Abstract
Background
Addition of cytarabine to high-dose methotrexate (HD-MTX) chemotherapy improves outcome of primary CNS lymphoma (PCNSL); however, the combination therapy increases toxicity. Sequential chemotherapy and cranial radiation may decrease toxicity without altering efficacy.
Methods
This was a single-center, retrospective cohort study of consecutive newly diagnosed immunocompetent PCNSL patients treated with HD-MTX (5 cycles of 3 g/m2 every 2 weeks) followed by consolidation whole-brain radiotherapy (WBRT) and cytarabine (2 cycles of 3 g/m2/d for 2 days every 3 weeks) from January 2013 to December 2020. Initial WBRT before HD-MTX was allowed in patients with significant disability or brain edema at presentation. Primary outcome was progression-free survival (PFS). Key secondary outcomes were response rate, treatment-related toxicity, and overall survival (OS).
Results
Of 41 patients, 25 patients had a complete response (CR) and ten patients had a partial response, inferring an overall response rate (ORR) of 85.4% and a CR rate of 60.9%. More than 90% of patients were able to tolerate and complete the HD-MTX. The incidence of ≥ grade 3 hematologic and non-hematologic toxicities were 4.8% and 17.1%, respectively. Treatment-related mortality rate was 2.4%. There was no difference in toxicity between patients with age < 60 and ≥ 60 years. At the median follow-up duration of 39.8 months, the median PFS was 35.2 months (95% CI 12.4–69.3) and median OS was 46.5 months (95% CI 21.8–NR).
Conclusion
High-dose methotrexate followed by consolidation whole-brain radiotherapy and cytarabine has acceptable efficacy, great tolerability, and low toxicity in newly diagnosed PCNSL patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, the standard induction of primary central nervous system lymphoma (PCNSL) is high-dose methotrexate (HD-MTX)-based chemotherapy [1, 2]. Addition of high-dose cytarabine (HD-Ara-C) to HD-MTX 3.5 g/m2 followed by whole-brain radiotherapy (WBRT) led to a significantly higher complete response rate (46% vs 18%, p = 0.006) and became a new standard of treatment since 2009 [3]. However, the addition of cytarabine also increased grade 3–4 hematological toxicity and treatment-related mortality.
In an RTOG 93–10 study [4], DeAngelis and her colleagues used WBRT and HD-Ara-C as a consolidation after HD-MTX 2.5 g/m2 in combination with vincristine and procarbazine. The separation of ‘combination chemotherapy’ (HD-MTX + HD-Ara-C → WBRT) to ‘sequential chemotherapy’ (HD-MTX → WBRT → HD-Ara-C) was rather effective with a reduced incidence of grade 3–4 hematologic toxicity.
The current most effective induction for PCNSL is the MATRix regimen (Methotrexate, Ara-C, Thiotepa, Rituximab), thiotepa is, however, very costly and non-reimbursable in Thailand. Besides, the addition of rituximab to HD-MTX and HD-Ara-C without thiotepa did not improve survival outcomes in a phase 3 study IELSG-32 [5]. Thus, most newly diagnosed PCNSL patients in Thailand are still treated with only HD-MTX in combination with HD-Ara-C and cranial irradiation.
Since 2013, our center has started using the sequential chemoradiotherapy for all patients with newly diagnosed PCNSL. However, the efficacy and toxicity of this regimen are still not known. Thus, this analysis aims to report disease response, survival outcomes, and treatment-related adverse events in patients with newly diagnosed primary CNS lymphoma after high-dose methotrexate followed by consolidation whole-brain radiotherapy and cytarabine.
Methods
Study design and participants
This was a retrospective cohort study from January 2013 to December 2020 at the Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University. All consecutive newly diagnosed PCNSL patients who were eligible for chemotherapy and received at least one cycle of chemotherapy were enrolled. Only patients treated with sequential chemoradiotherapy protocol were included. Patients with HIV seropositivity were excluded. Collected demographic data were age at diagnosis, sex, creatinine clearance (CrCl), Karnofsky performance status score (KPS), and MSKCC prognostic score [6]. Interesting disease characteristics were diameter of the largest lesion, single or multiple lesions, deep region involvement (defined as periventricular, basal ganglia, brainstem, or cerebellum involvement [7]), ocular involvement, serum LDH level, and tumor pathology. Treatment sequence, total WBRT dose, toxicity during treatment, and response after treatment were reviewed from electronic medical records, radiographic results, and laboratory results.
