Abstract
For the first time, a simple and rapid method for simultaneous determination of gentamicin sulfate and colistin sulfate in two pharmaceutical formulations for children and adults by ion-pairing reverse phase chromatography and low-UV detection at 215 nm has been developed. This simultaneous analysis is thus a challenge due to the multicomponent mixture of high polar, non volatile and non UV absorbing chromophores. Rapid separation required less than 5 min on a Waters X-Terra® C18 MS column (50 mm × 4.6 mm i.d., 2.5 μm) with temperature maintained at 35 °C. A linear gradient from 15/85 to 40/60 acetonitrile/water (v/v) with constant hexafluorobutyric acid (HFBA) concentration of 0.05 % (v/v) was used as pairing reagent at 1.5 mL min−1. In pharmaceutical analysis, the basic and polar compounds are separated by ion-pairing chromatography and the detection of analytes with weak chromophores requires working at low wavelengths. This application is an example of troubleshooting, i.e. baseline drift, due to gradient elution and absorbance of the ion-pairing agent. Baseline drift was minimized by optimizing the HFBA concentration gradient and its slope. Complete analytical validation was carried out according to the International Conference of Harmonization, and real samples were analyzed to demonstrate the applicability of the proposed method for routine use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selective decontamination of the digestive tract should decrease the burden of nosocomial infectious agents attributable to the translocation of enteric flora, endotoxin absorption, and subsequent cytokinemia [1]. In France, the combination of two antibiotics, gentamicin sulfate and colistin sulfate, is recommended by the ANSM (the French National Agency for Medicine and Health Products Safety) for selective digestive decontamination [2]. Decontamination of the digestive tract is selective because there is no digestive tract absorption of these two antibiotics [2]. The approach is based on reducing the concentration of Gram negative bacilli (enterobacteriaceae and Pseudomonas aeruginosa) by in situ decontamination of the digestive tract. This drug is given orally in a hard capsule form which containing the both active substances (gentamicin sulfate and colistin sulfate) and the excipients (pregelatinized maize starch, microcrystalline cellulose, magnesium stearate and colloidal silica).
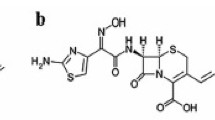
Gentamicin sulfate is a mixture of aminoglycoside antibiotic substances produced by Micromonospora purpurea and it is composed primarily of five closely related compounds [3] designated C1, C1a, C2, C2a and C2b (Fig. 1). On the other hand, colistin sulfate antibiotic substance produced by Bacillus polymyxa var. colistinus is a complex mixture of closely related polypeptides and is active against Gram negative bacteria. General structure of these polypeptides is a cyclic heptapeptide moiety bonded to a tripeptide side chain with an N-terminal fatty acyl residue (Fig. 2). It is a complex mixture of at least 30 different compounds, the main components of which are colistin A (polymyxin E1) and colistin B (polymyxin E2) that differ only in the fatty acid side chain. The sum of polymyxins E1, E2, E3, E1-1 and E1-7MOA accounts for a minimum of 77 % of the dried substance.
Consequently, simultaneous analysis of gentamicin sulfate and colistin sulfate is a by far real challenge, due to the multicomponent mixture of highly polar, non-volatile and non UV absorbing chromophores.
Detection
The chemical structures of gentamicin and colistin reveal the lack of a chromophore in the molecules. For colistin, direct low-UV detection at 212 nm is described in the European [4] and the US [5] pharmacopoeias and has been previously used to assay colistin in pharmaceutical formulations [6]. Derivatization methods with a chromophore or fluorophore were also described to increase sensitivity of the method [7] and mass spectrometry has been extensively used for stability studies [8].
