Abstract
In this work, a new immobilized enzyme reactor (IMER) containing gelatinase enzyme (a mixture of MMP-2 and -9) was developed and then inserted into a HPLC system to evaluate the inhibitory potency of novel compounds towards gelatinase. The gelatinase was immobilized on an epoxy-silica column by in situ technique. To monitor the performance of the newly developed method, the kinetic constant (K m) of immobilized gelatinase as well as the inhibition constant (IC50) of a known inhibitor, i.e., LY52, were determined and these results were compared with those obtained by classical succinyl-gelatin assay. The IMER-HPLC system was subsequently applied to the screening of 39 synthetic compounds. The inhibitory potential of these inhibitors (expressed in IC50) was found to be in agreement with the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Matrix metalloproteinases (MMPs), a multigene family of zinc-dependent endopeptidases, are crucial biomarkers for the development of cancer [1]. Under normal physiological conditions, MMPs are minimally expressed and well regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs), while in the presence of specific stimuli exemplified by growth factors and cytokines, they are up-regulated destroying the balance between MMPs and TIMPs, which can lead to a variety of pathological disorders ranging from cardiovascular disease to cancer [2–4]. Over-expression of MMPs is reported to be associated with degradation of the extracellular matrix (ECM) and basement membrane (BM), tumor proliferation, invasion, angiogenesis, and metastasis [5, 6]. To date, more than 26 subfamily members have been identified and can be loosely grouped into five classes according to their primary structure and function and substrate specificity: collagenases, gelatinases, membrane-type, stromelysins and matrilysins [7, 8]. Among all the MMP subtypes, MMP-2 and -9, having similar bio-activity and also known as gelatinases A and B, respectively, have been implicated in a number of cancers [9–11], which make them attractive therapeutic targets for anticancer drug design. Accordingly, these reports stimulate the development of truly selective inhibitors capable of blocking the unwanted activities of gelatinases for the inhibition of tumor vascularization and growth [12, 13].
The availability of appropriate screening methods allowing the selection of synthesized compounds is an essential prerequisite in such a drug discovery process. The spectrophotometric assay using succinylated gelatin as substrate is the most common method to screen gelatinase inhibitors. The assay is based on measurement of primary amines exposed by hydrolysis of the substrate by gelatinases. The exposed primary amines are detected by reacting with 2,4,6-trinitrobenzene sulfonic acid (TNBSA) that produces a quantifiable color reaction with a λmax at 450 nm [14]. However, this assay is limited by the reactivity of the TNBSA with primary amines present in the protease solutions or buffers used in the assay. In recent years, immobilized enzyme reactors (IMERs) have been employed for the screening of enzymatic inhibitors via affinity chromatography reflecting the interaction characteristics of biomacromolecules with the compounds. A human recombinant acetylcholinesterase micro-immobilized enzyme reactor was prepared by using an in situ immobilization procedure for online inhibition studies [15]. The inhibitory activities of tyrosinase inhibitors were determined by both in situ and in batch immobilization procedures using epoxy-silica as support [16]. Yun et al. [17] developed a ligands screening method based on enzyme-immobilized amine-terminated magnetic nanoparticles integrated with HPLC. Firstly, the magnetic nanoparticles were prepared and characterized, and α-amylase was immobilized on their surface. The enzyme-immobilized magnetic nanoparticles were used as target-guided tools to rapidly locate the potential active components in the extracts of Garcinia xanthochymus. A new IMER containing human recombinant butyrylcholinesterase was used for the online kinetic characterization of specific, pseudo-irreversible and brain-targeted butyryl-cholinesterase inhibitors as potential drug candidates for Alzheimer’s disease [18]. Spross et al. [19] prepared a capillary trypsin-immobilized monolithic