Abstract
Herein we report two sensitive and accurate UHPLC and TLC-densitometric methods for the simultaneous determination of amprolium HCl, ethopabate, and sulfaquinoxaline-Na. A UHPLC isocratic elution method was adopted with a mobile phase of sodium 1-hexanesulfonate aqueous solution–methanol–acetonitrile in a ratio of (1500:400:100, v/v/v) adjusted to a pH of 5.1 with phosphoric acid. Results showed R2 = 0.9999 over concentration ranges of 0.5–25.0 μg mL−1, 1.0–30.0 μg mL−1, and 1.0–30.0 μg mL−1 for the three drugs, respectively. The accuracy was 100.58% ± 0.52 for amprolium HCl and 98.78% ± 0.54 and 100.14% ± 0.11 for ethopabate and sulfaquinoxaline-Na. Additionally, a TLC-densitometric method was adopted to separate the three cited drugs with a developing system of chloroform:methanol:33% ammonia solution (6:4:0.5 v/v/v) and UV detection at 263 nm. Results showed Rf values of 0.34, 0.65, and 0.95 for amprolium HCl, sulfaquinoxaline-Na, and ethopabate, respectively. The linearity range was 1.0–30.0 μg/band, 0.5–20.0 μg/band, and 1.0–25.0 μg/band for amprolium HCl, ethopabate, and sulfaquinoxaline-Na, respectively. The proposed TLC-densitometric method was utilized for the simultaneous determination of the three drugs in spiked biological matrices with acceptable recoveries. The proposed methods were applied for simultaneous quantitation of three drugs in veterinary formulation and the results were in accordance with those obtained by the reported methods. In conclusion, the two suggested methods were sensitive and accurate for the simultaneous quantitation of the three drugs in both their dosage forms and in biological matrices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
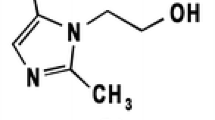
Coccidiosis is a parasitic disease that can affect poultry (broilers, layers, breeding hens, turkeys, pigeon, and ducks), animals (cattle, sheep, goats, dogs, and rabbits), and it therefore causes significant economic losses. [1] Anticoccidial drugs are either polyether ionophorous antibiotics that are derived from fermentation products, or synthetic compounds, produced by chemical synthesis. Drugs are prescribed based on disease severity, resistance patterns of strains in the area of acquisition, efficacy, and adverse effects of drugs available. [2] The three cited drugs are anticoccidial with different mechanisms of action. Amprolium hydrochloride is thiamine (vitamin B1) antagonist used as coccidiostat. [3] Ethopabate is used in the prophylaxis and treatment of coccidiosis via competition of para-aminobenzoic acid (PABA) for absorption [4], whereas sulfaquinoxaline-Na is a sulfonamide antibacterial [5] Fig. 1.
A novel and new combination of amprolium-HCl with ethopabate and sulfaquinoxaline-Na in new combined dosage form both extended and strengthened the spectrum of activity, and the combined dosage form was also recommended for treatment of outbreaks [6].
A literature survey revealed many analytical techniques for the analysis of the three drugs, including spectrophotometry [7,8,9,10], spectrofluorometry [11,12,13], electrochemistry [14, 15], TLC–densitometry [16,17,18], liquid chromatography [19,20,21,22,23], and electrophoresis [24, 25]. Up to our knowledge, there was no analytical method for simultaneous determination of the three drugs.
In this work, a TLC–densitometric and UHPLC methods were described for the simultaneous determination of amprolium HCl, sulfaquinoxaline-Na, and ethopabate in pure forms (Fig. 1), in their tertiary mixture and in dosage form. Additionally, the suggested methods were applied to chicken liver samples that usually contain high reservoir of the three drugs.
In addition, the greenness of the proposed methods was assessed according to the analytical Eco-Scale where an ideal green analysis has a value of 100. [26, 27]. Another green tool; Green Analytical Procedure Index (GAPI) was also applied to the present work. GAPI evaluates 15 parameters of any analytical procedure. It applies a pictogram to classify the greenness of each stage of an analytical procedure, using a color scale, with three levels of evaluation for each stage [28, 29].