Considerable work has been conducted to enhance the detection of gentamicin [9]. Refractive index detection proved to be insufficiently sensitive and incompatible with gradient elution. UV-detection after pre-column derivatization with o-phthalaldehyde is described in the US Pharmacopeia [10]. In most cases, pre- and post-column derivatizations with UV or fluorescence detection were found to be tedious, time-consuming and furthermore quantitative analysis is difficult due to the instability of derivatives or incomplete reaction of derivatization. Mass spectrometry is valuable for identifying and quantifying major and minor components, as well as for impurities or degradation products, but needs high cost instrument for routine analysis of pharmaceutical formulations [11]. Direct detection of gentamicin with electrochemical detection is the current official method prescribed in the European Pharmacopoeia [12]. However, this method is difficult in routine use and requires specific and costly instrumentation. Other direct detection methods can also be used, such as evaporative light scattering detection (ELSD) [9, 13, 14] or more recently, charged aerosol detector [15]. One drawback of the ELSD is that the detector response is exponential, rather than linear and explains why the range of linearity is restricted. Charged aerosol detector provides good linear signal and sensitivity with but this detector is relatively recent and is not yet commonly found in quality control laboratories. Direct capillary electrophoresis with low-UV detection of underivatized gentamicin has been conducted at 195 nm but was limited to the detection of gentamicin concentration range of 2–6 mg mL−1 [16].
Separation
Ion-pairing Chromatography
Colistin sulfate and gentamicin sulfate are both mixture of basic and polar species and are difficult to separate using reverse phase liquid chromatography. In an attempt at reaching successful separation, ion-pairing agents can be used to form ion pairs with the multiple amine functional groups of both gentamicin and colistin. The major compounds of gentamicin contain five amino groups (Fig. 1). The amino groups on ring I are the most basic (pKa ~ 9.6), whereas the pKa values of those on ring II (pKa ~ 5.6–8.0) and ring III (pKa ~ 7.5) are closer to physiological pH [17]. Polymyxin complexes (i.e. colistin sulfate) contain a polar moiety of five unmasked amino groups in each 2,4-diaminobutanoic acid with pKa about 10 [18]. The pKa of basic compounds such as gentamicin and colistin are shifted to lower values with increasing organic contents [19], while acidic species such as heptafluorobutyric acid (HFBA) are shifted to higher values. The shift is approximately 0.3 pH units per 10 % v/v organic phase and these basic compounds containing amino groups are positively charged in low pH mobile phases. Consequently, these compounds are necessarily retained by a combination of electrical (charge–charge) and hydrophobic interactions with the stationary phase and with the ions of the mobile phase. The mixed mechanism involves ion-pairing, ion exchange and hydrophobic partitioning [20, 21]. The mechanism of ion-pairing chromatography can vary between two extremes, i.e. ion exchange and partitioning similar to that in reverse phase chromatography. A mixed mode mechanism seems likely for many types of ion-pairing chromatography [22].
Elution Gradient
Polymyxin complexes are amphiphilic compounds containing a hydrophobic fatty acid moiety. They are less polar than gentamicin. Polarity gradient elution was in fact necessary to conduct their simultaneous determination as described for the separation of tobramycin and colistin in a pharmaceutical formulation [23]. The elution gradient enabled rapid and simultaneous analysis of two active substances of this pharmaceutical form. The goal of the present development was to quantitatively assay each drug substance after separation of colistin and gentamicin without the separation of their different constituents.
The aim of this work was to develop a rapid and simple chromatographic method for simultaneous quantitative determination in hard capsule of both gentamicin sulfate and colistin sulfate with low-UV detection after ion-pairing chromatographic gradient. The method was validated using criteria according to the ICH guidelines [24] and was evaluated in routine use.
Materials and Methods
Chemicals and Reagents
Hexafluorobutyric acid (HFBA) was used as the pairing reagent for HPLC separation was obtained from Sigma (Steinheim, Germany). Acetonitrile (Hipersolv® Chromanorm®, super-gradient grade) was obtained from Prolabo-VWR International SAS (Fontenay-sous-Bois, France). Water for HPLC analysis and dilutions was produced in-house with a Milli-Q water purification system (Millipore, Bedford, MA, USA). Gentamicin sulfate was supplied by Unipex (La Defense, France) and colistin sulfate by Inresa (Bartenheim, France). Both formulations (pediatric and adult) contained the same excipients: pregelatinized maize starch (Lycatab C®, from IMCD, Saint Denis, France) and microcrystalline cellulose (Emcocel 50 M®, Rettenmaier, Saint-Germain-en-Laye, France) as diluent, magnesium stearate (Univar, Fontenay-sous-Bois, France) as glidant and hydrophilic fumed silica (AerosIl 200®, Azelis, Paris, France) as lubricant.
