Abstract
The necessity to develop automated methods for the fast screening of new libraries of compounds and the identification of active entities from natural mixtures has led to an increasing interest in the development of immobilized enzyme reactors (IMERs). This strategy overcomes some drawbacks of the in-solution methods and is, therefore, very attractive in the drug discovery field. This review gives an overview of IMER applications in the last decade. The reported examples concern conventional columns as well as capillary reactors integrated in liquid chromatography or capillary electrophoresis systems, coupled to spectroscopic or mass spectrometry detectors. The experimental setups and main features as well as characterization of new active entities are discussed. As a result of the growing importance of compounds from natural sources in drug discovery, particular attention is given to IMERs developed to be used for the identification of bioactive compounds.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The drug discovery process is based on the knowledge of the molecular aspects of different diseases and the development and application of techniques suitable to investigate the biological systems at molecular level. In this context many efforts have been made to obtain suitable bioanalytical platforms for the high-throughput screening (HTS) of libraries of synthetic or natural molecules towards specific molecular targets. The application of immobilized enzyme reactors (IMERs) in mid-throughput analysis during the last decades has demonstrated this approach to be an efficient alternative to in-solution methods [1,2,3,4]. IMERs have found application in different fields spanning from proteomics, genomics to medical research and drug discovery. Moreover, the pharmaceutical industries have been attracted by their potential application in high-throughput experimentations and in combinatorial synthesis. So much interest is due to the numerous advantages related to the “immobilization” of the target enzyme onto a solid support. The use of such system, indeed, allows one to overcome some drawbacks of in-solution assays related to the use of fluorogenic substrates or tandem enzymes that can lead to some false positive results. In-solution analysis based on the use of fluorescent substrates, as in FRET assay, can be affected by the contribution of uncleaved fluorophores to the background signal and by some intermolecular quenching phenomena. The in-solution methods also require long incubation times that can promote the detection of false positive results due to non-specific inhibitors or to aggregation-forming inhibitors. Moreover, the absence of a tandem enzyme (i.e., luciferase used for kinases inhibition assay) obviates the possibility for the tested compounds to interact with the secondary enzyme. The most appealing benefits offered by IMERs include very low sample consumption (usually 10–50 μL of sample at micromolar concentration is injected when chromatographic systems are used while few microliters are required for capillary electrophoresis (CE)-based systems), increased stability of the immobilized enzyme, efficient catalysis, and short analyses (usually within minutes). The last feature is appealing when large libraries need to be screened for hit selection. The success of IMERs is also linked to the possibility to be connected to different separation systems such as HPLC and CE. Moreover, the recent hyphenation to mass spectrometry (MS), resulting in lower sample consumption and high sensitivity though holding the advantages of conventional enzyme immobilization, has raised great interest [5, 6]. Designing new IMERs first involves the selection of a suitable support material (inorganic or organic, natural, or synthetic) and format (disks, capillaries, columns, microchannels). In this respect, it must be kept in mind that there is no universal support for all enzymes and the best suitable material depends on the enzyme characteristics and IMER application. However, a set of desirable characteristics are common to any material considered for enzyme immobilization [7]. These include high surface area to ensure high-density enzyme loading, chemical stability, and suitable mechanical properties as well as suitable surface chemistry to allow immobilization but to avoid strong non-specific interactions with substrates or tested ligands. Finally, the chemical and physical nature of the binding has to be carefully selected to ensure that enzyme’s activity is retained after immobilization. Immobilization strategies can be roughly divided into two major categories: covalent and non-covalent. The main advantages of covalent immobilization are related to the strong binding which ensures negligible leakage of enzyme from the support during usage. On the other hand, covalent immobilization usually implies that the enzyme is in contact with reagents (even if mild conditions are used) which may cause enzyme denaturation, alter enzyme function, or change enzymatic activity or affinity for the substrate [8]. A number of immobilization strategies have been explored during recent decades and have been commented on in several reviews [2, 7, 9]. In general, techniques that could be used for enzyme immobilization can be divided into enzyme entrapment [10, 11], cross-linking [12,13,14], adsorption [15], covalent bonding [16,17,18], affinity linkage [19, 20], and a combination of these methods. Among these, covalent immobilization is the one most exploited thanks to the high stability of the obtained IMER which ensures negligible enzyme leakage during use. In parallel to the progress in microreactor development, the functional linkage of the target enzyme on the inner wall of silica capillaries [21,22,23] and nanoparticles [24] has reached a very broad appeal [25]. In fact the ongoing development of new technologies has been matched with the production of better-performing materials and IMER miniaturization which have enlarged their application in different fields. Notwithstanding several advantages, it must be mentioned that developing a new IMER is a challenging procedure which requires time to complete. Therefore the development of a new IMER is worth being pursued if it is estimated to be cost- and effort-effective, i.e., the advantages of reusing the same amount of enzyme over multiple cycles is advantageous in terms of costs, the high IMER stability ensures long life and its use for hundreds of injections, and the on-line setup helps to circumvent drawbacks of established in-solution assays such as the need for report enzymes or complex assay procedures. Finally it must also be underlined that it is not possible to develop an immobilized enzyme system for all target enzymes. Specific feasibility needs to be evaluated case-by-case.
This review reports the application of on-line IMERs in the drug discovery process during the last decade. An overview of the investigated targets, immobilization approaches, and analytical conditions for screening small molecules with particular attention to the application on natural products in drug discovery will be given.
Determination of Enzymatic Activity after Immobilization, Chromatographic Data Elaboration for IC50, and Kinetic Constant Determination
The enzyme immobilization process is based on three different steps: (1) the choice of most suitable support and immobilization strategy; (2) the evaluation of the immobilization yield and residual enzyme activity; (3) the validation with known reference compounds and screening of enzyme inhibitors [7]. Immobilization conditions can significantly affect the enzyme leading to enzyme denaturation and consequent loss of activity. Furthermore when a non-oriented immobilization procedure is used, access to binding site(s) may be hindered and prevent substrate binding and conversion. Therefore, other than the determination of the amount of protein retained on the support after immobilization, the quantitation of the amount of active units of enzyme is also a crucial step. It is worth mentioning that, other than immobilization conditions, the activity of immobilized enzyme could also be affected by limitations in substrate accessibility to the active sites or by the selected chromatographic conditions such as pH, composition of the mobile phase, and percentage of organic solvent. These aspects need to be taken into account when a new IMER is developed.
While quantification of the amount of protein immobilized can be carried out off-line by using different methods including colorimetric and fluorescent assays or chromatographic methods [26] and comparing the amount of protein in the immobilization buffer before and after immobilization, the determination of active units in the IMER apparatus requires that an on-line activity assay be set up first. As a general procedure, the estimation of retained active units is achieved by injecting increasing concentrations of the natural substrate or of a substrate analogue and determining the velocity of substrate conversion into the product. The apparent Michaelis–Menten constant (KMapp) and vmaxapp can be calculated by constructing the Michaelis–Menten graph (catalysis rates versus normalized substrate concentration) or corresponding Lineweaver–Burk plots. The normalized substrate concentration takes into consideration the distribution of the substrate within the IMER and it is calculated by the following formula:
where BV is the bed volume of the IMER, Vinj is the injected volume, and Cinj is the injected substrate concentration.
From the vmaxvalue the immobilized active units can be determined. The inhibition studies are usually performed in zonal mode, i.e., injecting increasing inhibitor concentrations in the presence of a fixed amount of substrate, most commonly at saturating concentration. Percentage inhibition is calculated by comparing the peak areas obtained in the presence and in the absence of inhibitor. Specifically, the percentage inhibition is obtained by the following expression:
where AUCi and AUC0 are the product peak area calculated in the presence and in the absence of the tested inhibitor, respectively. The construction of a correlation curve is also recommended in order to compare the IC50 values obtained with the IMER with those reported in literature, calculated by classical in-solution assay. Inhibition constant (Ki) values can also be determined simply by adapting the in-solution assay to the on-line approach, i.e., by injecting mixtures of increasing concentrations of substrate and tested inhibitor and processing data to get Lineweaver–Burk, Cornish-Bowden, or Dixon plots [27,28,29]. As a further consideration, although monitoring a specific feature, i.e., product formation or substrate disappearance, non-specific interactions between tested inhibitors and the chromatographic support may arise and alter the contact time. To account for such interactions, the use of a blank column, which has been prepared following the same immobilization procedure but without active immobilized enzyme, is strongly suggested.