Materials and Methods
Instruments
-
Agilent 1100 UHPLC with binary pump and UV detector, equipped with Phenomenex Kinetex C18 column (100 mm, 4.6 mm i.d., 2.6 µm); USA.
-
Ultrasonic bath (Wised clean, China).
-
Densitometer model 3; equipped with WINCATS software.
-
Camag TLC scanner 3, Camag-linomat 5 autosampler (Switzerland).
-
Chromatographic tank 10.0 × 20.0 cm for TLC development.
-
Digital pH meter with double-junction glass electrode (Hanna, Romania).
-
Corning® syringe filters, regenerated cellulose membrane, diam. 4 mm, pore size 0.5 and 0.22 μm (CLS431212-50EA), (Merck, Germany).
Chemical and Reagents
-
Pure amprolium HCl (CAS: 137-88-2); B.N. WS/20180717, was kindly supplied by Zhejiang K-sheng., Bio-pharmgroup Co. Ltd., Egypt; with purity of 99.8% purity as referred by the supplier and it was tested by TLC–densitometry.
-
Pure ethopabate (CAS: 59-06-3); B. N. 20,190,327, was kindly supplied by Zhejiang Huangyan Vet Pharma Factory, China, with purity of 99.5% as referred by the supplier and it was tested by TLC–densitometry.
-
Pure sulfaquinoxaline-Na (CAS: 967-80-6); B. N. BL160725, was kindly supplied by Wujiang Bolin Industry Co. Ltd., Egypt; with purity of 99.5% as referred by the supplier and it was tested by TLC–densitometry
-
Amproethoquine® powder; B.N. ATQN5097, labeled to contain amprolium HCl 200 gm, ethopabate 10 gm sulfaquinoxaline-Na 128.78 gm per 1 kg, the product of Biovet, Cairo, Egypt.
-
Chloroform, acetone (BDH Chemicals Ltd, England).
-
Ethyl acetate, n-butanol, triethylamine, dichloromethane, tetrahydrofuran, isopropanol, ammonia solution 33%, toluene, glacial acetic acid, propanol, formic acid, acetic acid (Adwic. Cairo, Egypt).
-
Pentanol (Alfa Chemicals, Egypt).
-
Ethanol (Sigma-Aldrich, Germany).
-
Acetonitrile HPLC grade, methanol HPLC grade (Fisher scientific, USA).
-
Sodium 1-hexanesulfonate (Sigma-Aldrich, Germany)
-
Phosphoric acid (Adwic, Cairo, Egypt).
-
TLC plates pre-coated with silica gel 60 F254, aluminum sheets 20 × 20 cm and 0.25 mm thickness, part number; 1,003,900,001 (Merck, Germany).
Preparation of the Mobile Phase
Preparation of mobile phase: Dissolve 3.0 gm sodium 1-hexanesulfonate in distilled water to prepare 1L solution. Mix 3.0 g/L sodium 1-hexanesulfonate aqueous solution-methanol- acetonitrile (1500:400:100, v/v/v). Adjust the pH to 5.1 using phosphoric acid. Filter under reduced pressure to remove insoluble substance (0.5 μm pore size).
Preparation of Standard Solution for UHPLC
Standard solutions of the three drugs (0.1 mg mL−1) were prepared and diluted with the diluent (mobile phase). All aqueous solutions were stored at 4–6 °C, whereas chicken liver samples were stored in deep freezer maintained at − 80 °C.
Preparation of Standard Solution for TLC–Densitometry
Standard solutions of amprolium HCl, ethopabate, and sulfaquinoxaline-Na (5.0 mg mL−1) were prepared in a mixture of methanol and water (50:50, v/v).