Treatment protocol
We have used our PCNSL treatment protocol since January 2013. In brief, after patients were diagnosed with PCNSL by pathology, phase I which consisted of methotrexate (MTX) 3 g/m2 infusion intravenously in 2 h every 2 weeks for 5 cycles was given. Vigorous hydration and urine alkalinization using 7.5% sodium bicarbonate 100 ml in 5% dextrose in water 1000 ml intravenously at a rate of 80 ml/m2/h were given 24 h before MTX infusion until completion of MTX for 48 h. Leucovorin rescue was initiated 12 h after methotrexate administration at a dose of 100 mg/m2 intravenously then 15 mg/m2 orally every 6 h for 12 doses or until serum MTX level was 0.1 µmol/L. Serum MTX level was monitored after methotrexate administration 24 h and every morning until less than 0.1 µmol/L.
Consolidation WBRT (photons of 6–10 MeV; five fractions per week; fraction size 200 cGy) was initiated within 4 weeks after completion of phase I. Whole-brain was irradiated by two opposite lateral fields including the first two cervical vertebrae and the posterior two-thirds of the orbits with 30–36 Gy. The additional 10 Gy boost at tumour-bed with 2 cm of margin around the lesion was performed in patients with residual disease. Orbits were shielded after 30 Gy (or 36 Gy in the case of intraocular disease).
Initial WBRT (either partial or full dose) before phase I was allowed in patients with significant disability (e.g. bedridden in more than 50% of the time) or significant brain edema at presentation (e.g. symptomatic increased intracranial pressure, presence of brain herniation from brain imaging). In patients who developed progression of disease during phase I, rescue WBRT (either partial or full dose) was allowed and the protocol was restarted only in patients with the radiosensitive disease. In patients who received partial WBRT at the beginning or during phase I, the residual dose of cranial irradiation was performed after the completion of phase I treatment.
After completion of HD-MTX and WBRT, patients received phase II treatment which consisted of two cycles of cytarabine 3 g/m2/d infusion intravenously in 3 h for 2 consecutive days every 3 weeks. The summarized treatment protocol is shown in Table 1.
Outcomes
The primary outcome was progression-free survival (PFS). Secondary outcomes were response after completion of treatment, incidence and severity of treatment-related toxicity according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) v4.0, treatment-related mortality (TRM), the cumulative incidence of relapse, and overall survival (OS). Treatment response was classified according to International Primary CNS Lymphoma Collaborative Group response criteria [8]. Complete response (CR) was defined as complete disappearance of all enhancing abnormalities on contrast-enhanced CT/MRI. Partial response (PR) was defined as ≥ 50% decrease in the contrast-enhancing lesion seen on CT/MRI as compared with baseline imaging and no new sites of disease. Progressive disease (PD) was defined as more than 25% increase in the contrast-enhancing lesion seen on CT/MRI as compared with baseline (or best response) or appearance of any new lesion or site of disease. Unconfirmed complete response (CRu), which included patients fulfilled the criteria for CR but had minimal persistent abnormality on brain imaging, was classified into CR group. PFS was defined as the time from the date of the beginning of treatment until progression after phase I treatment, relapse, or death from any cause. OS was defined as the time from the date of the beginning of treatment until death from any cause.
Statistical analysis
Categorical data were reported as count and percentage. Continuous data were reported as mean ± standard deviation (SD) or median with interquartile range (IQR) depending on the distribution of the data. Comparison of data was performed using Chi-square or Fisher’s exact test for categorical variables and independent t test for continuous variables. Kaplan–Meier method was used to estimate survival probabilities, with exact 95% CIs. Log-rank test was used to compare survival curves between groups. Cox proportional hazards model was used to explore clinical factors that possibly influence survival outcomes. All tests were two-sided and P values less than 0.05 were considered significant. Statistical analysis was performed using Stata 16.1 (StataCorp LLC, USA).
The study was conducted with approval from the Institutional Research Ethics Committee at the Faculty of Medicine, Chiang Mai University (Reference No. 019/2021).
Results
Patient characteristics and treatment
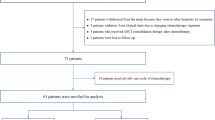
Forty-one immunocompetent newly diagnosed PCNSL patients were included. Twenty-two (56%) patients were female, and the mean age was 60 years (SD 10.5 years). All cases were diagnosed with diffuse large B-cell lymphoma by pathology. Pretreatment characteristics and details of treatment are shown in Table 2.