Immobilized Enzyme Reactors Coupled with HPLC and UV–Vis Detection
The choice of coupling IMER with HPLC derives from the combination of high selectivity and sensitivity with the possibility to perform automated, fast, and reproducible analysis. Thanks to the advantages in terms of low pressure drop, mechanical and chemical stability, and suitable mass transfer properties, macroporous monolithic materials have become increasingly popular for applications not only in chromatographic separations but also in IMER preparation. Monolith materials have been prepared and used in various geometrical formats, including disks, columns, or tubes. In particular, CIM™ disks are monolithic disk-shaped chromatographic columns which have been successfully used as support matrices for the preparation of IMERs owing to the low resistance to mass transfer and fast analysis time [30]. CIM™ disks are of small dimensions (12 mm in diameter and 3 mm in thickness) and are available with different surface chemistries, which can be exploited for target immobilization. Epoxy–CIM disks have been widely used for enzyme immobilization thanks to the easy one-step immobilization procedure which involves a nucleophilic attack on the epoxy groups by amine, thiol, or hydroxy groups on the target enzyme [31]. Immobilization on ethylendiamine (EDA)–CIM disk usually involves reaction with glutaraldehyde in order to introduce reactive aldehyde groups which can subsequently form Schiff bases upon reaction with nucleophilic residues from the target enzyme, most commonly amino residues in the side chain of lysines, followed by reduction of the imine groups by a mild reducing agent in order to stabilize the linkage (Fig. 1).
Other than offering a reactive group for easy immobilization in mild conditions, the use of glutaraldehyde also grants the insertion of a spacer which, in most cases, has been shown to be fundamental to (1) ensure a suitable degree of flexibility, which is important in the case of enzyme rearrangements upon substrate binding; (2) achieve a higher availability of the enzyme binding site(s) [32]; and (3) keep the enzyme away from the matrix surface in order to preserve it from adsorption phenomena or loss of the native conformation. After immobilization, both the amount of immobilized protein and the activity of the enzyme must be assessed. Since enzymes can undergo denaturation during the immobilization process, the amount of immobilized protein often does not correspond to the residual amount of active units. While determination of the amount of immobilized enzyme can be carried out using several techniques generally involving quantitation of the enzyme in the immobilization solution before and after immobilization, the activity assessment by an on-line activity assay should first be set up. This might be a tricky step that requires a good knowledge of separation techniques but, most importantly, of the biochemical and catalytic characteristics of the target enzyme. Once analysis conditions are defined, in the most common zonal function-based approach [26], increasing concentrations of the substrate, or of a substrate analogue, are injected into the IMER, after its insertion into an HPLC system, in order to obtain the Michaelis–Menten curves and calculate the vmax and Km values, by determining the peak area of the eluting unmodified substrate or formed product. The evaluation of Km for each substrate is critical to compare the behavior of the enzyme after immobilization with that in solution and determine the best conditions to perform inhibition studies. Then, in screening experiments, inhibition studies are carried out by injecting mixtures of substrate and inhibitor and calculating the percentage inhibition from the product peak area obtained in the presence of the tested inhibitor and after injection of the substrate solution, as reported in “Determination of Enzymatic Activity after Immobilization, Chromatographic Data Elaboration for IC50, and Kinetic Constant Determination” (Fig. 2).

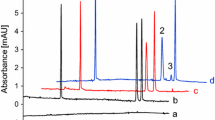
Reprinted with permission from J. Pharm. Biomed. Anal. [33]
Overlaid chromatograms obtained after injection of selected inhibitor at increasing concentration in the presence of substrate. The chromatographic peaks refer to M-2420 BACE1 substrate hydrolysis product.
β-Site Amyloid Precursor Protein-Cleaving Enzyme-Based IMERs
One of the major class of IMERs based on chromatographic columns and developed in the last decade focuses on target enzymes involved in Alzheimer disease (AD) drug discovery. Following the positive results from the previous preparation and application of AChE- and BuChE-IMERs [32, 34], the human recombinant β-site amyloid precursor protein-cleaving enzyme (hrBACE-1) was covalently immobilized on an EDA–CIM disk. The obtained IMER was used for screening molecules selected through a virtual screening approach and of natural origins [33, 35, 36]. The strategy for BACE1-IMER preparation was based on covalent enzyme immobilization [37]. The hrBACE1-IMER was inserted into an HPLC system coupled to a fluorescence detector. The M-2420 substrate and substrate IV, both mimicking the peptide sequence of the Swedish-mutated APP sequence, targeted by BACE-1, were used [33, 36]. The retained active units were then evaluated as previously described [38] by injecting the fluorogenic substrates which, upon cleavage by immobilized BACE-1, generated fluorescent products that could be easily quantitated (Table 1). The best chromatographic conditions were established for both substrate and product. In inhibitor screenings, according to the spectroscopic features of the tested molecules [39], either M-2420 or substrate IV was employed. The ability of the tested compounds to inhibit BACE-1 was related to the reduction of chromatographic peak of the fluorescent enzymatic product. The use of M-2420 substrate allowed the discovery of a bis-indanone derivative and its characterization in terms of IC50 and Ki (Table 1). This compound was selected from a small library of 38 compounds obtained by a virtual screening approach. The IC50 value of 5.1 μM and a Ki of 17.5 μM established by the IMER-HPLC system resulted in agreement with those obtained by the in-solution FRET assay.
Of great interest is the application of BACE1-IMER to the screening of natural compounds. The first reported study was based on the use of Corydalis cava in folk medicine for the treatment of memory dysfunction. A series of 15 isolated alkaloids were characterized in terms of inhibitory potency by both in-solution and on-line methods. The results obtained by monitoring the decrease in M-2420 substrate peak product were in agreement with those obtained by in-solution FRET assay. As reported by Chlebeck et al. the use of M-2420 substrate is not widely applicable for compounds of natural source because of possible fluorescence interference [35]. For instance, the use of substrate IV, whose cleavage product 1,5-EDANS (5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid) is characterized by higher excitation and emission wavelengths, is devoid of spectroscopic interference, allowing testing of a wider range of compounds (Fig. 3). That feature is important in order to avoid false positive or negative results related to quenching or absorption phenomena. This substrate was used for the characterization of uleine, a natural compound derived from Himatanthus lancifolius, a Brazilian native species [40]. The BACE1-IMER showed a higher reaction rate, likely owing to the high local concentration of enzyme and to the high accessibility of the active site. Moreover, the risk of precipitation of both substrate and tested compound is lower compared to the in-solution method.

Reprinted with permission from J. Chromatogr. B. [36]
Overlaid chromatograms obtained by injecting standard fluorogenic group 1,5-EDANS, substrate IV, and DMSO onto hrBACE1-IMER. The peak obtained after substrate injection and enzymatic hydrolysis was found to be consistent with that of standard product in terms of retention time.