Methods and Procedures
Linearity
UHPLC
Aliquots from the three drugs solutions (0.1 mg mL−1) equivalent to 0.005–0.25 mg, 0.01–0.30 mg, and 0.01–0.30 mg amprolium HCl, ethopabate, and sulfaquinoxaline-Na, respectively, were separately transferred into a series of 10 mL volumetric flasks and diluted to volume with the mobile phase. Triplicate 10 μL injections from each solution were chromatographed using six standard points under the last mentioned chromatographic conditions; 0.5, 1, 7, 15, 20, 25 µg mL−1 for amprolium HCl; 1, 3, 12, 20, 25, 30 µg mL−1 for ethopabate; 1, 3, 10, 12, 20, 30 µg mL−1 for sulfaquinoxaline-Na (supplementary material, Figure S2). The peak area was plotted versus the drug concentration and the regression parameters were deduced.
TLC–Densitometry
Aliquots of (5.0 mg mL−1) standard solutions of amprolium HCl equivalent to 1.0–30.0 mg, ethopabate equivalent to 0.5–20.0 mg and sulfaquinoxaline-Na equivalent to 1.0–25.0 mg were transferred into a series of 10 mL volumetric flasks and diluted to the volume with methanol:water (50:50,v/v) solution. 10 μL of each solution was applied to a TLC plate pre-coated with silica gel 60 F254 (10 × 20 cm) as a band of 6.0 mm width, 2.0 cm apart from the bottom edge of the plate. The plates were placed in a pre-saturated chromatographic chamber for 30 min with the mobile phase of methanol:chloroform:ammonia solution 33% (4:6:0.5, v/v/v) and allowed to develop at room temperature. The plates were dried in air and bands were scanned at 263.0 nm. Each calibration curve representing the recorded area under the peak versus drug concentration was constructed using six standard points; 0.1, 0.5, 1.0, 1.5, 2.0, 3.0 mg mL−1 for amprolium HCL; 0.05, 0.2, 0.3, 0.5, 1.0, 2.0 mg mL−1 for ethopabate; 0.1, 0.3, 0.5, 1.0,17.5, 2.5 mg mL−1 for sulfaquinoxaline-Na. (supplementary material, Figure S3). The corresponding regression equation was computed.
Assay of Laboratory Prepared Mixtures of the Three Drugs
UHPLC Method
Different aliquots of standard aqueous methanolic solutions of amprolium HCl, ethopabate and sulfaquinoxaline-Na (0.10 mg mL−1) equivalent to 0.005–0.25 mg, 0.01–0.3 mg, and 0.01–0.3 mg, respectively, were mixed in 10 mL volumetric flasks. Volumes were completed with methanol: water (50:50), v/v. 10 μL of the obtained mixtures were injected using the last mentioned chromatographic conditions.
TLC–Densitometric Method
Different aliquots of standard aqueous methanolic solutions of amprolium HCl, ethopabate, and sulfaquinoxaline-Na (5.0 mg mL−1) equivalent to 10.0–20.0 mg, 1.0–20.0 mg, and 10.0–20.0 mg, respectively, were mixed in 10-mL volumetric flasks. Volumes were completed with methanol:water (50:50, v/v). 10 μL of each solution was applied to the TLC plate analyzed by the TLC–densitometric method described under section “3.1. Linearity”.
Application to Veterinary Formulation
Accurately weighed 0.1 gm from Amproethoquine® powder equivalent to 20.0 mg amprolium-HCl, 1.0 mg ethopabate, and 12.8 mg sulfaquinoxaline-Na was added in a 100 mL volumetric flask. The powder was dissolved in 70.0 ml mixture of methanol and water (1:1) by shaking in ultrasonic bath at 25 °C for 10 min. Volume was completed with the same solvent and the solution was filtered to obtain a clear solution labeled to contain 2.0 mg mL−1 amprolium HCl, 0.1 mg mL−1 ethopabate, and 1.28 mg sulfaquinoxaline-Na. Several concentrations of three drugs within the linearity range were prepared and the prepared solution was chromatographed following the procedure mentioned under “3.1. Linearity”. The concentration of each drug was calculated from the corresponding regression equation.