Liquid Chromatographic Apparatus and Software
Chromatographic separations were carried out on an UltiMate® 3000 system (Dionex Corporation, USA) and consisting of a LPG-3400 A quaternary pump, an AS 3000 auto sampler fitted with a 300 μL loop, a SCM 400 vacuum degasser and a UV spectrophotometric multi-diode array detector. The detection wavelength was set at 215 nm and injection volume was 20 μL. The UltiMate® 3000 system was connected to a Chromeleon® 6.8 data acquisition and data processing software system. The analytical column was a Waters X-Terra® C18 MS (Wexford, Ireland) 2.5 μm, measuring 50 mm × 4.6 mm and was maintained at 35 °C. The mobile phase was a binary mixture of 0.05 % HFBA in water (phase A) and 0.05 % HFBA in acetonitrile (phase B) in a gradient elution mode at a flow-rate of 1.5 mL min−1. The mobile phases were filtered through 0.45 μm HVLP hydrophilic Millipore filters (Millipore, Molsheim, France) before use and gradient elution in final conditions is shown in Table 1.
Preparation of Standard and Assay Solutions
Standard solutions were prepared daily by dissolving the appropriate amount of gentamicin sulfate and colistin sulfate in water to obtain final concentrations in the range of 0.6–1.4 mg mL−1 and 0.3–1.3 mg mL−1 for colistin sulfate and gentamicin sulfate, respectively. When excipients were added to the spiked calibration standard solutions or were present in real samples, the corresponding solution was sonicated for 1 min and then filtered through an Acrodisc® syringe filter with a GHP membrane (13 mm diameter, 0.45 μm pore size).
Validation Procedure
The HPLC–UV analytical method was validated for specificity, precision (repeatability and intermediate precision), linearity and accuracy.
The validation protocol was conducted on three consecutive days by the same operator and fresh solutions were prepared daily. Two calibration curves with five calibration standards have been prepared in the presence or absence of excipients (with or without matrix). The calibration points have been prepared with respect of the next protocol routinely applied. The matrix was the addition of excipients to the pediatric hard capsules and was the worst case, i.e. with higher excipients percentages in the capsule composition (16.5 and 32.9 %, for adult and pediatric formulations, respectively). The range of the calibration curve was determined to enable the determination of colistin and gentamicin from 80 to 120 % of the capsule contents for pediatric and adult forms. Nine quality controls were prepared daily with excipients at three levels (0.6, 1.0 and 1.4 mg mL−1 for colistin sulfate and 0.3, 0.7 and 1.3 mg mL−1 for gentamicin sulfate) and were injected once. The e-noval software V2.0. (Arlenda, Liège, Belgium) was used to compute all validation results.
Results and Discussion
Development of the Chromatographic Method
Compatible Mobile Phase Composition with Low-UV Detection
UV detection is one of the most widespread detection techniques used with liquid chromatography, but its use is obviously limited since it requires a chromophore group in the analyte structure. Colistin sulfate and more so especially gentamicin sulfate lack highly absorbing π-electrons on their structures. Indeed, the specific absorbance (\( A_{{1{\text{cm}}}}^{1\% } \)), corresponding to the absorbance of a 10 g L−1 solution in a 1 cm cell [25] of colistin sulfate and gentamicin sulfate at 215 nm, was 48.2 and 0.40, respectively (Fig. 3). The wavelength was chosen for its highest signal/noise ratio.