Human Butyrylcholinesterase-Based IMER
An IMER based on human recombinant butyrylcholinesterase (rhBChE) was used in 2009 for the on-line characterization of some pseudo-irreversible BChE inhibitors as drug candidates for AD treatment [41]. The covalent immobilization of the enzyme on a monolithic column allows the drawbacks related to the use of silica-based beads to be overcome. The lack of diffusion resistance during mass transfer, high enzymatic efficiency, and reduction of analysis time are some of the advantages of the BChE-IMER [32, 34, 38, 42]. The enzyme immobilization occurred after derivatization of primary amino groups of a monolithic disk with glutaraldehyde. The amino groups of the lysine residues of enzyme reacted with the free aldehyde groups of the glutaraldehyde leading to a stable anchoring. The unreacted aldehyde groups were then inactivated by treatment with an ethanolamine solution. The BuChE-IMER activity was determined by injecting butyrylthiocholine as substrate and using a mobile phase containing Ellman’s reagent [43]. Butyrylthiocholine is hydrolyzed by the immobilized enzyme into butyric acid and thiocholine, the latter reacts with Ellman’s reagent in the mobile phase giving rise to a yellow product, whose formation is stoichiometrically related to the amount of hydrolyzed substrate and can be easily quantified by coupling the HPLC system with a UV–Vis detector. In that context, the use of 2-propanol at concentration ranging from 3% to 5% allowed the activity to be enhanced by 41%. Different flow rates were evaluated to optimize working conditions and contact time in order to improve catalytic efficiency. Apparent Km values were unaffected by the flow rate, while vmax slightly decreased increasing the flow rates likely as a result of increasing friction which can negatively influence enzyme catalytic efficiency. The applications of this BChE-IMER [41] included the study of the kinetics of enzyme inhibition by six carbamate derivatives which are known to act as pseudo-irreversible ChE inhibitors. This class of inhibitors is known to form a covalent adduct with the serine residue in the enzyme active site that is then hydrolyzed to regenerate the active enzyme. Indeed, for possible development as drugs, the determination of the carbamoylation (k2) and decarbamoylation (k3) values is of utmost importance to predict the duration of enzyme inhibition in vivo. The experimental workflow included the assessment of the initial enzymatic activity, followed by the evaluation of the kinetics of carbamoylation which was achieved by flushing the IMER with mobile phases containing different concentrations of inhibitor (Table 1). For each tested concentration, the apparent carbamoylation rate (kobs) was obtained. Among the studied inhibitors, the fastest inhibition was obtained for (−)-phenserine, in agreement with data obtained with the in-solution method. In general, the k2 values determined by the immobilized enzyme were lower (faster carbamoylation rate) than those obtained in solution likely as a result of the use of a flow-through system instead of a classic static stop-flow assay. After reaching the inhibition plateau the mobile phase was changed to clear mobile phase (without any inhibitor) and the regeneration of the enzyme occurred after hydrolysis of the carbamoyl-BChE adduct. This recovery phase was used to determine decarbamoylation rates (k3 values) by using Perola’s approach [44]. The recovery of the immobilized enzyme activity was obtained after a 2-h washing for the fastest inhibitor, i.e., (−)-phenserine. In contrast, a recovery time of 16 h was required to recover the initial activity after treatment with (−)-isobutylbisnorcymserine, the slower inhibitor. Inhibition constants obtained with the IMER were in agreement with those obtained in solution. Because in an IMER the enzyme is held in place, the use of the BChE-IMER allowed one to monitor the inactivation and regeneration steps in a single experiment with significant savings of time and enzyme compared with classic stop-time assays and dialysis. More interestingly, upon immobilization, BChE maintained its stereoselective properties, i.e., it was possible to discriminate the activities of the (−)- and (+)-enantiomers of phenserine.
Arginase-IMER
The strategy of a covalent immobilization of the target enzyme on an EDA-CIM disk was applied to prepare an arginase-IMER to be used for studying some plant-derived products with arginase activity. The arginase enzyme catalyzes the hydrolysis of arginine to ornithine and urea. The two isoforms of arginase were recently demonstrated to be expressed by vascular endothelial and smooth muscle cells, raising interest in this enzyme in cardiovascular physiology and pathology [45,46,47]. The petroleum ether extracts were obtained from the stem bark of Ficus glomerata Roxb. Arginase was covalently immobilized on an EDA-CIM disk (12 mm × 3 mm) after insertion of glutaraldehyde as spacer. The influence of pH and flow rate on arginase activity was investigated in order to assess the best enzymatic activity and chromatographic conditions. The results revealed a tenfold decrease in Km over the pH range 6.5–9.5 [48].
To validate the IMER, the IC50 values of some known inhibitors were evaluated by injecting solutions containing increasing concentration of inhibitor and saturating concentration (190 mM) of nitro guanidino benzene (NGB) as substrate. The formation of m-nitroaniline (m-NA) product was detected by UV, and peak area of m-NA at increasing inhibitor concentration was determined and plotted versus injected inhibitor concentration to obtain inhibition curves for all the tested inhibitors. The pIC50 values were in good agreement with those from in-solution experiments. Optimized chromatographic conditions were used to screen extracts from Ficus glomerata Roxb. and of procyanidins. The inhibitory activity of these natural products was evaluated by injecting mixtures of increasing concentrations of the tested extract (ranging from 0.1% to 0.9%) and a fixed concentration of the substrate NGB. The extracts were found to inactivate arginase by up to 70%, in agreement with previously reported data [49].Their effects on the kinetic parameters were also investigated. Procyanidins seemed to affect the vmax value without affecting the Km. This result suggested that procyanidins do not influence substrate binding and act as non-competitive inhibitors. The highest inhibition potency was found for the extract containing the largest amount of procyanidin (Table 1). These results open the possibility of treating NO-dependent smooth disorders with these plant extracts [45].
Immobilized Capillary Enzyme Reactors
In order to assess the activity and the selectivity of small library of coumarin derivatives, human [50] and Electric eel AChE and human BChE were immobilized on fused silica capillary supports using glutaraldehyde as a spacer [50,51,52]. The interest in coumarin derivatives is based on their biological activities [53,54,55,56]. The importance of BChE as target is based on the observation that imbalance in AChE and BChE activity in patients with AD makes BChE an appealing target for mild-to-moderate AD forms [57]. The use of immobilized enzymes is a great advantage since the analysis of coumarin derivatives may be altered by false positive results due to spectroscopic interference when classical in-solution methods are used. The enzymatic activity of the reactor was measured through the formation of the yellow anion (YA), resulting from the reaction between the product thiocholine, which was generated by enzymatic cleavage of the substrate acetylthiocholine (ATCh), and Ellman’s reagent [43]. Interestingly, immobilization increased enzyme stability toward organic modifier content. Indeed, cholinesterases maintained their activity at a high concentration of organic modifier (up to 80%). The inhibitory activity of the coumarins was evaluated for each enzyme. The results highlighted the good selectivity of an 8-hydroxycoumarin-3-carboxylic acid ethyl ester compound towards BChE. No significant difference between values obtained with the IMERs and those from in-solution assay [58] was observed.
Owing to the growing interest in natural products, many recent applications of capillary immobilized enzyme reactors have focused on the screening of natural extracts. The most recent application (2018) was reported by Cornelio et al. [59] who performed the immobilization of bovine cathepsin D (CatD) on fused silica open tubular capillary. CatD is an aspartic protease involved in peptide bond hydrolysis. Its overexpression has been implicated in degenerative processes and cancer [60]. For these reasons it is considered a good target in the search for new chemotherapeutics. The IMER showed advantageous features including low leakage of the immobilized enzyme, low back pressure, and high surface to volume ratio [59, 61]. For screening purposes, the CatD-IMER was placed in the first dimension of a 2D-liquid chromatography (LC) system that was used in zonal elution conditions to examine more than one compound per injection. The enzyme activity was measured using the fluorogenic undecapeptide 7-methoxycoumarin-4-acetic acid-(MOCAc)-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys(DNP)-d-Arg-NH2 (S-MOCAc) as substrate. Hydrolysis of the substrate leads to the formation of MOCAc-Gly-Lys-Pro-Ile-Leu-Phe (P-MOCAc). This substrate was used for both on-line and in-solution approaches. The kinetic parameters were evaluated before the screening of plant extract, mostly belonging to the Rutaceae family such as Almeidea sp., Hortia longifolia, Metrodorea nigra, Pilocarpus riedelianus, Neoraputia magnifica, and Lithraea molleoides. A Km value of 81.9 ± 7.49 μmol/L was obtained. This value is 30 times higher than that obtained by the in-solution method. The dried extracts were tested against CatD using both the IMER-based approach and the in-solution method. The percentage of inhibition obtained for the extracts with the on-line methods were three times lower compared with those obtained by in-solution methods. On the other hand, inhibition values obtained for the isolated phytocomponents evolitrine, 4-hydroxy-3-methoxycinnamadehyde, and (Z)-2-(pentadec-5-enyl)benzene1,4-diol from the tested extracts were in accordance with the in-solution inhibitory potencies.