enzyme reactor for a rapid and efficient digestion of proteins down to the femtomole level. Trypsin was immobilized on a poly monolith using the glutaraldehyde technique. At the same time, an online setup of the IMER with reversed phase nano-HPLC separation and nano-ESI–MS/MS analysis was established. An IMER containing human recombinant MMP-9 was developed by covalently immobilizing MMP-9 onto a glutaraldehyde derivatized EDA CIM® disk: the MMP-9 IMER was placed in a liquid chromatographic system where online chromatographic studies were performed for the screening of commercial model MMP-9 inhibitors [20]. The IMER containing human MMP-8 was investigated and used for the online screening of known MMP-8 inhibitors in zonal chromatography and inhibition experiments [21]. Based on the 3D structures of the known gelatinase inhibitors and binding modes of these compounds in complex with gelatinases, a series of compounds were designed and synthesized [22–24]. How to determine the inhibitory potency of synthetic test compounds against gelatinase targets selectively is the most difficult part. In this paper, we have addressed this issue by the development of an effective and rapid method by preparing a new immobilized gelatinase reactor containing both MMP-2 and -9 which were inserted into a HPLC system. The gelatinases were immobilized on an epoxy-silica column by an in situ technique. The enzyme activity was directly assayed by measuring the peak area of the product. The inhibition effect of the investigated compounds can be read out directly from the reduced peak area of the product in comparison with the peak area of the product obtained from the enzymatic reaction in the absence of an inhibitor in the substrate solution. The proposed method was validated by measuring gelatinase IMER inhibition from known compounds, and subsequently applied to quantitatively determine the inhibition of the enzymatic activity of some synthesized compounds.
Materials and Methods
Apparatus
Multiskan MK3 microplate reader (Thermolasystems), Vario EL3 elemental analyzer, NEXUS 470 Fourier transform infrared-based spectroscopy, Shimazu LC-10A HPLC system equipped with a Rheodyne sample valve (20 μL loop) and SPD-M 110A diode array detector. Phenomenex® LUNA C18 column (250 mm × 4.6 mm i.d., 5 μm).
Chemicals and Reagents
Gelatin was a GIBCO product, gelatinase (a mixture of MMP-2 and -9) and 5 % 2,4,6-trinitrobenzene sulfonic acid (AR) were purchased from Sigma Chemical; dialysis bags (molecular weight cut-off 10,000); succinic anhydride (98.0 %), (3-glycidoxypropyl) trimethoxysilane (≥96.0 %,), YQG chromatographic silica (10 μm), periodic acid (99.5 %), triacetoxy-sodium borohydride (98.0 %), hydroxamic acids compounds (Institute of Pharmaceutical Chemistry, Shandong University). Buffer components and other chemicals were of the highest purity available commercially.
Preparation of Aldehyde Silica Support
Silica gel was activated under vacuum at 180 °C for 15 h. After cooling, 15 g of silica gel were refluxed in 120 mL of toluene and 15 mL of (3-glycidoxypropyl) trimethoxy-silane for 12 h, from which epoxide silica was obtained. The surface coverage (α epoxy) of carbon was determined by elemental analyzer and calculated as Eq. (1), which has been introduced by Calleri et al. [25].
where n c is the numbers of carbon atoms per bonded ligand, P C is the percent carbon measured in the epoxy-modified silica, M Element is the mass of the calculated element and M Ligland is the molecular mass of the attached ligand, and S BET is the specific surface area m2 g−1 of the unmodified silica.
The epoxide silica (0.1 g) was packed in a 50 mm × 4.6 mm steel column by the dry packing method and the column was equilibrated using sulfuric acid (pH 3.0) at 0.5 mL min−1 for 2 h and 70 % acetic acid at 0.6 mL min−1 for 0.5 h, respectively, then 5 % periodic acid (pH 3.0) in 70 % acetic acid at 0.6 mL min−1 for 5 h, and finally washed using water to neutrality. The aldehyde group was tested by two methods:
-
1.
Chemical method: the aldehyde group can react with Fehling’s reagent as a test. The Cu2+ complex ions are reduced to a red brick-colored Cu2O precipitate.
-
2.
FT–IR method: FT–IR spectras of aldehyde and epoxide silica were recorded by the KBr pellet technique in the region of 4000–500 cm−1, and then compared.