Application to Chicken Liver Samples
An accurately weighed 1.0 gm of the chicken liver was transferred to centrifuge tubes to be spiked with different aliquots of amprolium-HCl, ethopabate, and sulfaquinoxaline-Na standard aqueous methanolic solutions (5.0 mg mL−1) equivalent to 1.0–30.0 mg, 0.5–20.0 mg, and 1.0–25.0 mg; respectively. The spiked samples were homogenized at 5000 rpm for 5 min. The homogenate was sonicated at 25 °C for 20 min and then centrifuged at 3500 rpm for 15 min. The samples were re‐extracted with methanol (2.0 × 3.0 mL). The extracts were combined, filtered through a syringe filter having 0.22 μm pore size in 10-mL methanol in volumetric flask. 10 μL of each solution was applied to a TLC plate, and the procedures described under “3.1. Linearity” for the TLC–densitometry method were adopted.
Results and Discussion
UHPLC Method
Three different chromatographic columns were compared: Kinetex-C18 (2.6 μm, 4.6 mm × 100 mm, 100 Å), ZIC-HILIC (3.5 μm, 4.6 mm × 150 mm, 100 Å), and Kromasil-C18 (5 μm, × 4.6 mm × 250 mm, 100 Å). Several mobile phase compositions were tested (butanol:methanol, butanol:acetonitrile, water:methanol and water:acetonitrile, various flow rates (0.5–1.5 mL min−1) and wavelengths (230–350 nm) were tested for simultaneous determination of the three drugs with satisfactory separation.
Fast separation over 1.5 min was obtained upon using ZIC-HILIC and Kromasil®, while the separation using Kinetex® column reached 3 min. However, higher resolution (Rs ˃ 4) was obtained upon using Kinetex® column (ZIC-HILIC and Kromasil Rs = 3.9). Thus, Kinetex column was selected for the simultaneous determination of the three drugs. Different mobile phases with different ratios were used. Using butanol:methanol (20:80, v/v/), only the peaks of amprolium HCl and sulfaquinoxaline-Na were eluted and detected. Whereas only sulfaquinoxaline-Na was eluted when butanol:acetonitrile (20:80, v/v/) used as the mobile phase. On the contrary, only amprolium HCl was eluted upon using water:acetonitrile (50:50 v/v/) and water:methanol (50:50, v/v/) mobile phases. Satisfactory separation of the tertiary mixture with a reasonable difference in retention time (about 2 min) was achieved with a mobile phase composed of acetonitrile—methanol–3.0 g/L sodium 1-hexanesulfonate aqueous solution in a ratio of (1:4:15, v/v/v) and pH = 5.1. The suggested mobile phase was pumped at 45 0C temperature, flow rate 1.0 mL min−1 and UV detection at 263 nm. These chromatographic conditions resulted in a stable baseline; sharp resolved peaks; tailing factor (T) ranged between 0.98 and 0.99 were obtained at Rt 4.17 ± 0.008 min for amprolium HCl, 2.325 ± 0.005 min for ethopabate, and at 6.25 ± 0.013 for sulfaquinoxaline-Na Fig. 2.
TLC–Densitometric Method
Simultaneous determination of amprolium HCL, ethopabate, and sulfaquinoxaline-Na was carried out using different developing systems in different ratios. Mobile phases tested were namely; toluene:ethylacetate (7:3 v/v) and methanol:chloroform:ethylacetate:glacial acetic acid (7:2:2:0.1 v/v/v/v); these mobile phases showed no baseline separation for amprolium HCl; however, both ethopabate and sulfaquinoxaline-Na showed remarkable baseline separation but unfortunately with similar elution pattern and same Rf values. Upon using methanol: H2O:glacial acetic acid (7:2.5:0.1 v/v/v), only ethopabate showed remarkable baseline separation. The use of ethylacetate:methanol: 33% ammonia solution (9:3:0.5, v/v/v); showed a significant difference in separation and Rf values for sulfaquinoxaline-Na and ethopabate, while amprolium HCl showed no baseline separation.
Only upon using these two mobile phases, chloroform methanol:glacial acetic acid:ethylacetate (5:8:1:1 v/v/v/v) and chloroform: acetonitrile:methanol:glacial acetic acid (9:2:1.5:0.5 v/v/v/v) amprolium HCl showed satisfactory baseline separation (Rf = 0.2).