Disease response, relapse, and survival outcomes
Of 41 patients, 25 patients had a complete response and ten patients had a partial response, which infers an overall response rate (ORR) of 85.4%. Nine patients (21.9%) developed progression of disease during phase I. Of 8 patients who received rescue WBRT, 5 patients had a radiosensitive disease and resumed the treatment protocol. Disease response at the end or cessation of treatment is shown in Table 3. The cumulative incidence of relapse at one year and two years were 18.5% and 33.2%, respectively. At the median follow-up duration of 39.8 months, median PFS was 35.2 months (95% CI 12.4–69.3) and median OS was 46.5 months (95% CI 21.8–NR) (Fig. 1). The 2-year PFS and OS were 55.3% and 62.6%, respectively.
Median PFS in patients with CR, PR, and PD were 42.6, 13.0, and 9.7 months, respectively (log-rank test, p = 0.008). Median OS in patients with CR, PR, and PD were 69.9, 29.4, and 10.6 months, respectively (log-rank test, p = 0.006) (Fig. 2). Univariable Cox proportional hazards analyses also revealed that depth of response affected survival outcomes (Table 3). However, age, sex, KPS, MSKCC prognostic score, the diameter of the largest mass, deep region involvement, ocular involvement, elevated serum LDH, or sequence of treatment did not significantly influence PFS or OS in this cohort (supplementary Table S1 and S2). In addition, disease progression during phase I was a risk of shorter PFS [hazard ratio (HR) 3.26, 95% CI 1.19–8.93] and a trend for shorter OS (HR 2.43, 95%CI 0.84–6.99).
Effects of treatment sequence
Twenty-one patients (51.2%) received initial WBRT before phase I chemotherapy. Between patients receiving initial WBRT and not receiving, there were differences in average MTX dose per cycle (2.33 ± 0.32 vs 2.61 ± 0.28 g/m2, p = 0.006) and proportion of patients with KPS less than 70% (100% vs 35%, p < 0.001). After adjusting for these factors, receiving of initial WBRT did not significantly affect both PFS (adjusted HR 3.57, 95% CI 0.45–28.24, p = 0.228) and OS (adjusted HR 1.93, 95% CI 0.41–9.20, p = 0.406).
Tolerability and safety profile
Thirty-seven patients (90.2%) were able to tolerate and complete HD-MTX at 3 g/m2 for 5 cycles. Only two patients developed serious hematologic adverse events. Seven patients (17%) developed a serious infection during treatment. Seven patients (17.1%) had to delay at least one treatment session due to toxicity. TRM rate was 2.4% (1 patient due to grade 5 infection). Treatment was stopped in a total of nine patients due to the progression of the disease (6 patients) and treatment-related toxicity (3 patients, 7.3%). Detailed treatment-related toxicity is presented in Table 4.
Between patients with age less than 60 years and 60 years or older, there was no difference in the incidence of any grade either hematologic (72.2% vs 86.9%, p = 0.237) or non-hematologic toxicity (66.7% vs 69.6%, p = 0.843). Also, incidences of grade 3–5 hematologic (5.6% vs 4.4%, p = 0.906) and non-hematologic toxicity (16.7% vs 17.4%, p = 0.951) were similar among two age groups.
Discussion
Relapsed and refractory PCNSL patients are associated with a dismal prognosis. Only a few months of survival after salvage treatment were observed in those with refractory diseases [9]. Thus, the quality of response, particularly CR, from the first-line treatment determines the survival outcomes, especially in areas with limited treatment options for relapsed/refractory PCNSL. Previous studies reported an ORR of 69–94% after HD-MTX-based chemotherapy combination with WBRT in newly diagnosed PCNSL [3, 4, 10,11,12,13,14,15]. Pooled analysis of three studies using the MPV regimen, which consisted of HD-MTX 2.5–3.5 g/m2, procarbazine, vincristine followed by WBRT with/without HD-Ara-C consolidation, revealed a CR rate of 58% (95% CI 48–67%) [16]. Our regimen was different from the original MPV regimen [4, 10, 11] by the omission of vincristine, procarbazine, and intrathecal MTX. Interestingly, our regimen yielded an ORR of 85.4% with a comparable CR rate of 60.9%. This finding confirms that this treatment regimen seems to be effective in newly diagnosed PCNSL, even without procarbazine, vincristine, and intrathecal MTX. The possible explanation could be the modest effect of vincristine, as demonstrated by no additional benefit from studies using CHOP-like chemotherapy before or after radiotherapy in PCNSL [17, 18]. Also, no improvement of response rates or survival by adding intrathecal MTX has been demonstrated in PCNSL patients treated with systemic HD-MTX [19,20,21].