Consequently, these molecules are barely detected and the sensitivity of UV-detection is very low. Since gentamicin fractions exhibit a very low specific absorbance and because absorbance is additive, co-elution of the five fractions should be envisaged to improve the detection sensitivity even with a large tailing peak. However, in our case, gradient elution is necessary for a shorten analysis run. Unfortunately, gradient elution caused drastic baseline drifts with low-UV detection. These drifts result from modifications of the composition of the mobile phase. Acetonitrile, which is a transparent mobile phase at 215 nm, exhibits moreover sufficient elution strength for paired biomolecules (Fig. 3) and was used in this study. HFBA is the ion-pairing agent which enables good retention of the two drug substances. The retention times of the two drugs increase with increasing hydrophobicity of the acid additive, explaining why HFBA was chosen because of the high polarity of gentamicin.
This ion-pairing has a specific absorbance at 215 nm (Fig. 3). The absorbance of an aqueous solution of 0.05 % (v/v) HFBA was 0.50 UA (\( A_{{1{\text{cm}}}}^{1\% } \) = 10) and concentration fluctuations during gradient elution resulted from baseline drift. The HFBA concentration and gradient elution were optimized as detailed in the following section.
Comparison of Two Gradient Elution Profiles
In the initial gradient (Fig. 4), the binary mixture consisted of aqueous solution with 0.1 % (v/v) HFBA, and ACN was HFBA free. First, gentamicin was eluted without baseline drift (Fig. 5a), then colistin was then eluted during baseline drift. These absorbance fluctuations of the mobile phase were related to variations of the HFBA concentration in the mobile phase. The hydrophobicity of HFBA anionic ion-pairing is due to hydrocarbon chain length (C3) and exhibits some amphiphilic properties [26]. Adsorption of HFBA on the stationary phase occurred and the hydrophobic chain was probably inserted in the bonded organic layer with the polar functional group protruding toward the mobile phase [26]. During gradient elution, the % of ACN increased after 3 min. The result on baseline drift was an increase of absorbance in the 3.7–4.5 min interval and then a linear decrease until 12 min. The increase of absorbance could be resulted from the change in HFBA partitioning between the stationary and mobile phases due to increasing elution. The HFBA concentration then decreased because ACN was HFBA free and could explain the decrease of absorbance after 4.5 min.
After optimization, the HFBA content was 0.05 % (v/v) (versus 0.1 % in chromatogram a). HFBA was added to ACN and the elution gradient profile was modified: baseline drift and analysis run time were minimized with good separation of gentamicin and colistin species (Fig. 5b).
The optimized gradient profile exhibited a number of improvements. First, the retention of gentamicin compounds was better (k = 3.2 versus k = 1.8 with the initial gradient). Second separation was more rapid (less than 10 min between two injections) and baseline fluctuations were controlled during colistin and gentamicin elution.
Interpretation of Negative Peaks
Three negative peaks (a, b and c) were observed on the chromatogram (Fig. 5b). The dwell time was estimated at 0.5 min by an injection of acetone, corresponding to the peak b. Peak a (tr = 0.3 min) was observed before the dwell time and the injection of aqueous sodium sulfate confirmed the presence of a negative sulfate peak at 0.3 min. This negative peak was proportional to the concentrations of colistin and gentamicin injected, since they are both sulfate salts. The chromatographic exclusion of sulfate (doubly negatively charged ion) probably resulted from the negative electrostatic repulsive force between sulfate anions and HFBA anions bonded to the stationary phase. Sulfate is a UV-transparent anion at 215 nm and indirect detection resulted from the absorbance of HFBA, reacting as a chromophoric probe. Peak c corresponded to the decrease of HFBA content in the mobile phase, since the solution injected is HFBA free. This negative peak disappeared when the injection solvent contained the same HFBA concentration than the mobile phase. On contrary, it became positive if the HFBA concentration in the injection solvent was higher than that of the mobile phase. The peak was eluted after the dwell time and illustrated the retention of HFBA on the stationary phase (k = 0.4).
