In this study, two cephalosporin groups of antibiotics (cefepime and cefixime) and their combinations with clavulanic acid, beta lactamase inhibitor, were simultaneously analyzed by HPLC method. The optimum separation was carried out on MZ C18 column (250 × 4.6 mm; i.d. 5 μm) with mobile phase consisting of an acetonitrile – 0.01% phosphoric acid (pH 2.5) (15 : 85, v/v) binary mixture. Detection was performed using a diode array detector at 228 nm. Under these experimental conditions, the analysis was accomplished in about 10 min. The developed method is specific, linear, sensitive, accurate, precise, and robust. The method was completely validated showing satisfactory data for all the parameters tested. Finally, the proposed method was successfully applied to two combined pharmaceutical dosage forms containing cefepime with clavulanic acid and cefixime with clavulanic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. INTRODUCTION
Antibiotics are medicines of great clinical significance that are used in the treatment and prophylaxis of infectious diseases caused by microorganisms. Bacteria are living things that can adapt quickly to changes that occur in the environment. Antibiotic resistance is an example of this adaptation. Resistance to a particular antibiotic means that this antibiotic cannot kill the drug-resistant bacteria in a treatment dose. Almost simultaneously with the discovery of antibiotics, they are predicted to lose their effectiveness in the treatment of infectious diseases if microorganisms can gain resistance to these drugs and the necessary precautions are not taken. Antibiotic resistance is a very important health problem that concerns the whole world and not just this day but the future as well. It is possible to reduce resistance rates by avoiding the use of unnecessary antibiotics and, if necessary, by producing rational policies. Different drug combinations can be used to reduce drug resistance by reducing antibiotic resistance.
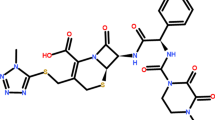
Cefixime (CFX) (Fig. 1a ) is commonly used to treat bacterial infections of the ear, urinary tract, and upper respiratory tract. Cefdinir (CDN) (Fig. 1b ) is highly effective against many Gram positive and Gram negative bacteria, and it is used to treat otitis media, soft tissue infections, and respiratory tract infections, including sinusitis, community-acquired pneumonia, and acute exacerbations of bronchitis. CFX and CDN are generally classified as third generation cephalosporin antibacterial drugs. Clavulanic acid (CLAV) (Fig. 1c ) is a major β-lactam antibiotic. CLAV enhances the activity of penicillin and cephalosporin antibacterials against many resistant strains of bacteria. Increasing resistance to cephalosporins, in recent years the combination of cephalosporins with beta-lactamase inhibitor such as CLAV becomes more common to enhance the antibacterial activity of cephalosporins [1, 2].
In the literature, several methods were reported for determining CFX [3, 4] and CDN [5,6,7] individually in tablets. Also there are CFX, CDN, and CLAV with other combinations in tablets (CFX [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22], CDN [23, 24], CLAV [25,26,27,28,29,30,31,32,33,34,35]). There are four methods reported for simultaneous analysis of CFX and CLAV in pharmaceutical dosage forms using HPLC (CFX-CLAV [36,37,38,39]). Basu, et al.[36] used 0.0075 M tetrabutyl ammonium hydroxide (pH 6.8) and methanol mixture (80 : 20 v/v) as mobile phase within 15 min while Joshi, et al. [37] analyzed the drugs within 20 min using 25 mM NaH2PO4 (pH 6.5) and methanol mixture (80 : 20 v/v). Khan, et al. [38] also developed an HPLC method for the simultaneous analysis of CFX and CLAV with shorter analysis time 8 min, but the method was not ap plied to any real samples. The study was only performed on synthetic mixture. Zade, et al. [39] simultaneously analyzed the two drugs using 30 mM NaH2PO4 (pH 3.0) and methanol mixture (70 : 30 v/v), but the method had lower sensitivity than the other methods. Now there are no methods in the literature for the simultaneous analysis of CDN and CLAV, although there are pharmaceutical dosage forms containing CDN in combination with CLAV. Thus, a fast, sensitive and fully validated method is needed for the analysis of CFX, CDN and CLAV in tablets. In the present work, a reversed phase HPLC method has been developed for simultaneous analysis of CFX, CDN and CLAV in pharmaceutical dosage forms.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
CFX, CDN and CLAV workingCFX, CDN and CLAV working standards were supplied from Sigma Aldrich. The tested pharmaceutical formulations (Cefbir® Plus and Molcef® Plus tablets) were procured from local markets. Acetonitrile (ACN) and phosphoric acid of analytical grade were purchased from Merck.