In 2012, de Moraes et al. [62] reported the immobilization of purine nucleoside phosphorylase (PNP) which catalyzes the cleavage of (deoxy)ribonucleosides to the corresponding purine bases and (deoxy)ribose-1-phosphate, in the presence of inorganic phosphate (Pi). PNP inhibitors represent a class of immunosuppressive agents useful in the treatment of a wide variety of T cell-mediated disorders [63].The most widely employed assay for the evaluation of PNP inhibitors is a coupled assay in which hypoxanthine (Hypo) released by inosine (Ino) phosphorolysis is oxidized by xanthine oxidase (XOx) to generate uric acid, which is spectrophotometrically monitored at 293 nm [64]. The method proposed by Kalckar in 1947 unfortunately presents some issues related to potential false positive outcomes due to the inhibition of the reported enzyme XOx. To achieve trustable data it is therefore important to develop and apply a direct method. This idea has prompted the development of a human PNP (hPNP)-IMER by covalently immobilizing the target enzyme on an activated silica capillary previously derivatized with glutaraldehyde as spacer. For IMER validation, the fourth-generation immunocillin derivative DI4G was used (Table 1). For on-line analysis the hPNP-IMER was inserted in the first dimension of a multidimensional chromatography system. To assess enzyme activity, the substrate (Ino) and the product (Hypo) were separated and quantified after elution from the IMER by a reversed-phase analytical column (10 cm × 0.46 mm I.D.) which was coupled to the hPNP-IMER by a three-way switching valve. Affinity of the immobilized enzyme for the substrate was slightly different when compared to that of the enzyme in solution (Km value calculated for the IMER was about 1.9 higher). The IMER was used for the determination of the inhibition potency of DI4G, a potent PNP inhibitor, as well as for the investigation of its mechanism of action. The IC50 value obtained with the IMER was about an order of magnitude higher than that obtained by in-solution assay. On the other hand, the results on the evaluation of the mechanism of action were in agreement with those obtained by in-solution assay (competitive inhibitor). The higher Ki parallels the higher value of Km found in the evaluation of the affinity of the immobilized enzyme for the substrate. The authors estimated that, thanks to the short analysis time and the use of an autosampler, approximately 100 compounds could be screened per day. Thus, on the basis of this and other advantages such as cost-saving and method robustness, and notwithstanding issues related to underestimation of the inhibitory potency, the authors claim that the system could find application in drug discovery campaigns.
IMERs Coupled with HPLC Tandem Mass Spectrometry
IMER assays were initially performed using both spectroscopic and MS detection modes. Spectroscopic methods allow a wider range of buffers, but suffer from potential interferences from strongly absorbing compounds, while MS/MS methods can be used in cases where colorimetric reagents are not available. On the other hand the use of low ionic strength buffers compatible with the electrospray process is necessary. Here, we briefly report some applications of IMER inserted in an LC system hyphenated with tandem MS. As reported in the first part of this review both AChE and BChE have been extensively explored as immobilized targets [41, 65]. So it is not surprising that one of the most recent applications of IMER coupled to LC–MS concerns this class of enzymes. Specifically, Cardoso and co-workers reported the development and application of capillary fused AChE-IMER and BChE-IMER [50, 66]. In the first application the AChE-IMER was used in an LC/tandem ion trap mass spectrometer. Methanol, delivered by a different pump through a T-shaped connection system, was used to improve ionization (Fig. 4). The enzyme activity was evaluated by multiple reaction-monitoring mode (MRM) selecting the transition of choline precursor ion [(M + H)+, m/z 104.0] and its ion fragment [(C2H3OH)(M + H)+, m/z 60.0] (Fig. 5). Both electric eel (ee)AChE and hAChE were immobilized. The evaluation of the kinetic parameters confirmed that cholinesterases maintained their activity toward the natural substrate acetylcholine (ACh). The ACER-LC–MS system was then used for the screening of some coumarin derivatives. As a final result, two hits were identified and their IC50 and Ki values were determined. More recently, Cardoso and co-workers reported the development of a capillary BuChE-IMER and its coupling with an MS detector [66]. The experimental conditions and instrument setup were the same used for the previously reported application. The same T-shaped connection was used to deliver both methanol and the running buffer. The KMapp values for butyrylcholine (BCh) and ACh were of 39.9 ± 3 and 44.8 ± 10 μM, respectively. Although BCh is the substrate of hBChE, the authors used ACh as substrate based on the consideration that BChE can also hydrolyze ACh, even if with a lower turnover rate [67]. The main goal of this work was to demonstrate the capability of the applied system to rank inhibitors with different potency. To validate the IMER, tacrine and galantamine were assayed as standard as well as uleine [40] (Table 1).The obtained results compared with those reported in the literature demonstrate the capability of the applied method to distinguish between inhibitory potency.

Reprinted with permission from Anal. Biochem. [66]
EIC (extracted ion chromatogram) and IT-MS spectrum obtained after the enzymatic hydrolysis using the LC–MS system with capillary hBChE-IMER after injection of ACh. The products of enzymatic hydrolysis Ch [M + H]+m/z 104.17 and acetate [M + H]+m/z 59.39; substrate ACh [M + H]+m/z 145.99 are detectable.
Other interesting applications of IMER-LC–MS were reported by Brennan and his research group [68, 69]. The IMER–tandem MS system was used for the screening of bioactive compound mixtures on AChE-IMER coupled to an ESI–MS/MS detector. In the work from 2011 [68] the IMER was obtained by a sol–gel entrapment method [70]. Studies on IMER were initially performed using both absorbance (Ellman’s assay) and MS detection modes. Indeed the use of a colorimetric method (coupling with UV–Vis detection) allows the use of a wider range of buffers, but suffers from potential interferences from strongly absorbing compounds. On the other hand, coupling with MS detector offers advantages in terms of selectivity and limited interferences but requires the use of low ionic strength buffers. MS/MS analyses were carried out in MRM mode, and suitable parent and daughter ions were identified for acetylthiocholine (ATCh) and thiocholine (TCh) as substrate and product of enzyme activity, as well as for galantamine and huperzine A as test inhibitors. The obtained signals were intense and no background interference or overlaps between the signals of different species were encountered. The product/substrate (P/S) ratios vs inhibitor concentration were used to assess inhibition. By monitoring the P/S ratios, the authors screened 52 randomly selected mixtures, consisting of 1040 compounds, from the Canadian Compound Collection. In the optimized assay conditions a full signal recovery was obtained after equilibration for weak inhibitors with Ki values in the high nanomolar or greater range, while in the presence of potent compounds, slower recovery times were registered leading to some carryover effects. In the presence of a covalent inhibitor in the screened mixture, the full recovery of the enzyme activity was not possible (irreversible inhibition) and thus required the preparation of a new IMER. Mixtures giving a decrease in the P/S signal ratio higher than 50% were selected to be further characterized for identification of individual components. This approach allowed the identification of physostigmine and 9-acridinamine as active phytocomponents. Note that analysis reproducibility was higher when single phytocomponents were assayed than when mixtures were evaluated. This has been attributed to the lower content of DMSO used in single-component analysis (0.1% versus 2%) which allows a higher enzymatic activity. Moreover, when single components are analyzed a lower ion suppression is encountered.