Immobilization of Gelatinase
The aldehyde silica column used for the in situ immobilization of gelatinase was connected to the HPLC system. An 0.5-mg mL−1 enzyme solution was prepared by dissolving gelatinase in 50 mM borate buffer (pH 6.0) containing of 50 mM ammonium sulfate and 0.5 M triacetoxy-sodium borohydride. The gelatinase solution was recycled through the aldehyde silica column at 0.2 mL min−1 for 10 h at room temperature. The amount of gelatinase in the elutes from the recycled enzyme solution was measured with the succinyl-gelatin method to investigate the online bonding time. After immobilization, the column was flushed using 10 mM borate buffer (pH 6.0) at 0.5 mL min−1 for 1.5 h in order to wash away the unbound enzyme. Finally, the unreacted aldehyde group was blocked using 1 M glycine buffer solution (pH 7.0) at 0.2 mL min−1 for 1.5 h. After immobilization, the enzyme solution and flushing solution were collected. Immobilization yield was determined and calculated by Eq. (2):
where m is the initial amount of enzyme, m f is the amount of enzyme in the flushing solution, m r is the residual amount of enzyme in the enzyme solution after immobilization, and m s is the amount of aldehyde silica support.
Determination of Activities of Free and Immobilized Gelatinase
Preparation of Substrate
Succinylated gelatin (substrate) was prepared by dissolving 500 mg gelatin in 50 mM borate buffer (pH 8.5) 25 mL, and adding 18 mg succinic anhydride (5 times in 1 h). During the reaction, the pH of the solution was kept at 8.0–8.5. The reaction lasted for 5 h at room temperature. Then, the product was dialyzed for 48 h with a borate buffer (pH 8.5). The concentration of the succinylated gelatin was determined by the Lowry method.
Immobilized Gelatinase Activity Assay
The activity of the immobilized gelatinase was assayed by using the online procedure. The gelatinase IMER was coupled with a Phenomenex® LUNA C18 column (250 mm × 4.6 mm i.d., 5 μm) and attached to a liquid chromatography system, then the IMER column was placed inside a column oven at 42 °C. The mobile phase was 50 mM borate buffer (pH 8.5):methanol (95:5); the flow rate was 0.2 mL min−1. The detecting wavelength was 210 nm. The chromatographic peak area of the product from enzymatic reaction in the IMER was directly proportional to the activity of immobilized gelatinase. The immobilized gelatinase column was stored at 4 °C in a 0.01 % (w/v) solution of sodium azide for a week. The activity of the immobilized gelatinase was assayed every other day.
Assay of Free Gelatinase Activity
Free gelatinase activity was determined by the succinyl-gelatin method using succinylated gelatin as substrate [14].
Michaelis–Menten of Free and Immobilized Gelatinase
A Michaelis–Menten plot describes the kinetics of the immobilized enzyme. The K m value was obtained using immobilized enzyme reactor columns from the initial velocity measurement by varying the substrate concentration (0.052–0.52 mM) and fitting the data to the Michaelis–Menten equation. Initial velocity data were obtained by quantitation of the formation of the hydrolysis product. K m can be caculated by Lineweaver–Burk plot method.
where V and V max are the initial and maximal reaction velocity, [S] is the substrate concentration, and K m is the Michaelis constant. Kinetic parameters values were obtained using the nonlinear curve fitting program PRISM 5.04 (GraphPad, San Diego, CA, USA).
Validation of the Inhibitor Screening Method
Precision of Screening System
Inhibition % of the positive control LY52 was determined at three concentrations (2.3, 11 and 110 μM). Intra-precision was calculated by injection five times in 1 day, and inter-precision was obtained by injection on 5 successive days.
Determination of IC50 of the Positive Control
The IC50 of LY52 was determined by an IMER-HPLC system. The succinylated gelatin solution and the mixtures of the substrate and LY52 with different concentrations were respectively injected into the IMER-HPLC system. The inhibition percentage of LY52 was calculated by evaluating the product peak area with and without inhibitor. The following expressions were used:
where A i is the peak area calculated in the presence of LY52, and A 0 is the peak area obtained with the substrate solution in the absence of LY52.
Inhibition curves were obtained for LY52 by plotting the inhibition % versus the logarithm of LY52 concentration. The IC50 value was determined.