2.2. Chromatographic System and Conditions
HPLC analyses were performed on an Agilent 1200 HPLC system. Separations were carried on a MZ C18 column (250 × 4.6 mm; i.d., 5 μm). The flow rate was 1 mL min-1 in isocratic elution mode with a mobile phase consisted of acetonitrile – phosphoric acid (0.01%, pH 2.5) mixture in a ratio of 15 : 85 (v/v). The injection volume was 20 μL and the UV detection was performed at 228 nm. Peak identity was confirmed by retention time comparison.
Ten Molcef® Plus (400 mg CFX /125 mg CLAV) tablets were weighed to get the average weight and ground. The amount of powder equivalent to average tablet weight was transferred to a 250 mL volumetric flask, and 150 mL of 0.1 KH2PO4:MeOH (1:1, v/v) was added and sonicated for 30 min. The volume was made up with solvent to obtain a solution containing 1600 μg mL-1 CFX and 500 μg mL-1 CLAV. An aliquot was taken and centrifuged at 5000 rpm for 10 min. The solution was filtered using 0.45 μm membrane filter paper, diluted with mobile phase, and injected into the HPLC system.
Preparation of placebo samples. Placebo solutions were prepared by mixing excipients (crospovidone, lactose monohydrate, magnesium stearate, povidone, starch, talc and titanium dioxide). Then an amount of the powder equivalent to average tablet weight was transferred 250 mL volumetric flask and then dissolved with mobile phase. The placebo solution also was centrifuged and filtered same as sample solution preparation.
3. RESULTS AND DISCUSSION
3.1. HPLC System Suitability
The chromatographic parameters, pH and organic phase ratio in mobile phase were optimized. For HPLC analysis, various mobile phases were tried in attempts to obtain the best separation and resolution between CFX, CDN and CLAV. Firstly, acetonitrile and water (20:80, v/v) mixture was used for mobile phase optimization but it was seen that the peaks were not separated from each other. For this reason, 25 mM KH2PO4 buffer solutions at various pH (7.4, 4.7, and 3.0) and phosphoric acid (0.01%, pH 2.5) were tested as aqueous phases in order to provide separation.
The optimum conditions for simultaneously analysis of CFX, CDN and CLAV were found with a mobile phase consisting of acetonitrile and phosphoric acid (0.01%, pH 2.5) in the ratio of 15:85 (v/v) at flow rate of 1 mL min-1. Results of the system suitability testing (capacity factor, asymmetry, column theoretical plate number, and resolution) showed that the proposed method met all requirement of the ICH guidelines [40] (Table 1).
3.2. Method Validation
The developed method has been validated according to ICH guidelines. The evaluated parameters included specificity, linearity, sensitivity, accuracy, precision, robustness and ruggedness.
Specificity. Specificity of the method was evaluated by preparing the analytical placebo sample, standard solution, and samples of commercial pharmaceutical formulations. A solution of analytical placebo was prepared as described in the sample preparation procedure and injected into the HPLC system. The chromatograms of placebo solution did not show any other peaks, which confirmed the specificity of the method. The typical chromatograms of placebo, standard, and tablet solutions are shown in Fig. 2.
Linearity. Calibration curves were constructed by plotting the peak areas of CFX, CDN and CLAV versus their concentrations. Seven different standard solutions within the linear range containing 1.0, 5.0, 10.0, 20.0, 25.0, 30.0 and 40.0 μg mL-1 of CFX, CDN and CLAV were prepared and injected into the HPLC system. The graph proved that the method was linear up to 40 μg mL-1. Characteristics of the calibration curves determined using the standard solutions for quantitative analysis are presented in Table 2 (n = 6).