A similar approach was applied by Forsberg and Brennan to the screening of bioactive mixtures on adenosine deaminase (ADA). This is an important enzyme in purine metabolism and catalyzes the deamination of adenosine to inosine [69]. Increased ADA levels are reported to be involved in various health disorders such as hereditary hemolytic anemias, systemic lupus erythematosus, inflammatory responses, rheumatoid arthritis, heart diseases, tuberculosis, and some types of carcinoma. Hence, the interest in the development of ADA inhibitors arises from the wide range of applications of these drugs in different pathologies [71,72,73]. The target enzyme was covalently immobilized onto monolithic silica capillary columns. The ADA-IMER was coupled with an ESI–MS detector. The identification of bioactive compounds from mixtures was achieved by coupling the IMER with bioselective solid-phase extraction (bioSPE) columns; the screening platform developed by the authors featured a two-step bioassay: (1) identification of mixtures containing bioactive compounds towards the target enzyme by ADA-IMER interfaced with ESI–MS detector, (2) isolation of active compounds from identified “hit” mixtures by bioSPE followed by their identification by ESI–MS. In the first dimension, specifically bound compounds were washed out by flushing the IMER with buffer, while the strongly bound ligands were eluted by washing with an acidic solution (3% acetic acid). The developed platform allowed the identification of the active compounds. EHNA and MAC-0038732, a potent and a weak ADA ligand, respectively, were successfully isolated and identified by bioSPE, both in MRM and data-dependent acquisition (DDA)-MS modes.
The previously reported human purine nucleoside phosphorylase fused silica capillary hPNP-IMER obtained by de Moraes and co-workers [62] was also used for affinity screening studies of new inhibitor exploiting MS detection [74]. These studies were carried out by frontal affinity chromatography tandem MS (FAC-MS). Different monolithic supports including Chromolith Speed Rod (0.1 mm I.D. × 5 cm) and a methacrylate-based monolithic polymeric capillary column (0.25 mm I.D. × 5 cm) with epoxy reactive groups were employed. Inosine (Ino) was used as substrate to determine and compare activity as well as Km values upon immobilization of hPNP on the selected columns. Results showed that kinetic parameters were influenced by the immobilization procedure [75,76,77]. The Km values obtained using polymeric monolithic capillary (PMC) and silica monolithic capillary (SMC) were respectively sevenfold and tenfold higher than values obtained by the in-solution method. The ability of the proposed IMERs to screen compounds with different affinity was tested by continuously infusing a mixture containing two inhibitors and one compound with no inhibitory activity into the system. The analytes were detected in positive ion mode by ESI–MS/MS. In agreement with expectations, the inactive compound was the less retained while the two inhibitors were retained more, in agreement with a significantly higher affinity for the target. The same ranking order was obtained for all the tested supports, thus demonstrating that the type of support did not influence the capability of the FAC-MS/MS method to discriminate inhibitors from a compound mixture.
CE-Based IMERs
Its peculiar analytic advantages including high separation efficiency, short analysis time, and minute sample consumption, make CE suitable for CE-based IMERs development. Optimized enzymatic assays based on CE, indeed, are rapid and automatable [9, 78, 79]. Although the species resolved by the electrophoretic approach could be directly revealed by several detectors including UV, LIF, and MS, the most exploited one still is UV–Vis detection [9]. This section focuses on works reporting on IMER development to be used in CE systems. The overall characteristics of the proposed IMERs are summarized in Table 2.
In 2010 Ji et al. [80] reported on the development of adenosine deaminase (ADA) microreactor (Table 2) useful for ADA inhibition studies. The protocol proposed by the authors to obtain the ADA microreactor was based on enzyme encapsulation in alginate, a linear anionic polysaccharide, and its immobilization on the surface of a fused-silica capillary pretreated with polyethylenimine (PEI), a strong cation exchanger (Fig. 6a). Evaluation of IMER activity was carried out by substrate (adenosine) and product (inosine) CE separation and UV quantification at 254 nm. Inhibition studies were performed by injecting the buffer containing the single inhibitor (or the natural extracts) into the capillary and leaving it in place for 3 min. After that time, a mixture of substrate and inhibitor was injected, and the separation voltage was applied. Inhibition studies were first performed using a known inhibitor, erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA), and the resulting Ki (12.1 nM) was in agreement with the previously reported values (1.5–6 nM) [81]. Finally the optimized method was applied for screening 19 natural extracts. Among these, the extract from Rhizome chuanxiong was the only one endowed with inhibitory activity toward ADA (inhibition percentage 48.0%) (Table 2).

Reprinted with permission from [80]
a Schematic representation of ADA-based capillary microreactor. b Electropherograms related to substrate (adenosine, A) and product (inosine, I) separation obtained upon injection into the ADA-IMER of adenosine alone (I), adenosine in the presence of EHNA 12 nM (II), or in the presence of Rhizome chuanxiong 0.6 mg/mL (III).
ADA was also used, along with xanthine oxidase (XOD), for the development of a dual IMER by Lin et al. (Table 2) [82]. XOD catalyzes the oxidation of hypoxanthine and xanthine to uric acid, hydrogen peroxide, and superoxide radicals [83]. Since uric acid is involved in the onset of gout and superoxide radicals are involved in a series of pathological inflammatory processes, the inhibition of XOD is recommended for the treatment of related diseases [84]. The capillary microreactor was prepared by co-immobilizing ADA and XOD on citrate-capped gold nanoparticles, which were then injected into the PEI-modified capillary, allowing the immobilization through electrostatic interaction between the negatively charged enzyme-functionalized gold nanoparticles and the positive charges of the capillary wall. In the CE optimized conditions, substrates and products were baseline-separated within 3 min. Inhibition studies were first performed by employing EHNA and 4-aminopyrazolo[3,4-d]pyrimidine as selective inhibitors for ADA and XOD, respectively. Interestingly, Ki values exactly matched the values previously reported in the literature [85]. Finally, the proposed dual IMER was applied to screen 20 natural extracts from medicinal plants for anti-ADA and anti-XOD activity. Results showed that natural extracts from Rhizome chuanxiong and Indigo naturalis were able to inhibit ADA, while extracts from Rhizome chuanxiong, Cortex Phellodendri, Rhizome Alpiniae officinarum, and Ramulus cinnamomi were endowed with inhibitory capacity toward XOD (Table 2).
In 2011 Iqbal [86] reported on the development of an IMER with immobilized alkaline phosphatases (ALPs). ALPs can be classified into tissue-specific ALPs (placental, intestinal, and germ cell) and tissue-nonspecific alkaline phosphatase (TNAP) [87]. Intestinal ALP plays a role in fat absorption in the gastrointestinal tract and in gut mucosal defense, while TNAP is able to hydrolyze inorganic pyrophosphate, a potent calcification inhibitor; hence, TNAP-selective inhibition could be beneficial in the treatment of osteoarthritis [88]. In this work, two distinct IMERs were prepared by immobilizing CIAP, a calf intestinal ALP, and TNAP on capillaries pretreated with a 1,5-dimethyl-1,5-diazaundecamethylene hexadimethrine bromide (polybrene, PB) solution which produced a positively charged coating layer which could be used for enzyme adsorption through ionic interactions. PB, acting as electroosmotic flow (EOF) modifier, also allowed an improved separation of substrate (4-nitrophenylphosphate, NPP) from the product (4-nitrophenol, NP). Interestingly, the introduction of the sample into the capillary by electro injection resulted in an increased assay sensitivity when compared with normal pressure injection. Inhibition studies on CIAP-based IMER were performed using three standard inhibitors, namely inorganic phosphate (competitive inhibitor), theophylline (non-competitive inhibitor), and arsenate (irreversible inhibitor), while inhibition of TNAP-IMER activity was investigated using different concentrations of levamisole. Resulting Ki values were close to those obtained using a 96-well microplate reader and to literature values [89].