Optimization of the Gradient Elution
The objectives of optimization were to minimize baseline drift and noise by optimizing the HFBA concentration, the proportion of ACN and the gradient profile; the retention factors of gentamicin and colistin depend on these parameters. Retention is in fact a combination of electrical (charge–charge) and hydrophobic interactions with the stationary phase and with the ions present in the mobile phase. The mixed mechanisms involved are (i) ion-pairing, (ii) ion exchange, and (iii) hydrophobic partitioning. The various equilibria were disrupted by variations in HFBA concentration as well as ACN content.
The quantities of HFBA adsorbed on the stationary phase increased with increasing HFBA concentration in the mobile phase [27], and thus increasing the baseline drift. It was important to note that equilibration time was not changed by the HFBA concentration and was short for HFBA ion-pairing due to the short chain length (C3) [27]. This short equilibration time was of interest in this gradient application to minimize the time of each run. Retentions of the two solutes increased with increasing HFBA concentration (from 0.01 to 0.1 % v/v) and decreasing of proportion of ACN.
From the point of view of detection, the ion-pairing agent affected detection because of its absorbance at 215 nm. The level of the ion-pairing agent may in fact have to be minimized to limit baseline noise, and baseline drift was primarily due to variations of the HFBA concentration during the analysis run.
Optimization of this separation had to take into account both constraints related to retention of the solutes and their detection. There are no simple rules to select the optimal concentration of HFBA and the gradient elution profile. To minimize baseline drift, the HFBA concentration had to be reduced with the risk to elute gentamicin in the void volume.
Initially, HFBA was added to the organic phase at the same concentration as that of the aqueous phase. The gradient profile and different HFBA concentrations were then evaluated.
Because of the differences in the retention behavior of gentamicin, colistin and HFBA, changing the gradient slope may alter adsorption of the ion-pairing reagent in the stationary phase to a different degree than gentamicin and colistin. HFBA selectivity may be explained by the concentration-dependent coating of the stationary C18 alkyl chain [28]. Gentamicin and colistin may elute at slightly different HFBA concentrations.
Initially, the gradient consisted of two main steps (Fig. 4): the first enabled isocratic elution of gentamicin and the second enabled the elution of colistin compounds, but retention of gentamicin was insufficient (k = 1.8). The isocratic elution of gentamicin with a lower proportion of ACN was associated with a tailing of the different peaks of gentamicin and a loss of sensitivity. For this reason, gradient elution was started by the injection with a lower ACN proportion, thereby eluting gentamicin in the gradient. Gentamicin could then be correctly retained (k = 3.2) and the various peaks of gentamicin tailing could also be reduced.
Influence of the Gradient Slope
Increasing the slope of the gradient increased baseline drift. Concomitantly, peak height increased and peak area remained constant.
Influence of the HFBA Concentration
With the optimized gradient slope described in Fig. 4, HFBA concentrations were evaluated from 0.01 to 0.10 % (v/v). Figure 6 illustrates the effect of HFBA concentration. It is known that the increase in retention resulted from interaction with acidic counter-anions. Increased retention depends on the concentration of the counter-ion in the mobile phase [19]. Retention factors, baseline drift and the background noise increased with increasing HFBA concentrations. The choice of an optimal HFBA concentration was a compromise between drift and the retention and is proposed at 0.05 % (v/v).
Validation of the Proposed Method
Specificity
The method was developed to measure the content of drug substances in the presence of other ingredients and excipients in a pharmaceutical product. The results showed no interferences between gentamicin and colistin and none of the excipients interfered with the drug substances.
Response Function
The response function is the relationship between the response observed and the concentration of the analyte in the sample. The best response function with or without excipients was obtained with linear regression without weighting. The response functions obtained by applying these regression models are shown in Table 2.
The slopes of the calibration curves in water or in the presence of excipients were not significantly different (α = 5 %) for gentamicin and for colistin. The Y intercepts with or without excipients were not different (α = 5 %) but were significantly different from zero for gentamicin (α = 5 %).
No matrix effects due to excipients were observed. The standards for calibration curves and quality controls were prepared in water in routine use for analysis of real samples.
