Abstract
Background
The treatment of primary CNS lymphoma (PCNSL) comprises high dose methotrexate (HDMTX) based chemotherapy followed by whole brain radiotherapy (WBRT), the major drawback of which is long term neurotoxicity. We intended to assess the feasibility of response adapted WBRT in PCNSL in the Indian setting.
Methods
We screened 32 patients and enrolled 22 eligible patients with PCNSL from 2015 to 2017 in a prospective phase II trial. The patients underwent five 2-weekly cycles of induction chemotherapy with rituximab, methotrexate, vincristine, procarbazine. Patients with complete response(CR) to induction chemotherapy were given reduced dose WBRT 23.4 Gy/13 fractions/2.5 weeks while those with partial response (PR), stable or progressive disease (SD or PD) were given standard dose WBRT 45 Gy/25 fractions/5 weeks. Thereafter two cycles of consolidation chemotherapy with cytarabine were given. The primary endpoints of the study were assessment of response rate (RR) and progression free survival (PFS). The secondary endpoints of the study were assessment of overall survival (OS), toxicity profile of treatment and serial changes in quality of life and neuropsychological parameters.
Results
Out of 19 patients who completed HDMTX based chemotherapy, 10 (52.63%) patients achieved CR, 8 (42.11%) patients had PR and 1 patient had PD. After a median follow-up period of 11.25 months, the estimated median OS was 19 months. The actuarial rates of PFS and OS were respectively 94.1 and 68.2% at 1 year and 50.2 and 48.5% at 2 years. Three patients in reduced dose WBRT arm had recurrence and two of them died of progressive disease, whereas there was no recurrence or disease related death in standard dose WBRT arm. On univariate analysis of PFS, age ≤ 50 years and use of standard dose WBRT (45 Gy) led to significantly improved outcome (p value 0.03 and 0.02 respectively).
Conclusion
In patients with PCNSL, reduced dose WBRT after CR to HDMTX based chemotherapy may lead to suboptimal clinical outcome due to higher risk of recurrence, progression and early death. Trial Registration No CTRI/2015/10/006268
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system lymphomas (PCNSL) are extranodal malignant lymphomas that arise within the brain, eyes, leptomeninges or spinal cord in the absence of systemic lymphoma at the time of diagnosis [1]. There are controversies regarding the optimal treatment strategy [2, 3]. High dose methotrexate (HDMTX) based chemotherapy is accepted to be the mainstay of treatment. Whole brain radiotherapy (WBRT) helps in prolonging the progression free survival (PFS) but its role in improving overall survival (OS) is not clear [4,5,6,7]. The major limiting factor for using WBRT is treatment related late neurotoxicity in patients who achieve long term disease control [8, 9]. This can manifest as progressive cognitive deterioration which may lead to dementia and death. This late effect is more pronounced in elderly patients. In an attempt to decrease the treatment related neurotoxicity, some researchers have opted to defer radiotherapy until recurrence even at the cost of sub-optimal disease control [6, 10,11,12]. Partial brain radiation is another option but this approach results in high rates of relapse within the brain, outside of the irradiated area, suggesting that the whole brain must be treated when radiotherapy is used in PCNSL [13]. Some groups have tried to reduce the dose of WBRT [14, 15] and dose reduction in patients with complete response (CR) to induction chemotherapy has yielded excellent outcomes [16, 17]. The feasibility of this approach merits revalidation in larger and ethnically different cohorts of patients with PNCSL to determine the optimal treatment strategy in these patients.
Patients and methods
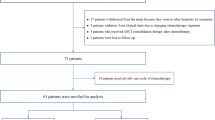
We screened a total of 32 patients and enrolled 22 eligible patients with PCNSL (age 18–80 years, ECOG PS 0–3, HIV seronegative, biopsy proven PCNSL, no significant end-organ dysfunction, immunocompetent) attending our institute from 2015 to 2017 in a prospective phase II trial (Fig. 1). Pre-treatment evaluations included complete blood counts, liver and kidney function tests, serum lactate dehydrogenase (LDH) level, hepatitis B, C and human immunodeficiency virus (HIV) screening, ophthalmologic examination to assess for ocular involvement, lumbar puncture to assess for leptomeningeal involvement and cerebrospinal fluid (CSF) protein levels, contrast enhanced magnetic resonance imaging (CMRI) of the brain, contrast enhanced computed tomography (CECT) of neck, chest, abdomen and pelvis, ultrasonogram (USG) of testis in males, bone marrow aspirate and biopsy to rule out systemic lymphoma. The study was approved by the institutional ethics committee and all patients or guardians signed an informed consent form before participating in the study.