In patients with significant disability or brain edema at first presentation, initial WBRT rapidly improved patients’ physical condition and increased the proportion of patients which could receive HD-MTX-based chemotherapy. Although half of the patients in this cohort received initial WBRT, we found that the WBRT sequence did not affect survival outcomes even after adjustment for unbalanced risk factors. Also, this finding suggested that if the patients had improved performance status from initial WBRT, their survival outcomes would be equivalent to those with good performance status at first after treatment with this regimen. Thus, according to patients’ conditions, initial WBRT before HD-MTX could be a valuable alternative way to the standard therapeutic course (HD-MTX before WBRT).
Current major consolidation options for PCNSL response to HD-MTX-based chemotherapy are WBRT (with or without chemotherapy) and autologous stem cell transplantation (ASCT) with a thiotepa-based conditioning regimen [1, 2]. Both options appeared to have the same efficacy in two randomized clinical trials [22, 23]. As mentioned before, ASCT is limitedly performed in our country due to its cost and relatively old age of patients, while WBRT is widely used as a part of consolidation. A recent systematic review revealed the pooled 2-year PFS and OS in patients receiving WBRT together with chemotherapy consolidation were 56% and 72% [16]. Consistently, the 2-year PFS and OS in our study were 55.3% and 62.6%, respectively. This finding affirms that WBRT and HD-Ara-C is an effective consolidation approach.
Moreover, the HD-MTX-based polychemotherapy including MATRix regimen, which is considered a current standard of care for newly diagnosed PCNSL, provided a CR rate of 23–49% and ORR of 53–87% before consolidation in a randomized phase II study [5]. Our study was planned to assess the disease response after consolidation, so it was difficult to compare the efficacy in terms of response rate. Besides, only patients with the responsive disease proceeded to the second randomization with either WBRT or ASCT consolidation [22]. Thus, the survival outcomes from the first randomization should be more representative of the real efficacy. Surprisingly, our study showed comparable survival outcomes to the reported 2-year PFS and OS, which were 36–61% and 42–69%, respectively [5]. Of note, this should be carefully interpreted due to unbalanced baseline characteristics of populations.
Concurrent HD-MTX and HD-Ara-C for four courses followed by WBRT provided a CR rate of 64% in randomized phase II trial [3]. Even though our sequential chemoradiotherapy protocol offered a similar CR rate, there was a remarkable reduction in grade 3–4 hematologic toxicity (92% vs 4.8%) and TRM (8% vs 2.4%). However, our study reported a lower median average MTX dose (2.5 vs 3.5 g/m2), which might be compensated by a more frequent of HD-MTX infusion (every 14 days vs 21 days). In addition, the reported toxicities in the HD-MTX arm in the mentioned study were comparably low [3]. So, the treatment toxicity would be mainly reduced by sequential use of chemotherapy (HD-MTX → HD-Ara-C).
Since PCNSL is usually found in the older age groups, treatment-related toxicity should be carefully weighed to the efficacy. Most treatment-related toxicities in our cohort were grade 1–2 cytopenia and abnormal liver enzymes which were manageable and self-limited. Moreover, patients with age 60 or older had no increased incidence of treatment-related toxicity. Treatment was interrupted due to toxicity only in three patients (7.3%). These ensure that the sequential strategy has good tolerability, low toxicity profile, and can be used in PCNSL patients with older age.
The strength of our analysis was all patients received the same treatment. The data was homogenous with the long follow-up period. So, the results could reflect the real-world efficacy of this sequential treatment protocol. On the other hand, there are several limitations to our study. First, this was a retrospective study. However, there was little missing data. Also, even we enrolled every newly diagnosed PCNSL patient receiving at least one cycle of chemotherapy, a certain number of patients who had radioresistant after initial WBRT were not included in this cohort. Second, the relatively small sample size of the study may be underpowered to infer that the selected risk factors did not influence the survival outcomes. Lastly, we did not periodically perform the neurocognitive assessment in survived PCSNL patients, which could have the potential risk of long-term neurotoxicity from cranial irradiation. Further study with continual evaluation of cognitive function and quality of life after this treatment regimen is warranted.
Conclusion
High-dose methotrexate followed by consolidation whole-brain radiotherapy and cytarabine has acceptable efficacy, great tolerability, and low toxicity in newly diagnosed PCNSL patients.
Data availability
De-identified raw data available upon request.
Code availability
Not applicable.