An on-line immobilized glucose oxidase (GOx) capillary microreactor was developed in 2012 by Wang et al. [90]. GOx has been commonly used as a model enzyme in several disciplines. Among other applications, GOx was successfully used for the determination of several heavy metals as environment contaminants with health implications [91]. GOx was immobilized at the inlet end of the capillary using a cross-linking method. Activity of immobilized GOx was determined by monitoring the reduction of 1,4-benzoquinone to hydroquinone. For inhibition studies, Ag+ and Cu+ were used as model inhibitors. The calculated Ki values were higher than those obtained with free GOx in solution.
In 2013 Jiang [92] proposed a method for the development of a capillary tyrosinase (TRS) reactor to screen TRS inhibitors from traditional Chinese medicine. TRS is a multifunctional type-3 copper-containing metallo enzyme capable of catalyzing two distinct reactions of melanin biosynthesis: (1) the o-hydroxylation of the amino acid l-tyrosine to l-3,4-dihydroxyphenylalanine (L-DOPA) and (2) the subsequent oxidation of L-DOPA to the corresponding o-dopaquinone. TRS is involved in the enzymatic browning of fruits and vegetables and in the onset of various dermatological disorders. Hence, TRS inhibitors have aroused interest in the food, medicine, and cosmetics industries. TRS was immobilized on the inner wall of a fused-silica capillary, premodified by treatment with the cationic polyelectrolyte hexadimethrine bromide (HDB) via ionic binding. The assessment of IMER activity was performed by filling the capillary with the running buffer and injecting the substrate (l-tyrosine) in the presence and in the absence of inhibitor. After a brief incubation, application of an opportune voltage allowed the substrate to be separated from the product (L-DOPA). The inhibition kinetics of the immobilized TRS were validated using kojic acid, a known TRS inhibitor, and the corresponding Ki value (24.54 µM) was consistent with what was previously reported in the literature [93]. Finally, the inhibitory potencies of 20 traditional Chinese medicines were evaluated. Among the screened extracts, those from Fructus crataegi and Radix angelicae pubescentis were able to inhibit the target enzyme (Table 2).
Camara et al. [94] recently (2015) developed a microreactor immobilizing glucose-6-phosphate dehydrogenase (G6PDH). G6PDH is the rate-limiting enzyme that catalyzes the first step in the pentose phosphate pathway. G6PDH is involved in the etiology of some metabolic disorders including obesity and diabetes; therefore, it represents a potential therapeutic target for novel inhibitors, which are required to probe the causative mechanisms and to treat the related pathologies [95, 96]. G6PDH immobilization was achieved by exploiting a two-step protocol based on electrostatic assembly. Specifically, the inner surface of the capillary was first treated with the cation exchanger poly-(diallyl dimethyl ammonium chloride) (PDDA), a water-soluble polyelectrolyte bearing strong cation groups; then, a solution of G6PDH was injected into the capillary to get adsorbed. Enzyme activity was evaluated by monitoring the formation of NADH (resulting from the cofactor conversion during the enzymatic reaction) at 340 nm. G6PDH inhibition by six inhibitors was investigated and the Ki values were found to be consistent with those obtained using the off-line assays. Investigation of the inhibition mechanism showed that metals such as Cu2+, Pb2+, and Cd2+, vancomycin, and urea exerted a competitive inhibition.
In 2016, Schejbal et al. [97] proposed the development of a magnetic particle-based IMER for kinetic and inhibition studies of the 2C9 isoform of cytochrome P450 (CYP2C9) and its hyphenation with CE. CYP2C9 isoform constitutes approximately 20% of all CYPs in human liver, and it is responsible for the metabolism of more than 10% of commonly used drugs. Microsomal recombinant CYP2C9 (Baculosomes®) was used for IMER preparation. Prior to enzyme immobilization, carboxy groups on magnetic microparticles were activated by treatment with N-hydroxysulfosuccinimide (S-NHS) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDAC); EDAC is able to create an amine-reactive O-acylisourea intermediate which subsequently reacts with the amino groups of proteins to form a stable amide bond. Finally, CYP2C9 microparticles were loaded into a silica capillary. CYP2C9-capillary IMER was validated using diclofenac as model substrate and sulfaphenazole as a model inhibitor, in the presence of NADPH as cofactor. Although the stability of CYP2C9 upon immobilization was significantly higher than in solution, a significant loss of CYP2C9 activity (about 90% loss in 21 injections) was observed during IMER use, thus the authors opted for rinsing out the immobilized enzyme after nine runs, which correspond to a loss of about half of activity in the best-performing IMER. A great advantage of the system is the very low enzyme consumption. Indeed, only 0.5 pmol of enzyme was required for six subsequent analyses (83 fmol per analysis). However, an in-depth investigation of kinetic parameters including apparent Michaelis–Menten constant, apparent vmax, Hill coefficient, apparent Ki, and IC50 values for the conversion of diclofenac in the absence or presence of the inhibitor sulfaphenazole suggested that the characteristics of CYP2C9 were affected when nanoparticles with the immobilized enzyme were inserted into the capillary IMER, resulting in a lower affinity towards ligands. The authors related this phenomenon to a spatial distortion of the membrane of the Baculosomes® with consequent alteration of the enzyme properties. This lower affinity must be taken into account when the system is used for metabolism studies in order to properly assess the clinical relevance of the obtained data.
Conclusions
The most commonly used assays for high-throughput in vitro screening of new potential enzyme inhibitors involve in-solution assays in multiwell plates. Although based on different reaction and detection systems, these assays all share common shortcomings related to risk of contamination within multistep protocols, waste of expensive target enzyme, and low automation level unless expensive robot-based protocols are available.
Immobilized enzyme-based systems can be considered as a valid alternative choice to in-solution assays when robust, automated, and cost-effective analyses are required. These aspects are greatly beneficial in the early phase of the drug discovery process in which a large number of new compounds need to be screened. A number of recently published papers supports the idea that IMERs are quite versatile tools and can be developed for a variety of different targets and successfully used in different drug discovery areas. An overall analysis of recently published manuscripts also shows a clear trend toward miniaturization. Such an interest arises from some appealing features of miniaturized IMERs which include lower (nanoliter or microliter) sample consumption, lower amount of target enzyme needed for IMER production, and shorter run time which also translates into a higher throughput. Coupling with MS detection offers further advantages in terms of high sensitivity and higher information content. However, coupling is not always feasible and might require complex instrumental setups because of the opposite requirements of IMER for optimal activity and MS for optimal detection.
Because of the higher degree of automation, IMER-based platforms have also been shown to be particularly advantageous when complex plant extracts need to be assayed, since the bio-guided fractionation process leading to the identification of active phytocomponents is a long and complex procedure. However, despite clear benefits related to the use of IMERs in screening campaigns and the efforts in developing high-performance screening tools based on immobilized enzymes, most of the proposed IMER-based approaches are still at the development/academia level. This attrition to a widespread use of IMER-based approaches for screening campaigns at industrial level might be simply due to the preference for consolidates intramural protocols and the need for an initial time investment in building the specific expertise required to properly handle the IMER and set up the on-line analytical platform.