Screening for Gelatinase Inhibitory Activity
The inhibition of gelatinase using IMER was examined. Stock solutions were prepared by dissolving the respective 39 synthetic compounds in the borate buffer solution (pH 8.5) to appropriate concentrations. Each assay solution was prepared by mixing an equal amount of the respective stock solution of the test inhibitor with the substrate solution to obtain a mixture containing the test inhibitor and substrate (0.54 mM). The substrate solution and the assay solution were injected into the IMER-HPLC system and the IC50 values of compounds were calculated from a sigmoidal dose response nonlinear regression equation using PRISM 5.04.
Results and Discussion
Gelatinase Immobilization
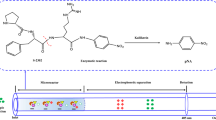
Most studies of immobilization techniques focus on the formation of covalent bonds between the enzyme and the support. Functional groups should be previously introduced onto the surface of silica, and then the enzyme is covalently attached to the functional groups. In our experiment, silica was modified by the epoxy group, the surface coverage (α epoxy) of carbon on the silica was 2.24 μmol m−2, then the epoxide group was oxidized by periodic acid and changed to an aldehyde group. The scheme of the gelatinase immobilization is illustrated in Fig. 1. The aldehyde group was identified by chemical and FT–IR methods. In the chemical method, the aldehyde group reacted with CuSO4 under alkaline condition and the brick-red precipitate was formed. Figure 2 shows FT–IR spectra comparisons of epoxide-silica and aldehyde-silica. The wavenumbers at 1737.06 and 2941.38 cm−1 were attributed to the stretching vibrations of the C=O and C–H on the aldehyde group. The chemical experiments and FT–IR indicated that the silica was modified by the aldehyde group.
The aldehyde column was used for the in situ immobilization of gelatinase. The immobilization of enzyme was carried out directly in a packed activated column to avoid the packing problem of a protein silica gel and to give high loading, good stability and good reproducibility from column to column [26]. The reactivity of amine groups on enzyme molecules appears to be universal, and the reaction between the amine groups and aldehyde groups allowed in situ covalent incorporation of the enzyme and the support into a new stationary phase (Fig. 1).
The immobilization conditions were optimized via the reaction of 0.1 g of aldehyde silica with the gelatinase solution at 25 °C for 12 h in batch. A four-factor, two-level orthogonal experimental design L8(27) was used to find the suitable parameters that could give the best results of the optimization function. The factors related to gelatinase activity and immobilization yield included the total amounts of gelatinase, concentration of gelatinase, concentration and pH value of borate buffer, as shown in Table 1. Eight experiments were arranged to obtain high immobilized yields. The results of the orthogonal test indicated that the highest immobilized yield can be obtained when the optimal immobilization condition was 2 mg of gelatinase, 0.5 mmol L−1 of gelatinase solution, and 0.05 mol L−1 of buffer solution with pH 6.0. It is necessary for the successful immobilization that the covalent bonding reaction can be performed under pH condition close to the enzyme iso-electric point, in which the dipole moment of enzymatic molecule decrease and enzyme is readily bonded with the support. The iso-electric points of MMP-2 and MMP-9 are 5.3 and 5.9, so pH 6.0 accords with the theoretical analysis. The gelatinase solution was recycled through the aldehyde silica column at 0.2 mL min−1 for 10 h at room temperature. The online bonding time was estimated by determining the amount of galatinase in the elutes from the recycled enzyme solution with the succinyl-gelatin method at different times (0–10 h). There was a constant decrease of the absorbance during the 0–8 h resulting from the reaction between the amino sites of the enzyme and the aldehyde groups. There was stabilized absorbance at successive 2-h intervals suggesting the completion of immobilization. This indicated that the online bonding time was 8 h. The immobilized yield was 15.6 mg g−1 online.
