan H, Lu WW, Liu W (2017) 3D-printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater Sci Eng 3(6):1109–1118. https://doi.org/10.1021/acsbiomaterials.7b00224
Camci-Unal G, Cuttica D, Annabi N, Demarchi D, Khademhosseini A (2013) Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 14(4):1085–1092. https://doi.org/10.1021/bm3019856
Ju Young P, Jong-Cheol C, Jin-Hyung S, Jung-Seob L, Hyoungjun P, Sung Won K, Junsang D, Dong-Woo C (2014) A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 6(3):035004
Levett PA, Melchels FP, Schrobback K, Hutmacher DW, Malda J, Klein TJ (2014) A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater 10(1):214–223. https://doi.org/10.1016/j.actbio.2013.10.005
Shaoquan B, He M, Junhui S, Cai H, Sun Y, Liang J, Fan Y, Zhang X (2016) The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture, vol 140. doi:https://doi.org/10.1016/j.colsurfb.2016.01.008
Riccardo L, Jetze V, Josep AP, Elisabeth E, Jos M, Miguel AM-T (2014) Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 6(3):035020
Chameettachal S, Midha S, Ghosh S (2016) Regulation of Chondrogenesis and hypertrophy in silk fibroin-gelatin-based 3D bioprinted constructs. ACS Biomater Sci Eng 2(9):1450–1463. https://doi.org/10.1021/acsbiomaterials.6b00152
Levett PA, Melchels FP, Schrobback K, Hutmacher DW, Malda J, Klein TJ (2014) Chondrocyte redifferentiation and construct mechanical property development in single-component photocrosslinkable hydrogels. J Biomed Mater Res A 102(8):2544–2553. https://doi.org/10.1002/jbm.a.34924
Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci-Unal G, Dokmeci MR, Peppas NA, Khademhosseini A (2014) 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv Mater 26(1):85–124. https://doi.org/10.1002/adma.201303233
Hynes WF, Doty NJ, Zarembinski TI, Schwartz MP, Toepke MW, Murphy WL, Atzet SK, Clark R, Melendez JA, Cady NC (2014) Micropatterning of 3D microenvironments for living biosensor applications. Biosensors (Basel) 4(1):28–44. https://doi.org/10.3390/bios4010028
Pereira RF, Bartolo PJ (2015) 3D bioprinting of photocrosslinkable hydrogel constructs. J Appl Polym Sci 132(48). https://doi.org/10.1002/app.42458
Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA (2009) Hydrogels in regenerative medicine. Adv Mater 21(32–33):3307–3329. https://doi.org/10.1002/adma.200802106
Duan B, Kapetanovic E, Hockaday LA, Butcher JT (2014) Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater 10(5):1836–1846. https://doi.org/10.1016/j.actbio.2013.12.005
Kim BS, Jang J, Chae S, Gao G, Kong JS, Ahn M, Cho DW (2016) Three-dimensional bioprinting of cell-laden constructs with polycaprolactone protective layers for using various thermoplastic polymers. Biofabrication 8(3):035013. https://doi.org/10.1088/1758-5090/8/3/035013
Axpe E, Oyen ML (2016) Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci 17(12). https://doi.org/10.3390/ijms17121976
Nguyen D, Hagg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, Kalogeropoulos T, Zaunz S, Concaro S, Brittberg M, Lindahl A, Gatenholm P, Enejder A, Simonsson S (2017) Cartilage tissue engineering by the 3D bioprinting of iPS cells in a Nanocellulose/alginate bioink. Sci Rep 7(1):658. https://doi.org/10.1038/s41598-017-00690-y
Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P (2015) 3D bioprinting human chondrocytes with Nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 16(5):1489–1496. https://doi.org/10.1021/acs.biomac.5b00188
Bakarich SE, Gorkin R, in het Panhuis M, Spinks GM (2014) Three-dimensional printing fiber reinforced hydrogel composites. ACS Appl Mater Interfaces 6 (18):15998–16006. doi:https://doi.org/10.1021/am503878d
Wang Y, Wu S, Kuss MA, Streubel PN, Duan B (2017) Effects of hydroxyapatite and hypoxia on Chondrogenesis and hypertrophy in 3D bioprinted ADMSC laden constructs. ACS Biomater Sci Eng 3(5):826–835. https://doi.org/10.1021/acsbiomaterials.7b00101
Yang J, Zhang YS, Yue K, Khademhosseini A (2017) Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta biomaterialia 57 (supplement C):1-25. https://doi.org/10.1016/j.actbio.2017.01.036
Cui X, Breitenkamp K, Lotz M, D'Lima D (2012) Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation. Biotechnol Bioeng 109(9):2357–2368. https://doi.org/10.1002/bit.24488
Kesti M, Eberhardt C, Pagliccia G, Kenkel D, Grande D, Boss A, Zenobi-Wong M (2015) Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv Funct Mater 25(48):7406–7417. https://doi.org/10.1002/adfm.201503423
Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ (2010) Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376(9739):440–448. https://doi.org/10.1016/S0140-6736(10)60668-X
Hung K-C, Tseng C-S, Dai L-G, Hsu S-H (2016) Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 83(Supplement C):156–168. doi:https://doi.org/10.1016/j.biomaterials.2016.01.019
Gao G, Zhang XF, Hubbell K, Cui X (2017) NR2F2 regulates chondrogenesis of human mesenchymal stem cells in bioprinted cartilage. Biotechnol Bioeng 114(1):208–216. https://doi.org/10.1002/bit.26042
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Mora-Boza, A., Lopez-Donaire, M.L. (2018). Preparation of Polymeric and Composite Scaffolds by 3D Bioprinting. In: Oliveira, J., Pina, S., Reis, R., San Roman, J. (eds) Osteochondral Tissue Engineering. Advances in Experimental Medicine and Biology, vol 1058. Springer, Cham. https://doi.org/10.1007/978-3-319-76711-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-76711-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76710-9
Online ISBN: 978-3-319-76711-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)