References
Bertucci C, Bartolini M, Gotti R, Andrisano V (2003) J Chromatogr B Analyt Technol Biomed Life Sci 797:111–129
Girelli AM, Mattei E (2005) J Chromatogr B Analyt Technol Biomed Life Sci 819:3–16. https://doi.org/10.1016/j.jchromb.2005.01.031
Jason-Moller L, Murphy M, Bruno J (2006) Curr Protoc Protein Sci 45:19.13.1–19.13.14. https://doi.org/10.1002/0471140864.ps1913s45
Choi JW, Oh BK, Kim YK, Min J (2007) J Microbiol Biotechnol 17:5–14
Lee J, Soper SA, Murray KK (2009) Anal Chim Acta 649:180–190. https://doi.org/10.1016/j.aca.2009.07.037
Krenková J, Foret F (2004) Electrophoresis 25:3550–3563. https://doi.org/10.1002/elps.200406096
Fang SM, Wang HN, Zhao ZX, Wang WH (2012) J Pharm Anal 2:83–89. https://doi.org/10.1016/j.jpha.2011.12.002
Brena BM, Irazoqui G, Giacomini C, Batista-Viera F (2003) Effect of increasing co-solvent concentration on the stability of soluble and immobilized beta-galactosidase. J Mol Catal B Enzym 21:25–29
Schejbal J, Glatz Z (2018) J Sep Sci 41:323–335. https://doi.org/10.1002/jssc.201700905
Vilanova E, Manjon A, Iborra JL (1984) Biotechnol Bioeng 26:1306–1312. https://doi.org/10.1002/bit.260261107
Luckarift HR, Johnson GR, Spain JC (2006) J Chromatogr B Analyt Technol Biomed Life Sci 843:310–316. https://doi.org/10.1016/j.jchromb.2006.06.036
Hu F, Deng C, Zhang X (2008) J Chromatogr B Analyt Technol Biomed Life Sci 871:67–71. https://doi.org/10.1016/j.jchromb.2008.06.036
Freije R, Klein T, Ooms B, Kauffman HF, Bischoff R (2008) J Chromatogr A 1189:417–425. https://doi.org/10.1016/j.chroma.2007.10.059
Wu S, Sun L, Ma J, Yang K, Liang Z, Zhang L, Zhang Y (2011) Talanta 83:1748–1753. https://doi.org/10.1016/j.talanta.2010.12.011
Fossati T, Colombo M, Castiglioni C, Abbiati G (1994) J Chromatogr B Biomed Appl 656:59–64
Yamato S, Kawakami N, Shimada K, Ono M, Idei N, Itoh Y, Tachikawa E (2004) Biol Pharm Bull 27:210–215
Shu HC, Wu NP (2001) Talanta 54:361–368
Markoglou N, Wainer IW (2002) J Chromatogr A 948:249–256
Mattiasson B (1988) Methods Enzymol 137:647–656
Gast FU, Franke I, Meiss G, Pingoud A (2001) J Biotechnol 87:131–141
Luckarift HR, Ku BS, Dordick JS, Spain JC (2007) Biotechnol Bioeng 98:701–705. https://doi.org/10.1002/bit.21447
Betancor L, Luckarift HR (2008) Trends Biotechnol 26:566–572. https://doi.org/10.1016/j.tibtech.2008.06.009
Subramanian A, Kennel SJ, Oden PI, Jacobson KB, Woodward J, Doktycz MJ (1999) Comparison of techniques for enzyme immobilization on silicon supports. Enzyme Microbial Technol 24:26
He P, Greenway G, Haswell SJ (2008) Nanotechnology 19:315603. https://doi.org/10.1088/0957-4484/19/31/315603
Kim D, Herr AE (2013) Biomicrofluidics 7:41501. https://doi.org/10.1063/1.4816934
Andrisano V, Bartolini M (2010) Immobilisation of enzymes on monolithic matrices: applications in drug discovery. In: Wang PG (eds) Monolithic chromatography and its modern applications. ILM, London
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Soc 56:658–666
Dixon M (1953) The determination of enzyme inhibitor constants. Biochem J 55:170–171
Cornish-Bowden A (1974) A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J 137:143–144
Vodopivec M, Podgornik A, Berovic M, Strancar A (2003) J Chromatogr B Analyt Technol Biomed Life Sci 795:105–113
Wheatley JB, Schmidt DE (1999) J Chromatogr A 849:1–12
Bartolini M, Cavrini V, Andrisano V (2005) J Chromatogr A 1065:135–144
De Simone A, Mancini F, Cosconati S, Marinelli L, La Pietra V, Novellino E, Andrisano V (2013) J Pharm Biomed Anal 73:131–134. https://doi.org/10.1016/j.jpba.2012.03.006
Andrisano V, Bartolini M, Gotti R, Cavrini V, Felix G (2001) J Chromatogr B Biomed Sci Appl 753:375–383
Chlebek J, De Simone A, Hošťálková A, Opletal L, Pérez C, Pérez DI, Havlíková L, Cahlíková L, Andrisano V (2016) Fitoterapia 109:241–247. https://doi.org/10.1016/j.fitote.2016.01.008
De Simone A, Seidl C, Santos CA, Andrisano V (2014) J Chromatogr B Analyt Technol Biomed Life Sci 953–954:108–114. https://doi.org/10.1016/j.jchromb.2014.01.056
Mancini F, Andrisano V (2010) J Pharm Biomed Anal 52:355–361. https://doi.org/10.1016/j.jpba.2009.07.012
Mancini F, Naldi M, Cavrini V, Andrisano V (2007) J Chromatogr A 1175:217–226. https://doi.org/10.1016/j.chroma.2007.10.047
Mancini F, De Simone A, Andrisano V (2011) Anal Bioanal Chem 400:1979–1996. https://doi.org/10.1007/s00216-011-4963-x
Seidl C, de Moraes Santos CA, De Simone A, Bartolini M, Weffort-Santos AM, Andrisano V (2017) Curr Alzheimer Res 14:317–326. https://doi.org/10.2174/1567205013666161026150455
Bartolini M, Greig NH, Yu QS, Andrisano V (2009) J Chromatogr A 1216:2730–2738. https://doi.org/10.1016/j.chroma.2008.09.100
Nicoli R, Bartolini M, Rudaz S, Andrisano V, Veuthey JL (2008) J Chromatogr A 1206:2–10. https://doi.org/10.1016/j.chroma.2008.05.080
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) Biochem Pharmacol 7:88–95
Perola E, Cellai L, Lamba D, Filocamo L, Brufani M (1997) Biochim Biophys Acta 1343:41–50
André C, Herlem G, Gharbi T, Guillaume YC (2011) J Pharm Biomed Anal 55:48–53. https://doi.org/10.1016/j.jpba.2011.01.003
Morris SM (2002) Annu Rev Nutr 22:87–105. https://doi.org/10.1146/annurev.nutr.22.110801.140547
Wu G, Meininger CJ (1995) Am J Physiol 269:H1312–H1318. https://doi.org/10.1152/ajpheart.1995.269.4.H1312
Kuhn NJ, Ward S, Piponski M, Young TW (1995) Arch Biochem Biophys 320:24–34. https://doi.org/10.1006/abbi.1995.1338
Raman NN, Khan M, Hasan R (1994) Bioactive components from Ficus glomerata. Pure Appl Chem 66:2287–2290
Vanzolini KL, Vieira LC, Corrêa AG, Cardoso CL, Cass QB (2013) J Med Chem 56:2038–2044. https://doi.org/10.1021/jm301732a
Vilela AF, da Silva JI, Vieira LC, Bernasconi GC, Corrêa AG, Cass QB, Cardoso CL (2014) J Chromatogr B Analyt Technol Biomed Life Sci 968:87–93. https://doi.org/10.1016/j.jchromb.2013.11.037
da Silva JI, de Moraes MC, Vieira LC, Corrêa AG, Cass QB, Cardoso CL (2013) J Pharm Biomed Anal 73:44–52. https://doi.org/10.1016/j.jpba.2012.01.026
Orhan IE (2012) Curr Med Chem 19:2252–2261