Identification of the Peak of the Gelatinase Hydrolysis Product
The substrate solution containing the test compound was injected into the IMER-HPLC system, then the chromatograms showing three different peaks were obtained. The inhibition effect of the investigated compounds can be read out directly from the reduced peak area of the product in comparison with the peak area of the product obtained from the enzymatic reaction in the absence of an inhibitor in the substrate solution. So only the product peak needs to be identified. The product peak was identified by the succinyl-gelatin and HPLC methods. The principle of the succinyl-gelatin method is described in Fig. 3.
The peak of the product was identified by two steps. First, the substrate solution was injected into the IMER-HPLC system. The effluents at 20.916, 22.629, and 37.631 min were collected and identified by the succinyl-gelatin method, respectively (Fig. 4a). Only the product could react with TNBSA to form the colored product. Compared with the absorbance of the blank group (A: 0.035), that of the elute at 37.631 min increased to 0.256, and there was no obvious difference to the other two elutes (Table 2). It was shown that the peak at 37.631 min was the peak of the product. Second, the mixtures of succinylated gelatin and LY52 with different concentrations (2.27, 5.68, 22.7 μmol L−1) were injected into the IMER-HPLC system as the positive control. It was found that the peak areas at 37.631 min decreased gradually with the increase of LY52 concentration (Fig. 4b). The peak at 37.631 min was also proved to be the peak of the product.
Optimization of Conditions for Gelatinase IMER
The back-pressure will influence the activity of immobilized enzymes in the IMER. Two extreme flow rate values (from 0.1 to 0.4 mL min−1) were chosen to represent a compromise between the detection of the product and the chromatographic system constraints (i.e., back-pressure). A flow rate of 0.1 mL min−1 is considered to be the best condition for lower back-pressure, but the product retention time is too long. A flow rate of 0.4 mL min−1 is the best for retention time, but the column pressure is too high, which has an adverse effect on enzyme activity. Therefore, a flow rate of 0.2 mL min−1 was chosen by considering both the activity and back-pressure (Table 3).
The suitable pH and temperature of the liberated and immobilized enzyme activity were also investigated by the succinyl-gelatin method. The optimal pH value of 8.5 for the highest liberated gelatinase activity was unchanged after immobilization (Fig. 5a). The relationship between temperature and enzymatic activity (Fig. 5b) indicated that the optimum temperatures for the activity of immobilized gelatinase increase to 42 °C from 37 °C for liberated gelatinase. This result showed that the heat stability of immobilized enzyme had increased.
Kinetic Parameters of Free and Immobilized Gelatinase
Under the “optimized” conditions, the Michaelis–Menten constant of immobilized gelatinase was determined using seven different substrate concentrations ranging from 0.052 to 0.52 mM. Each concentration was measured in triplicate. The kinetic characteristics of immobilized enzyme were evaluated by plotting the enzyme reaction velocity versus the substrate concentration [S] (Fig. 6). From the Michaelis–Menten plot, the K m value of the immobilized gelatinase was found to be 0.319 mM, which was different from that of the free gelatinase determined by the succinyl-gelatin method (K m: 0.0105). Although the binding reaction was simple and mild because of no addition of organic solvents and other catalysts, the stereochemical structure of immobilized enzyme active sites may be affected, and the steric hindrance effect of the supports may reduce the chance of the enzyme binding with the substrate.
The Feasibility Validation of IMER-HPLC Screening System
LY52 as the positive control is a kind of N 1-substituent pyrrolidine derivative, which exhibits a strong inhibitory effect on gelatinase. So LY52 was selected to evaluate the feasibility of the developed IMER-HPLC screening system for determining the inhibitory potency of the compounds. The IC50 value was calculated from a sigmoidal dose–response nonlinear regression equation by PRISM 5.04. Using the IMER-HPLC methodology, this value was determined as 23 μM by measuring the initial velocities at different inhibitor concentrations within a range from 2.27 to 114 μM at 0.54 mM substrate concentration. In comparison, the IC50 value obtained from the free enzyme was 18 μM. These results were in a good agreement with those reported in the literature (IC50: 26.9 μM) when free gelatinase was used [27].