The separation of the three drugs was achieved only using a mobile phase composed of chloroform:methanol: 33% ammonia solution (6:4:0.5 v/v/v) where the Rf values were found to be 0.34, 0.65, and 0.95, for amprolium HCl, sulfaquinoxaline-Na, and ethopabate; respectively. Chromatogram of the drug was scanned densitometrically at different wavelengths (220.0, 230.0, and 254.0 nm) where optimum peak shape (tailing factor; 1.0), capacity factor (K´ = 0.08–1.9), resolution (Rs ˃3.5), good linearity, and reproducible response were obtained at 263.0 nm Fig. 3.
Method Validation
The two proposed methods were validated according to ICH guidelines.
Linearity
A linear correlation was obtained between each response and the corresponding drug concentration in the range of 0.5–25.0 μg mL−1, 1.0–30.0 μg mL−1, and 1.0–30.0 μg mL−1 for UHPLC method. A linear correlation in the range of 1.0—30.0 μg/band, 0.5–20.0 μg/band, and 1.0–25.0 μg/band for amprolium HCl, ethopabate, and sulfaquinoxaline-Na, respectively, for TLC–densitometric method. The regression parameters were calculated Table 1.
Accuracy
The mean accuracy of the proposed methods were tested using a triplicate of three concentrations of the cited drugs within the linearity range. Mean accuracy was found to be 100.58 ± 0.52, 98.78 ± 0.54, and 100.14 ± 0.11, respectively, for the UHPLC method. Whereas for the TLC–densitometric method, the mean accuracy was found to be 99.4% ± 0.83, 100.25% ± 0.54, and 99.82% ± 1.64 for amprolium HCl, ethopabate, and sulfaquinoxaline-Na, respectively.
Precision
Intraday was performed over a period of 10 min, whereas interday was performed within-laboratories variations (different days; 3 days intervals). The intra- and inter-precision ranges were 0.53–0.71%, 0.43–0.89% for amprolium-HCL, 0.53–0.71%, 0.21–1.14% for ethopabate, and 0.21–0.41%, 0.04–1.14% for sulfaquinoxaline-Na using UHPLC method. The TLC/densitometric method precision values ranged between 0.11 and 1.89%, 0.27 and 1.41% for amprolium HCL, 0.15–1.14%, 0.11–0.78% for ethopabate, and 0.23–0.61%, 0.48–0.68% for sulfaquinoxaline-Na over a period of 3 weeks (supplementary material, Tables S3, S4). These results indicated the repeatability and reproducibility of the proposed methods Table 1.
Robustness
UHPLC Method
It was estimated either by altering the volume of acetonitrile (± 2%), the volume of water (± 2%), or by changing flow rate (± 0.1 mL min−1). No significant changes in the system suitability parameters were observed. Moreover, the resolution between amprolium HCl, ethopabate, and sulfaquinoxaline-Na was not changed and the RSD percentage was ≤ 1.23%; verifying the robustness of the method Table 2a.
TLC–Densitometric method
There was no significant change in Rf values upon introduction of small variations in the volume of the mobile phase compositions within (± 2%). The Rf value gave RSD% not exceeding 1.71% illustrating the robustness of the method Table 2b.
Specificity
Five laboratory prepared mixtures in different ratios of amprolium HCl, ethopabate, and sulfaquinoxaline-Na were analyzed. The proposed UHPLC method was valid for simultaneous determination of amprolium HCl, ethopabate, and sulfaquinoxaline-Na with mean recoveries of 100.03 ± 1.34 for amprolium HCl, 99.41 ± 0.86 for ethopabate, and 100.97 ± 0.6 sulfaquinoxaline-Na. While for the TLC–densitometric, the mean recoveries were 100.19% ± 1.30, 100.17% ± 0.85, and 99.75% ± 1.4 for the three drugs, respectively.
Stability of Standard Solution
The stability of the aqueous methanolic solutions of the three drugs at (5 mg mL−1) was evaluated by the TLC–densitometric methods. The solutions were found to be stable for 2 weeks either at room temperature or in the refrigerator.