Precision
The precision of the method was determined by computing the relative standard deviations (RSDs, %) for repeatability and intermediate precision at each concentration level of quality control. The precision at each concentration level did not exceed 3.5 and 2.1 % for colistin and for gentamicin, respectively (Table 3), confirming that the method is convenient for quality control use.
Accuracy
Accuracy was determined by measuring the relative bias of quality control and mean recovery according to ICH recommendations (2005). As can been seen in Table 4, the relative bias (trueness) was lower than 2.4 % and thus acceptable. The confidence intervals (α = 5 %) of mean recovery included 100 %.
Analysis of Real Samples
Six home-made batches of pediatric (n = 3) and adult (n = 3) capsules were evaluated by the validated analytical method (Table 5). The three pediatric batches complied with the test of uniformity of dosage units (n = 10) [29]. Two of the three batches tested for adults complied with the test after 10 or 30 dosage units. Batch A for adult was out of specification according to the specification of the European pharmacopeia (2.09.40): means and RSDs were equivalent for gentamicin and colistin, indicating a suitable blend of drug substances and excipients but incorrect capsule filling.
Conclusion
A very rapid and simple method for the simultaneous assay of gentamicin and colistin in hard capsules by ion-pairing gradient chromatography with low-UV detection has been developed. The method has been validated for specificity, precision, accuracy and linearity. The chromatographic method has been shown suitable for the quantification of gentamicin and colistin in routine use, for pediatric and adult hard capsule formulations.
In pharmaceutical analysis, the detection and quantification of analytes with weak chromophores is highly problematic and requires working at a low-UV wavelength. This application is an example of troubleshooting, i.e. baseline drift, due to gradient elution and absorbance of the ion-pairing agent and illustrates that the mixed mechanism involves ion-pairing, ion exchange and hydrophobic partitioning. Baseline drift was minimized by optimizing the HFBA concentration and the gradient slope and could be applied to other substances with low-UV absorbing chromophores.
References
de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay LH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ (2009) Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31
(2011) Utilisation de préparations hospitalières dans la décontamination digestive séléctive. http://nosobase.chu-lyon.fr/recommandations/afssaps/2011_DDS_AFSSAPS.pdf
(2011) European Pharmacopeia 7th edn, Gentamicin sulfate, Monograph 331. European Department for the Quality of Medicines, Strasbourg, France
(2011) European Pharmacopeia, 7th edn, Colistin sulfate, Monograph 320. European Department for the Quality of Medicines, Strasbourg, France
(2010) United States Pharmacopeia 32 NF 27, Colistin sulfate, Official monograph. The United States Pharmacopeial Convention, Rockville
Perez-Lozano P, Garcia-Montoya E, Orriols A, Minarro M, Tico JR, Sune-Negre JM (2007) Application of a validated method in the stability study of colistin sulfate and methylparaben in a veterinary suspension formulation by high-performance liquid chromatography with a diode array detector. J AOAC Int 90:706–714
Morales-Munoz S, de Castro MD (2005) Dynamic ultrasound-assisted extraction of colistin from feeds with on-line pre-column derivatization and liquid chromatography-fluorimetric detection. J Chromatogr A 1066:1–7
Orwa JA, Govaerts C, Gevers K, Roets E, Van Schepdael A, Hoogmartens J (2002) Study of the stability of polymyxins B(1), E(1) and E(2) in aqueous solution using liquid chromatography and mass spectrometry. J Pharm Biomed Anal 29:203–212
Manyanga V, Grishina O, Yun Z, Hoogmartens J, Adams E (2007) Comparison of liquid chromatographic methods with direct detection for the analysis of gentamicin. J Pharm Biomed Anal 45:257–262