Anand N, Singh P, Sharma A, Tiwari S, Singh V, Singh DK, Srivastava KK, Singh BN, Tripathi RP (2012) Bioorg Med Chem 20:5150–5163. https://doi.org/10.1016/j.bmc.2012.07.009
Catto M, Pisani L, Leonetti F, Nicolotti O, Pesce P, Stefanachi A, Cellamare S, Carotti A (2013) Bioorg Med Chem 21:146–152. https://doi.org/10.1016/j.bmc.2012.10.045
Peng XM, Damu GL, Zhou C (2013) Curr Pharm Des 19:3884–3930
Nordberg A, Ballard C, Bullock R, Darreh-Shori T, Somogyi M (2013) Prim Care Companion CNS Disord 15:1–8. https://doi.org/10.4088/pcc.12r01412
Kruskal WH, Wallis WA (1952) J Am Statist Assoc 47:583–621
Cornelio VE, de Moraes MC, Domingues VC, Fernandes JB, da Silva MFDG, Cass QB, Vieira PC (2018) J Pharm Biomed Anal 151:252–259. https://doi.org/10.1016/j.jpba.2018.01.001
Benes P, Vetvicka V, Fusek M (2008) Crit Rev Oncol Hematol 68:12–28. https://doi.org/10.1016/j.critrevonc.2008.02.008
Cardoso CL, Lima VV, Zottis A, Oliva G, Andricopulo AD, Wainer IW, Moaddel R, Cass QB (2006) J Chromatogr A 1120:151–157. https://doi.org/10.1016/j.chroma.2005.10.063
de Moraes MC, Ducati RG, Donato AJ, Basso LA, Santos DS, Cardoso CL, Cass QB (2012) J Chromatogr A 1232:110–115. https://doi.org/10.1016/j.chroma.2011.10.056
Galmarini CM (2006) IDrugs 9:712–722
Kalckar HM (1947) J Biol Chem 167:429–443
Bartolini M, Cavrini V, Andrisano V (2007) J Chromatogr A 1144:102–110. https://doi.org/10.1016/j.chroma.2006.11.029
Vilela AFL, Seidl C, Lima JM, Cardoso CL (2018) Anal Biochem 549:53–57. https://doi.org/10.1016/j.ab.2018.03.012
Darvesh S, Walsh R, Kumar R, Caines A, Roberts S, Magee D, Rockwood K, Martin E (2003) Alzheimer Dis Assoc Disord 17:117–126
Forsberg EM, Green JR, Brennan JD (2011) Anal Chem 83:5230–5236. https://doi.org/10.1021/ac200534t
Forsberg EM, Brennan JD (2014) Anal Chem 86:8457–8465. https://doi.org/10.1021/ac5022166
Besanger TR, Hodgson RJ, Green JR, Brennan JD (2006) Anal Chim Acta 564:106–115. https://doi.org/10.1016/j.aca.2005.12.066
La Motta C, Sartini S, Mugnaini L, Salerno S, Simorini F, Taliani S, Marini AM, Da Settimo F, Lavecchia A, Novellino E, Antonioli L, Fornai M, Blandizzi C, Del Tacca M (2009) J Med Chem 52:1681–1692. https://doi.org/10.1021/jm801427r
Alunni S, Orrù M, Ottavi L (2008) J Enzyme Inhib Med Chem 23:182–189. https://doi.org/10.1080/14756360701475233
Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, Camaioni E (2001) Med Res Rev 21:105–128
de Moraes MC, Temporini C, Calleri E, Bruni G, Ducati RG, Santos DS, Cardoso CL, Cass QB, Massolini G (2014) J Chromatogr A 1338:77–84. https://doi.org/10.1016/j.chroma.2014.02.057
Brekkan E, Lundqvist A, Lundahl P (1996) Biochemistry 35:12141–12145. https://doi.org/10.1021/bi9603231
Haneskog L, Lundqvist A, Lundahl P (1998) J Mol Recognit 11:58–61. https://doi.org/10.1002/(SICI)1099-1352(199812)11:1/6%3c58:AID-JMR390%3e3.0.CO;2-S
Haneskog L, Zeng CM, Lundqvist A, Lundahl P (1998) Biochim Biophys Acta 1371:1–4
Ouimet CM, D’amico CI, Kennedy RT (2017) Expert Opin Drug Discov 12:213–224. https://doi.org/10.1080/17460441.2017.1268121
Iqbal J, Iqbal S, Müller CE (2013) Analyst 138:3104–3116. https://doi.org/10.1039/c3an00031a
Ji X, Ye F, Lin P, Zhao S (2010) Talanta 82:1170–1174. https://doi.org/10.1016/j.talanta.2010.06.029
Haynes J, Killilea DW, Peterson PD, Thompson WJ (1996) J Pharmacol Exp Ther 276:752–757
Lin P, Zhao S, Lu X, Ye F, Wang H (2013) J Sep Sci 36:2538–2543. https://doi.org/10.1002/jssc.201300315
Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V (2016) Int J Cardiol 213:8–14. https://doi.org/10.1016/j.ijcard.2015.08.109
Kim SC, Schneeweiss S, Choudhry N, Liu J, Glynn RJ, Solomon DH (2015) Am J Med 128:616–657. https://doi.org/10.1016/j.amjmed.2015.01.013
Zhang L, Hu K, Li X, Zhao S (2018) CE method with partial filling techniques for screening of xanthine oxidase inhibitor in traditional Chinese medicine. Chromatographia 73:583–587
Iqbal J (2011) Anal Biochem 414:226–231. https://doi.org/10.1016/j.ab.2011.03.021
Lanier M, Sergienko E, Simão AM, Su Y, Chung T, Millán JL, Cashman JR (2010) Bioorg Med Chem 18:573–579. https://doi.org/10.1016/j.bmc.2009.12.012
Teriete P, Pinkerton AB, Cosford ND (2013) Methods Mol Biol 1053:85–101. https://doi.org/10.1007/978-1-62703-562-0_5
Narisawa S, Harmey D, Yadav MC, O’Neill WC, Hoylaerts MF, Millán JL (2007) J Bone Miner Res 22:1700–1710. https://doi.org/10.1359/jbmr.070714
Wang S, Su P, Yang Y (2012) Anal Biochem 427:139–143. https://doi.org/10.1016/j.ab.2012.05.014
Guascito MR, Malitesta C, Mazzotta E, Turco A (2008) Inhibitive determination of metal ions by an amperometric glucose oxidase biosensor: study of the effect of hydrogen peroxide decomposition. Sensors Actuators B Chem 131:394–402
Jiang TF, Liang TT, Wang YH, Zhang WH, Lv ZH (2013) J Pharm Biomed Anal 84:36–40. https://doi.org/10.1016/j.jpba.2013.05.023
Gao X, Luo W, Xie G, Xue C, Ding Q (2004) Characteristics and kinetics of inhibition of polyphenol oxidase from Spodoptera exigua (Lepidoptera: Noctuidae). Sci Agric Sin:687–691
Camara MA, Tian M, Guo L, Yang L (2015) J Chromatogr B Analyt Technol Biomed Life Sci 990:174–180. https://doi.org/10.1016/j.jchromb.2015.03.019
Ham M, Choe SS, Shin KC, Choi G, Kim JW, Noh JR, Kim YH, Ryu JW, Yoon KH, Lee CH, Kim JB (2016) Diabetes 65:2624–2638. https://doi.org/10.2337/db16-0060
Zhang C, Zhang Z, Zhu Y, Qin S (2014) Anticancer Agents Med Chem 14:280–289
Schejbal J, Řemínek R, Zeman L, Mádr A, Glatz Z (2016) J Chromatogr A 1437:234–240. https://doi.org/10.1016/j.chroma.2016.01.081
Funding
The authors receive no funds to develop this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Published in the topical collection Rising Stars in Separation Science, as part of Chromatographia’s 50th Anniversary Commemorative Issue.
Rights and permissions
About this article
Cite this article
De Simone, A., Naldi, M., Bartolini, M. et al. Immobilized Enzyme Reactors: an Overview of Applications in Drug Discovery from 2008 to 2018. Chromatographia 82, 425–441 (2019). https://doi.org/10.1007/s10337-018-3663-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3663-5