Precision of Screening System
The precision and accuracy of the screening system were demonstrated by the mean RSDs at the optimized conditions. The intra-precision of the screening system was evaluated by five runs on the same day, and inter-precision was obtained by injection on 5 successive days measuring the inhibition % of the positive control LY52 at three concentrations (2.3, 11 and 110 μM). The intra-day RSD values for the inhibition % of the positive control LY52 at three concentrations (2.3, 11 and 110 μM) were 1.7, 1.4 and 0.54 %, respectively. The corresponding inter-day RSDs were 2.2, 1.3 and 0.83 %, respectively.
Inhibitor Screening
The developed IMER-HPLC method was applied to the investigation of the gelatinase inhibitory activity of 39 compounds. They were three series of pyrrolidine derivatives A (A3a, A3b, A4a, A4b, A5a, A5b, A6a, A7c, A7i, A7 m, A8a, A8c, A8d, A8e, A9), derivatives B (B3a, B3b, B3c, B4a, B4b, B4c, B5a, B5b, B5d, B5e, B6a, B6c, B6d, B6e, B6f) and derivatives C (C3a, C3c, C4a, C4f, C4i, C4 m, C5a, C5b, C5c). The structures of the compounds are given in Table 4. Among them, IC50 values of 13 compounds were within the range of 1–18 μM, while the others were 352.4–2050 μM. Therefore, these 13 compounds, A8a, A8c, A8d, A8e, A9, B6a, B6c, B6d, B6e, B6f, C5a, C5b and C5c, were identified to be potential inhibitors for gelatinases. The IC50 values of LY52 and these 13 inhibitors obtained with the IMER were compared with those obtained with the free enzyme. They correlated well with those obtained from the succinyl-gelatin method. The results are summarized in Table 5. These results confirmed the feasibility of online chromatographic screening of gelatinase inhibitors using IMER containing MMP-2 and -9. In addition, this technique can be used to evaluate the inhibitory capacity of various compounds to mixtures of MMP-2 and -9 indicating their contribution to the total gelatinolytic activity. One key advantage was that the mixture of MMP-2 and -9 was available for direct purchase for its immobilization onto the support, compared the high cost and difficulty to express and purify human recombinant enzyme in published IMER containing human recombinant MMP-9 [20]. Another key advantage of this online screening system was that the inhibition effect of the investigated compounds can be read out directly from the reduced peak area of the product with no need for adding a chromogenic agent to produce a quantifiable color reaction.
Conclusion
In this study, gelatinases were immobilized onto an epoxy-silica column. The gelatinase IMER containing both MMP-2 and -9 was connected to a HPLC system for online screening of potential gelatinase inhibitors. The screening system exhibited good precision, which will guarantee the accuracy and feasibility of the screening. Moreover, the system has the potential to be automated, allowing large numbers of compounds to be analyzed continuously. The IMER-HPLC method described in this study provides an application example of a rapid, simple, automatic, and economic approach for the middle-throughput screening of gelatinase inhibitors in early drug discovery.
References
Roy R, Yang J, Moses MA (2009) Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol 27:5287–5297
Hu JL, Van den Steen PE, Sang QXA, Opdenakker G (2007) Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 6:480–498
MacFadyen RJ (2007) Can matrix metalloproteinase inhibitors provide a realistic therapy in cardiovascular medicine? Curr Opin Pharmacol 7:171–178
Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P (2004) Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hemat 49:179–186
Fink K, Boratynski J (2012) The role of metalloproteinases in modification of extracellular matrix in invasive tumor growth, metastasis and angiogenesis. Postep Hig Med Dosw 66:609–628
Krstic J, Santibanez JF (2014) Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. Sci World J 2014:521754
Bourboulia D, Stetler-Stevenson WG (2010) Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 20:161–168