References
Ferreri AJM (2017) Therapy of primary CNS lymphoma: role of intensity, radiation, and novel agents. Hematol Am Soc Hematol Educ Program 2017(1):565–577
Grommes C, DeAngelis LM (2017) Primary CNS lymphoma. J Clin Oncol 35(21):2410–2418
Ferreri AJ, Reni M, Foppoli M et al (2009) High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 374(9700):1512–1520
DeAngelis LM, Seiferheld W, Schold SC, et al. Radiation Therapy Oncology Group S (2002) Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: radiation therapy oncology group study 93–10. J Clin Oncol 20(24):4643–4648
Ferreri AJ, Cwynarski K, Pulczynski E et al (2016) Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the international extranodal lymphoma study group-32 (IELSG32) phase 2 trial. Lancet Haematol 3(5):e217–e227
Abrey LE, Ben-Porat L, Panageas KS et al (2006) Primary central nervous system lymphoma: the Memorial Sloan-Kettering cancer center prognostic model. J Clin Oncol 24(36):5711–5715
Ferreri AJ, Blay JY, Reni M et al (2003) Prognostic scoring system for primary CNS lymphomas: the international extranodal lymphoma study group experience. J Clin Oncol 21(2):266–272
Abrey LE, Batchelor TT, Ferreri AJ et al (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23(22):5034–5043
Langner-Lemercier S, Houillier C, Soussain C et al (2016) Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol 18(9):1297–1303
Abrey LE, Yahalom J, DeAngelis LM (2000) Treatment for primary CNS lymphoma: the next step. J Clin Oncol 18(17):3144–3150
Ferreri AJ, Reni M, Dell’Oro S et al (2001) Combined treatment with high-dose methotrexate, vincristine and procarbazine, without intrathecal chemotherapy, followed by consolidation radiotherapy for primary central nervous system lymphoma in immunocompetent patients. Oncology 60(2):134–140
Glass J, Gruber ML, Cher L (1994) Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg 81(2):188–195
Glass J, Won M, Schultz CJ et al (2016) Phase I and II study of induction chemotherapy with methotrexate, rituximab, and temozolomide, followed by whole-brain radiotherapy and postirradiation temozolomide for primary CNS lymphoma: NRG oncology RTOG 0227. J Clin Oncol 34(14):1620–1625
Morris PG, Correa DD, Yahalom J et al (2013) Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 31(31):3971–3979
Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H et al (2003) High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European organization for research and treatment of cancer lymphoma group phase II trial 20962. J Clin Oncol 21(24):4483–4488
Yu J, Du H, Ye X (2021) High-dose methotrexate-based regimens and post-remission consolidation for treatment of newly diagnosed primary CNS lymphoma: meta-analysis of clinical trials. Sci Rep 11(1):2125
Mead GM, Bleehen NM, Gregor A et al (2000) A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer 89(6):1359–1370
Schultz C, Scott C, Sherman W et al (1996) Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88–06. J Clin Oncol 14(2):556–564
Ferreri AJ, Reni M, Pasini F et al (2002) A multicenter study of treatment of primary CNS lymphoma. Neurology 58(10):1513–1520
Khan RB, Shi W, Thaler HT et al (2002) Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol 58(2):175–178
Sierra Del Rio M, Ricard D, Houillier C et al (2012) Prophylactic intrathecal chemotherapy in primary CNS lymphoma. J Neurooncol 106(1):143–146
Ferreri AJM, Cwynarski K, Pulczynski E et al (2017) Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the international extranodal lymphoma study group-32 phase 2 trial. Lancet Haematol 4(11):e510–e523
Houillier C, Taillandier L, Dureau S et al (2019) Radiotherapy or autologous stem-cell transplantation for primary cns lymphoma in patients 60 years of age and younger: results of the Intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol 37(10):823–833
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
PP designed the research, collected and analyzed the data, wrote and revised the manuscript. TR, SH, CC, ER, AT, and LN revised the manuscript and gave critical comments. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
Approval was obtained from the Institutional Research Ethics Committee at the Faculty of Medicine, Chiang Mai University (Reference No. 019/2021).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Piriyakhuntorn, P., Rattanathammethee, T., Hantrakool, S. et al. Outcome of patients with newly diagnosed primary CNS lymphoma after high-dose methotrexate followed by consolidation whole-brain radiotherapy and cytarabine: an 8-year cohort study. Int J Clin Oncol 26, 1805–1811 (2021). https://doi.org/10.1007/s10147-021-01982-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01982-0