Sensitivity. The limit of detection (LOD) and limit of quantification (LOQ) of the proposed method are estimated for the signal to noise ratio 3:1 and 10:1, respectively. The results are shown in Table 2.
Accuracy. Accuracy of the developed method was investigated by intra-day and inter-day analysis. Three different concentrations of the standard CFX, CDN and CLAV (5.0, 20.0 and 30 μg mL-1) solutions in the linear range were analyzed during six consecutive days (inter-day accuracy) and six times in the same day (intra-day accuracy). Bias values within the acceptable values for intra- and inter-day studies signify that the developed method is accurate. The results are summarized in Table 3.
The accuracy of the method was also evaluated through recovery studies. For the recovery studies, synthetic tablet solutions (20 μg/mL-1) were prepared as explained before. The recovery of CFX, CDN and CLAV was calculated as 99.00%, 98.20% and 95.10%, respectively. The closeness of bias values to zero and high recovery shows that the method is accurate.
Precision. Precision of the developed method was investigated by intra-day and inter-day analysis. Three different concentrations of the standard CFX, CDN and CLAV (5.0, 20.0 and 30.0 μg mL-1) solutions on the linear range were analyzed six consecutive days (inter-day precision) and six times in the same day (intra-day precision). The relative standard deviation (RSD) values within the acceptable values for intra- and inter-day studies signify that the developed methodis precise. The results are summarized in Table 3. Intermediate precision of the developed method was also evaluated using results of two analysts. There is no statistically significant difference between the data according to Wilcoxon paired two sample test (p > 0.05) (Table 4).
Robustness. The robustness of determining CFX, CDN and CLAV using HPLC was studied by introducing small changes in mobile phase acetonitrile amount (%14 – %16), mobile phase pH (2.3 – 2.4) and flow rate (0.9 – 1.1 mL min-1). The changes in results were statistically comparable with the results obtained under optimum conditions, which indicated that the method was robust (Tt = 2.776 > Tc, p > 0.05; Tc = Calculated Tt = Table t-Test).
3.3. Application of Developed Method
Pharmaceutical preparations containing CDN with CLAV [Cefbir® Plus tablet (300 mg CDN /125 mg CLAV)] and CFX with CLAV [Molcef® Plus tablet (400 mg CFX/125 mg CLAV)] were analyzed by the developed and validated HPLC method (Fig. 2). The results of tablet analysis are presented in Table 5.
Thus, according to results of the validation studies, the developed HPLC method is specific, linear, accurate, precise, sensitive, and robust. Therefore, this method can be applied to determination of CFX, CDN and CLAV in pharmaceutical preparations.