Park SY, Shin HW, Lee KB, Lee MJ, Jang JJ (2010) Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in thioacetamide-induced chronic liver injury. J Korean Med Sci 25:570–576
Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P et al (1999) Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol 17:768–774
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174
Lee M, Celenza G, Boggess B, Blase J, Shi Q, Toth M et al (2009) A potent gelatinase inhibitor with anti-tumor-invasive activity and its metabolic disposition. Chem Biol Drug Des 73:189–202
Auge F, Hornebeck W, Laronze JY (2004) A novel strategy for designing specific gelatinase A inhibitors: potential use to control tumor progression. Crit Rev Oncol Hemat 49:277–282
Kleifeld O, Kotra LP, Gervasi DC, Brown S, Bernardo MM, Fridman R et al (2001) X-ray absorption studies of human matrix metalloproteinase-2 (MMP-2) bound to a highly selective mechanism-based inhibitor. comparison with the latent and active forms of the enzyme. J Biol Chem 276:17125–17131
Baragi VM, Shaw BJ, Renkiewicz RR, Kuipers PJ, Welgus HG, Mathrubutham M et al (2000) A versatile assay for gelatinases using succinylated gelatin. Matrix Biol 19:267–273
Bartolini M, Cavrini V, Andrisano V (2004) Monolithic micro-immobilized-enzyme reactor with human recombinant acetylcholinesterase for online inhibition studies. J Chromatogr A 1031:27–34
de Oliveira KB, Mischiatti KL, Fontana JD, de Oliveira BH (2014) Tyrosinase immobilized enzyme reactor: development and evaluation. J Chromatogr B 945:10–16
Li Y, Chen Y, Xiao C, Chen D, Xiao Y, Mei Z (2014) Rapid screening and identification of alpha-amylase inhibitors from Garcinia xanthochymus using enzyme-immobilized magnetic nanoparticles coupled with HPLC and MS. J Chromatogr B 960:166–173
Bartolini M, Greig NH, Yu QS, Andrisano V (2009) Immobilized butyrylcholinesterase in the characterization of new inhibitors that could ease Alzheimer’s disease. J Chromatogr A 1216:2730–2738
Spross J, Sinz A (2010) A capillary monolithic trypsin reactor for efficient protein digestion in online and offline coupling to ESI and MALDI mass spectrometry. Anal Chem 82:1434–1443
Ma X, Chan EC (2010) On-line chromatographic screening of matrix metalloproteinase inhibitors using immobilized MMP-9 enzyme reactor. J Chromatogr B 878:1777–1783
Mazzini F, Nuti E, Petri A, Rossello A (2011) Immobilization of matrix metalloproteinase 8 (MMP-8) for online drug screening. J Chromatogr B 879:756–762
Cheng XC, Wang Q, Fang H, Tang W, Xu WF (2008) Design, synthesis and preliminary evaluation of novel pyrrolidine derivatives as matrix metalloproteinase inhibitors. Eur J Med Chem 43:2130–2139
Cheng XC, Wang Q, Fang H, Tang W, Xu WF (2008) Design, synthesis and evaluation of novel sulfonyl pyrrolidine derivatives as matrix metalloproteinase inhibitors. Bioorgan Med Chem 16:5398–5404
Cheng XC, Wang Q, Fang H, Tang W, Xu WF (2008) Synthesis of new sulfonyl pyrrolidine derivatives as matrix metalloproteinase inhibitors. Bioorgan Med Chem 16:7932–7938
Calleri E, Massolini G, Lubda D, Temporini C, Loiodice F, Caccialanza G (2004) Evaluation of a monolithic epoxy silica support for penicillin G acylase immobilization. J Chromatogr A 1031:93–100
Liu YQ, Lipponen K, Cilpa-Karhu G, Oorni K, Riekkola ML (2012) Plasma low-density lipoprotein immobilized silica as stationary phase in nano-liquid chromatography. J Chromatogr A 1270:104–110
Yuan YX, Xu WF, Liu J, Chen MH, Meng H, Qu XJ (2006) Inhibitory effects of matrix metalloproteinase (MMP) inhibitor LY52 on expression of MMP-2 and MMP-9 and invasive ability of human ovarian carcinoma cell line SKOV3. Chin J Cancer 25:663–670
Acknowledgments
This work was financially supported by the Shandong Provincial Natural Science Foundation of China (2009ZRB02230).
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, W., Hou, Z., Wang, H. et al. Immobilized Enzyme Reactor Chromatography for Online Gelatinase Inhibitors Screening. Chromatographia 78, 763–773 (2015). https://doi.org/10.1007/s10337-015-2904-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-015-2904-0