Trial profile: 32 patients with primary CNS lymphoma were evaluated for inclusion in the study from 2015 to 17, out of which 22 patients were found eligible; 14 patients underwent RMPV regimen and 8 patients underwent MPV regimen; 2 patients on RMPV regimen died due to sepsis, 1 patient on RMPV regimen was lost to follow-up after 2 cycles and subsequently died at home; 19 patients were eligible for response assessment after induction chemotherapy; 10 patients had CR, 8 patients had partial response (PR) and 1 patient had progressive disease(PD); 1 patient with CR died due to subarachnoid haemorrhage before cranial RT; 9 patients with CR received reduced dose (23.4 Gy) and 9 patients with PR/PD received standard dose(45 Gy) whole brain radiotherapy (WBRT); the first 3 patients who received reduced dose WBRT had recurrence in brain and 2 of them died due to progressive disease, whereas there was no recurrence or cancer related death in patients who received standard dose WBRT
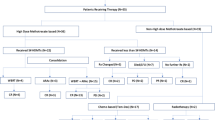
The patients underwent five 2-weekly cycles of MPV induction chemotherapy with methotrexate 3.5 g/m2 IV D1 with hydration, alkalinisation and leucovorin rescue (25 mg IV every 6 h D2–D4), vincristine 1.4 mg/m2 (capped at 2 mg) IV D1, procarbazine 100 mg/m2 P.O. D1–7 in odd number cycles. Rituximab 375 mg/m2 IV D1 q2 weeks was added in 14 patients as per patient-preference and affordability. Intrathecal methotrextae 12 mg bi-weekly was added in patients with CSF+ve disease till two consecutive negative CSF cytology specimens. Primary prophylaxis with granulocyte colony stimulating factor (G-CSF) was not allowed, but secondary prophylaxis at a dose of 5 µg/kg/day was allowed. Toxicity assessment was done on day 1 and day 8 of cycle 1 and subsequently on day 1 of cycles 2–5 using Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). Before each cycle the following parameters had to be met-total leucocyte count > 3000/mm3, absolute neutrophil count > 1500/mm3, platelet count > 100,000/mm3, serum creatinine < 2 mg/dl, calculated glomerular filtration rate > 50 ml/min and urinary pH > 7. All non-haematological toxicities had to resolve to ≤ grade 1 before administration of the subsequent cycle. In case of grade 3/4 myelosuppression (thrombocytopenia and/or neutropenia), the dose of the offending drug i.e. procarbazine was reduced by 25 and 50% on 1st and 2nd occurrences of toxicity respectively and procarbazine was permanently discontinued on 3rd occurrence of severe haematological toxicity. Patients with CR to induction chemotherapy were given reduced dose WBRT 23.4 Gy/13 fractions/2.5 weeks while those with partial response (PR), stable or progressive disease (SD or PD) were given standard dose WBRT 45 Gy/25 fractions/5 weeks by bilateral parallel opposed skull fields using German Helmet portal with Co60 gamma rays. The radiation portal encompassed the entire brain with the meningeal reflections, posterior one-third of bilateral orbits (to include bilateral optic nerves up to optic disc), cribriform plate and spinal cord till the lower border of C2 vertebrae. In patients of PCNSL with ocular involvement, who obtained CR (in brain and eye) to induction chemotherapy, the entire brain (as described before) and bilateral orbits were included in the radiation portal to a dose of 23.4 Gy/13 fractions/2.5 weeks. In patients with ocular involvement with PR/SD/PD to induction chemotherapy, bilateral orbits in entirety were included in the radiation portal up to a dose of 30.6 Gy/17 fractions/3.5 weeks and thereafter the entire brain and posterior one-third of orbits were irradiated up to a total dose of 45 Gy. Thereafter two cycles of consolidation chemotherapy with cytarabine 3 g/m2/day (maximum daily dose 6 gm), IV D1 and D2 were given 1 month apart. Patients were kept on 3 monthly follow-up for the first year after completion of treatment. CMRI of brain was done at baseline, 2–4 weeks after completion of induction chemotherapy, 3 months after completion of WBRT and subsequently on each follow-up visit. The primary endpoints of the study were assessment of response rate (RR) and PFS. The secondary endpoints of the study were assessment of OS, toxicity profile of treatment, molecular subtype of lymphoma, EBV