References
H. S. Sader,M. R. Jacobs, and T. R. Fritsche, Diagn. Microbiol. Infect. Dis., 57(3), 5 – 12 (2007).
P. S. Saudagar, S. A. Survase, and R. S. Singhal, Biotechnol. Adv., 26(4), 335 – 351 (2008).
P. B. Shah and K. Pundarikakshudu, J. AOAC Int., 89(4), 987 – 994 (2006).
Z. Talebpour, H. Pourabdollahi, H. Rafati, et. al., Sci. Pharm., 81(2), 493 – 504 (2013).
H. Hashem, A. A. Gouda, and W. Hassan, J. Liq. Chromatogr. Relat. Technol., 35(12), 1638 – 1648 (2012).
M. Kumudhavalli, R. M. Chandira, B. Jayakar, et. al., J. Pharm. Res., 2(6) (2009).
W. Xing-lin, Chin. New Drugs J., 2, 010 (2003).
S. D. Bhinge and S. M. Malipatil, J. Taibah Univ. Sci., 10(5), 734 – 744 (2016).
P. Deekonda and M. S. Reddy, Der Pharm. Chem., 6(2), 31 – 37 (2014).
G. Devika, M. Sudhakar, and J. V. Rao, Orient J. Chem, 28(4), 1743 – 1750 (2012).
M. V. Dhoka, S. J. Sandage, and S. C. Dumbre, J. AOAC Int., 93(2), 531 – 535 (2010).
G. Rathinavel, J. Valarmathy, L. Samueljoshua, et. al., E-J. Chem., 5(3), 648 – 651 (2008).
S. Kathiravan and S. Mathankumar, Asian J. Chem, 3(4), 865 – 868 (2010).
G. J. Kher, V. R. Ram, G. G. Pandiya, et. al., Int. J. Chemtech. Res., 4(3), 1124 – 1136 (2012).
M. M. Mahesh, V. S. Kasture, and S. A. Gosavi, Eurasian J. Anal. Chem., 5(3), 227 – 238 (2010).
S. Natesan, Int. J. Pharm. Sci. Rev. Res, 2(2), 219 – 224 (2016).
N. Nyola and S. Jeyabalan, Indo Am. J. Pharm. Res., 2(12), 1472 – 1481 (2013).
N. S. Patel, F. B. Tandel, Y. D. Patel, et. al., Indian J. Pharm. Sci., 76(6), 535 (2014).
S. A. Patel and J. V. Patel, Int. J. Pharm., Chem. Biol. Sci., 3(2), 372 – 379 (2013).
S. A. Patel and J. V. Patel, Int. J. Pharm. Tech. Res., 3(4), 1958 – 1962 (2011).
K. J. Trivedi, P. V. Chokshi, and N. S. Patel, Int. J. Chem. Tech. Res., 4(4), 1628 – 1632 (2012).
A. R. Wankhede, Int. J. Pharm. Bio. Arch., 1(3) (2010).
S. R. Narala and K. Saraswathi, J. Pharm. Sci. Res., 3(1), 1002 – 1004 (2011).
Z. G. K. X. R. Ping and G. S. C. Hui, China Pharm., 9, 012 (2008).
M. A. Abounassif, E. M. Abdel-Moety,M. E. Mohamed, et. al., J. Pharm. Biomed. Anal., 9(9), 731 – 735 (1991).
A. Aghazadeh and G. Kazemifard, J. Sci. I. R. Iran, 12(2), 127 – 131 (2001).
A. Aghazadeh and G. Kazemifard, J. Pharm Bio. Anal., 25(2), 325 – 329 (2001).
S. B. Dighe, Int. J. Pharm. Sci. Res., 5(4), 1566 (2014).
F. Hasanpour, A. A. Ensafi, and T. Khayamian, Anal. Chim. Acta, 670(1 – 2), 44 – 50 (2010).
P. L. Ingale, S. D. Dalvi, D. D. Jadav, et. al., J. Pharm. Pharm. Sci., 5(4), 179 – 181, (2013).
S. Jadhav, V. Salunkhe, and S. Bhinge, Curr. Pharm. Res., 3(4), 994 (2013).
S. Malathi, R. Dubey, and R. Venkatnarayanan, Indian J. Pharm. Sci., 71(1), 102 (2009).
M. R. Sengar, S. V. Gandhi, U. P. Patil, et. al., Int. J. Chem. Tech. Res, 1(4), 1105 – 1108 (2009).
G. Yu-long, Hebei Chem. Eng. Ind., 5, 034 (2008).
J. Zhou, T. Shen, and X. Lu. Chinese Pharm. J., 39(7), 538 – 539 (2004).
A. Basu, K. Basak, M. Chakraborty, et. al., J. Pharm Res., 4(5), 1319 – 1321 (2011).
M. P. Joshi, K. G. Gamit, V. K. Parmar, et. al., World J. Pharm. Sci., 3(9), 1259 – 1271 (2014).
I. U. Khan, S. Sharif, M. Ashfaq, et. al., J. AOAC Int., 91(4), 744 – 749 (2008).
S. Zade, V. Bhatpalliwar, N. Mendhule, et. al., Int. J. Pharm. Sci. Nanotech., 6(2), 2033 – 2039 (2013).
ICH Guidelines. Validation of Analytical Procedures: Text and Methodology Q2 (R1), International Conference on Harmonization, Geneva (2005), pp. 11 – 12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reçber, T., Özkan, E., Nemutlu, E. et al. Simultaneous Determination of Cefixime, Cefdinir and Clavulanic Acid by High Performance Liquid Chromatography. Pharm Chem J 54, 1186–1191 (2021). https://doi.org/10.1007/s11094-021-02341-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02341-z