The proposed method was successfully applied for the simultaneous determination of amprolium HCl, ethopabate, and sulfaquinoxaline-Na in Amproethoquine® powder. The obtained results revealed no interference by excipients or additives indicating specificity of the method. The mean recoveries ± SD were 100.92 ± 0.78, 99.67 ± 0.75, and 101.38 ± 1.01 and in the mentioned dosage form, respectively Table 3.
The validity of the proposed methods was further verified by applying the standard addition technique. Whereby three different concentrations of each standard solution of the three cited drugs were added directly to the aliquots of veterinary formulation containing the following ratio of the three drugs; 20:12.8:1 of amprolium HCl:ethopabate:sulfaquinoxaline-Na, respectively, for the proposed methods (Supplementary material S5 and S6). The obtained mean recoveries of added standards ± SD were 100.48 ± 1.93 for amprolium HCl, 101.51 ± 0.14 for ethopabate, and 98.80 ± 0.84 for sulfaquinoxaline-Na for UHPLC. The TLC–densitometric method showed mean recoveries of the added standard to be 101.05 ± 0.94, 99.46 ± 0.62, and 99.52 ± 0.79, respectively Table 4.
These results were statistically compared with those obtained from the reported method [8]. As shown in Table 4, calculated t values were 1.75, 0.58, 1.81, 1.62, 0.85, and 0.63 for UHPLC and TLC–densitometric methods for amprolium HCL, ethopabate, and sulfaquinoxaline-Na, respectively. While F-values were 1.04, 0.95, 3.31, 3.15, 0.32 and 0.95 for UHPLC and TLC–densitometric methods for amprolium HCL, ethopabate, and sulfaquinoxaline-Na, respectively. Hence, such results were less than the theoretical ones (2.31) for t test and (6.39) for F test, indicating that there was no significant difference between the proposed and reported methods. While, the proposed methods had the advantage of being successfully applied for simultaneous determination of the three drugs.
The applicability of the established TLC densitometric methods was extended to the analysis of the three drugs in chicken liver samples after homogenization with methanol, sonication, centrifugation, and filtration. Well-resolved symmetrical peaks with linear correlation were obtained between the average peak areas and the drug concentration over the range 1.0–30.0 μg/band, 1.0–25.0 μg/band, and 0.5–20.0 μg/band for amprolium HCL, ethopabate, and sulfaquinoxaline-Na, respectively Table 5.
Satisfactory recoveries of the three cited drugs from biological samples were obtained, and the results were 99.71–100.45, 98.08–99.6, and 99.73–101.43%, respectively Table 6.
Assessment of Greenness of the Proposed Methods
According to analytical Eco-scale, Penalty points PPs for each reagent are calculated by multiplying number of GSH (globally harmonized system of classification and labeling of chemicals) hazard pictograms by degree of hazard (‘warning’ multiplication by 1 and ‘danger’ multiplication by 2). [30] Because the GSH hazard pictograms are placed on the reagent containers, the hazard related to utilization of chemicals is easy to calculate (supplementary material, table S2). [31] PPs obtained for the proposed methods were calculated. UHPLC and densitometric methods showed a score of 81 and 74, respectively, confirming that both methods are excellent, acceptable green methods respectively Table 7.
The proposed methods were applied for in-line sample collection; the samples did not require preservation, transport, or storage in addition no sample preparation was required, and hence, the method was considered as a direct analytical technique. In addition, both methods can be used for the simultaneous qualitative and quantitative analysis of the tertiary mixture. Section 9 was colored yellow, since the volume of the solvents used along the analytical methods was 10–100 mL. Section 10 and 11 were colored yellow as health hazard rating and the flammability score of the used solvents for both proposed methods were 2 or 3. Sections 14 and 15 were colored red as the waste volume in both methods was ˃ 10 mL and no waste treatment was applied. The results of the GAPI assessment were shown in Fig. 4 and Table 8. GABI pictograms suggest that both proposed methods are of green practice.