(2010) United States Pharmacopeia 32 NF 27, Gentamicin sulfate, Official monograph. The United States Pharmacopeial Convention, Rockville
Vucicevic-Prcetic K, Cserenak R, Radulovic N (2011) Development and validation of liquid chromatography tandem mass spectroscopy methods for the determination of gentamicin, lincomycin, and spectinomycin in the presence of their impurities in pharmaceutical formulation. J Pharm Biomed Anal 56:736–742
Ghinami C, Giuliani V, Menarini A, Abballe F, Travaini S, Ladisa T (2007) Electrochemical detection of tobramycin or gentamicin according to the European Pharmacopoeia analytical method. J Chromatogr A 1139:53–56
Clarot I, Chaimbault P, Hasdenteufel F, Netter P, Nicolas A (2004) Determination of gentamicin sulfate and related compounds by high-performance liquid chromatography with evaporative light scattering detection. J Chromatogr A 1031:281–287
Megoulas NC, Koupparis MA (2004) Development and validation of a novel LC/ELSD method for the quantitation of gentamicin sulfate components in pharmaceuticals. J Pharm Biomed Anal 36:73–79
Joseph A, Rustum A (2010) Development and validation of a RP-HPLC method for the determination of gentamicin sulfate and its related substances in a pharmaceutical cream using a short pentafluorophenyl column and a charged aerosol detector. J Pharm Biomed Anal 51:521–531
Curiel H, Vanderaerden W, Velez H, Hoogmartens J, Van Schepdael A (2007) Analysis of underivatized gentamicin by capillary electrophoresis with UV detection. J Pharm Biomed Anal 44:49–56
Wilson WL, Richard G, Hughes DW (1973) Chemical determination of component ratio and potency of gentamicin complex. J Pharm Sci 62:282–284
Kwa AL, Tam VH, Falagas ME (2008) Polymyxins: a review of the current status including recent developments. Ann Acad Med Singapore 37:870–883
Lobrutto R, Jones A, Kazakevich YV, McNair HM (2001) Effect of the eluent pH and acidic modifiers in high-performance liquid chromatography retention of basic analytes. J Chromatogr A 913:173–187
Bidlingmeyer BA (1983) Reversed-phase ion-pair liquid chromatography. LC Mag 1:344–349
Berthod A, Ruiz-Angel MJ, Carda-Broch S (2008) Ionic liquids in separation techniques. J Chromatogr A 1184:6–18
Fritz JS (2005) Factors affecting selectivity in ion chromatography. J Chromatogr A 1085:8–17
Clarot I, Storme-Paris I, Chaminade P, Estevenon O, Nicolas A, Rieutord A (2009) Simultaneous quantitation of tobramycin and colistin sulphate by HPLC with evaporative light scattering detection. J Pharm Biomed Anal 50:64–67
International Conference on Harmonisation (ICH) of technical requirements for registration of pharmaceuticals for human use, topic Q2 (R1) (2005) Validation of analytical procedures: text and methodology
(2011) European Pharmacopeia 7th edn, Absorption spectrophotometry. European Department for the Quality of Medicines, Strasbourg, France
Flieger J (2010) Application of perfluorinated acids as ion-pairing reagents for reversed-phase chromatography and retention-hydrophobicity relationships studies of selected beta-blockers. J Chromatogr A 1217:540–549
Petritis KN, Chaimbault P, Elfakir C, Dreux M (1999) Ion-pair reversed-phase liquid chromatography for determination of polar underivatized amino acids using perfluorinated carboxylic acids as ion pairing agent. J Chromatogr A 833:147–155
Pearson JD, McCroskey MC (1996) Perfluorinated acid alternatives to trifluoroacetic acid for reversed-phase high-performance liquid chromatography. J Chromatogr A 746:277–281
European Pharmacopeia 7th edn, Uniformity of dosage units, 2.09.40 (2011) European Department for the Quality of Medicines, Strasbourg, France
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caudron, E., Baghriche, S., Prognon, P. et al. Simultaneous Quantification of Gentamicin and Colistin Sulfate in Pharmaceuticals using Ion-Pairing and Polarity Gradient Chromatography with Low-UV Detection. Chromatographia 76, 747–755 (2013). https://doi.org/10.1007/s10337-013-2478-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-013-2478-7