Conclusion
Two sensitive, selective, and rapid chromatographic methods were developed for the separation and determination of amprolium HCl, ethopabate, and sulfaquinoxaline-Na. Both UHPLC and TLC–densitometric methods can assess the purity of the three drugs. Both methods also proved to be green according to analytical Eco-scale and GABI assessment tools. They were used for the first time to determine the three drugs in their tertiary mixture formulation.
References
Girardi C, Odore R (2008) Pharmacological treatments and risks for the food chain. Vet Res Commun 32(Suppl 1):S11–S18. https://doi.org/10.1007/s11259-008-9083-5
Capleton AC, Courage C, Rumsby P et al (2006) Prioritising veterinary medicines according to their potential indirect human exposure and toxicity profile. Toxicol Lett 163:213–223. https://doi.org/10.1016/j.toxlet.2005.10.023
Gao F, Yin C-X, Huo F-J, Yang P (2005) Ethopabate. Acta Crystallogr E Struct Rep Online 61:o3870–o3871. https://doi.org/10.1107/S1600536805034082
Clarke L, Fodey TL, Crooks SRH et al (2014) A review of coccidiostats and the analysis of their residues in meat and other food. Meat Sci 97:358–374. https://doi.org/10.1016/j.meatsci.2014.01.004
Bedaso K (2016) Comparative study on the efficacy of amprolium and sulfadimidine in coccidia infected chickens in Debre-Zeit agricultural research center poultry farm, Bishoftu, Ethiopia. SOJVS 2:1–5. https://doi.org/10.15226/2381-2907/2/2/00121
Fatoba AJ, Adeleke MA (2018) Diagnosis and control of chicken coccidiosis: a recent update. J Parasit Dis 42:483–493. https://doi.org/10.1007/s12639-018-1048-1
Hussein LA, Magdy N, Abbas MM (2015) Five different spectrophotometric methods for determination of Amprolium hydrochloride and ethopabate binary mixture. Spectrochim Acta A Mol Biomol Spectrosc 138:395–405. https://doi.org/10.1016/j.saa.2014.11.073
Abdelaziz S, Abdel Razeq S, Ahmed N (2021) Smart UV-Spectrophotometric methods for the simultaneous determination of amprolium-HCl, ethopabate and sulfaquinoxaline-Na in combined dosage forms. Azhar Int J Pharmaceutical Med Sci. https://doi.org/10.21608/aijpms.2021.62019.1046
Fink DW, deFontenay G, Bonnefille P et al (2004) Further studies on the spectrophotometric determination of amprolium. J AOAC Int 87:677–680
Catelani TA, Tóth IV, Lima JLFC et al (2014) A simple and rapid screening method for sulfonamides in honey using a flow injection system coupled to a liquid waveguide capillary cell. Talanta 121:281–287. https://doi.org/10.1016/j.talanta.2013.12.034
Alattar A (2018) Native spectrofluorimetric determination of amprolium in bulk and pharmaceutical formulation. Innoriginal: Int J Sci 5(3):42–45
Nasr JJ, Shalan S (2014) Spectrofluorimetric analysis of ethopabate in veterinary formulations with application to residue determination in chicken muscles and liver. Luminescence 29:1188–1193. https://doi.org/10.1002/bio.2683
El-Kosasy AM, Hussein LA, Magdy N, Abbas MM (2015) Sensitive spectrofluorimetric methods for determination of ethopabate and amprolium hydrochloride in chicken plasma and their residues in food samples. Spectrochim Acta A Mol Biomol Spectrosc 150:430–439. https://doi.org/10.1016/j.saa.2015.05.082
Natalia Z, Zholt K, Iryna A et al. (2019) Potentiometric sensor for determination of amprolium in pharmaceutical formulation
Basha MA, Abd El-Rahman MK, Bebawy LI, Salem MY (2017) Novel potentiometric application for the determination of amprolium HCl in its single and combined dosage form and in chicken liver. Chin Chem Lett 28:612–618. https://doi.org/10.1016/j.cclet.2016.11.012
Basha MA, Abd El-Rahman MK, Bebawy LI, Moustafa AA (2019) Validated TLC stability-indicating methods for the quantitative determination of some veterinary drugs. Microchem J 146:157–163. https://doi.org/10.1016/j.microc.2018.12.057
El-Kosasy AM, Lobna AH, Magdy N, Mahmoud MA (2016) Validated TLC–densitometric method for determination of amprolium hydrochloride and ethopabate in veterinary preparation. Anal Chem: Indian J 16(13):1–11
Thomas MH, Soroka KE, Thomas SH (1983) Quantitative thin layer chromatographic multi-sulfonamide screening procedure. J Assoc Off Anal Chem 66:881–883
Goessens T, Baere SD, Troyer ND et al (2020) Highly sensitive multi-residue analysis of veterinary drugs including coccidiostats and anthelmintics in pond water using UHPLC-MS/MS: application to freshwater ponds in Flanders, Belgium. Env Sci Process Impacts 22:2117–2131. https://doi.org/10.1039/d0em00215a
Baker MM, El-Kafrawy DS, Abdel-Khalek MM, Belal TS (2019) Comprehensive stability-indicating high-performance liquid chromatography coupled with diode array detection method for simultaneous determination of amprolium hydrochloride and ethopabate in powder dosage form for veterinary use. J Sep Sci 42:3340–3351. https://doi.org/10.1002/jssc.201900440
Ali MM, Ahmed MAA, Shinger MI (2017) Development and validation of RP-HPLC method for simultaneous determination of amprolium hcl and ethopabate in their combination drug. Chem Biomol Eng 2(1):51–56
Mantri AP, Rubeena MS, Nischal K, Shiva K (2015) Simultaneous estimation of sulfaquinoxaline sodium and amproliumhydrochloride by RP- HPLC. IJPSR 6:1097–1100
Ghanem M, Abu-Lafi S (2013) Validation of a stability-indicating assay of amprolium hydrochloride in water soluble powder formulation using hydrophilic interaction liquid chromatography. J Appl Pharm Sci 3(10):51–58. https://doi.org/10.7324/JAPS.2013.31009
Martínez-Villalba A, Núñez O, Moyano E, Galceran MT (2013) Field amplified sample injection-capillary zone electrophoresis for the analysis of amprolium in eggs. Electrophoresis 34:870–876. https://doi.org/10.1002/elps.201200579
Su HX, Tan HR, Shen HQ, Tian CQ (2013) Simultaneous separation and determination of four anticoccidial drugs in soil by high performance capillary electrophoresis. J Instrum Anal 2:5–10
Van Aken K, Strekowski L, Patiny L (2006) EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J Org Chem 2:3. https://doi.org/10.1186/1860-5397-2-3
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical eco-scale for assessing the greenness of analytical procedures. TrAC, Trends Anal Chem 37:61–72. https://doi.org/10.1016/j.trac.2012.03.013
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181:204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Gaber Y, Törnvall U, Kumar MA et al (2011) HPLC-EAT (environmental assessment tool): a tool for profiling safety, health and environmental impacts of liquid chromatography methods. Green Chem 13:2021. https://doi.org/10.1039/c0gc00667j
Mercer SM, Andraos J, Jessop PG (2012) Choosing the greenest synthesis: a multivariate metric green chemistry exercise. J Chem Educ 89:215–220. https://doi.org/10.1021/ed200249v
Tobiszewski M, Marć M, Gałuszka A, Namieśnik J (2015) Green chemistry metrics with special reference to green analytical chemistry. Molecules 20:10928–10946. https://doi.org/10.3390/molecules200610928
Funding
The authors declared no funding was provided for the work published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that for this manuscript, no funds, grants, or other support was received. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Razeq, S.A.A., Aziz, S.E.A. & Ahmed, N.S. TLC–Densitometry and UHPLC Methods for Simultaneous Determination of Amprolium HCl, Ethopabate, and Sulfaquinoxaline-Na in Their New Combined Dosage Form. Chromatographia 85, 563–574 (2022). https://doi.org/10.1007/s10337-022-04163-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04163-x