status (by immunohistochemistry for EBV LMP-1) and serial changes in quality of life (EORTC-QLQ-C30 and BN 20 module) and neuropsychological parameters (cognition, executive functions, motor speed, visual construction, language and mood). Response assessment was done according to the report of international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma (PCNSL) [18]. PFS was defined as the duration of time from the date of diagnosis to the date of clinical or radiological disease progression or death due to PCNSL, which ever occurred earlier. Patients without evidence of progression till last follow-up were censored. OS was defined as the duration of time from the date of diagnosis to the date of death or last follow-up. Patients alive at last follow-up were censored. Kaplan–Meier product limit method was used to evaluate PFS and OS. Univariate analysis of PFS and OS with respect to molecular subtypes of diffuse large B cell lymphoma (DLBCL) and other patient related, disease related and laboratory prognostic factors were done by Log Rank test. Multivariate analysis of PFS and OS with respect to different prognostic factors were done using Cox proportional hazard regression model. MedCalc software (version 11.3.0) was used for statistical analysis. Treatment related acute toxicity assessment was done using Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). Assessment of serial changes in neuropsychological and quality of life parameters (at baseline, before WBRT and 6 and 12 months after completion of treatment) was done by Friedman’s test. Comparison of these parameters between two follow up visits was done by using Wilcoxon signed rank test. Comparison of these parameters between standard dose and reduced dose WBRT arms was done by Mann–Whitney’s test. A detailed background comparison of patient related, tumour related and treatment related factors in patients receiving reduced dose WBRT versus standard dose WBRT and patients receiving MPV versus RMPV regimens was made using Fisher’s exact test.
Results
The median age at diagnosis was 51.5 years (range 31–67 years). The male: female ratio was 13:9. The ECOG PS was 3, 2 and 1 in 13 (59.09%), 5 (22.73%) and 4 (18.18%) patients respectively. The median duration of symptoms was 4 months (range 0.5–36 months). The major presenting symptoms were motor impairment in 14 (63.64%), cognitive impairment in 13 (59.09%), headache in 11 (50%), vomiting in 9 (40.91%), visual symptoms in 7 (31.82%) and seizure in 6 (27.27%) patients. Baseline MRI was done in all patients. Deep-seated lesions were present in 15/20 (75%) patients which included areas like basal ganglia, corpus callosum, brainstem, cerebellum and periventricular locations. Ocular involvement was present in 5/22 (22.73%) patients, in which 2 (9.09%) had exclusive ocular involvement at presentation and the remaining 3 (13.64%) patients had synchronous ocular and brain involvement. CSF cytology was positive for malignant cells in 3/22 (13.64%) patients. CSF protein was raised (> 50 mg/dl) in 19/21 (90.48%) patients. The median value of CSF protein was 127 mg/dl (range 34–473 mg/dl). Serum LDH was raised (> 420 U/L) in 10/22 (45.45%) patients. The median value of serum LDH was 415.5 U/L (mean: 465.64 U/L; range 170–1060 U/L). Histopathological diagnosis was established after stereotactic biopsy from the brain lesion, or inadvertent surgery or vitreal tap in case of ocular lymphoma. 8 (36.36%) patients underwent inadvertent surgery, out of which two patients underwent gross total excision, one patient had near total excision and five patients had subtotal excision of tumour. Three patients underwent vitreal tap for the diagnosis of intraocular lymphoma as the only histopathological diagnosis. All the remaining 11 patients underwent stereotactic biopsy from brain lesion. One patient was reported to have NHL from vitreal tap, where further characterisation was not possible. 3 (13.64%) patients (vitreal tap in two and tumour decompression in one patient) were reported to have B-cell NHL, which were positive for CD20. 18 (81.82%) patients were reported to have diffuse large B-cell lymphoma, which were further subclassified as germinal centre type in 3 patients and activated B cell type/post-germinal centre type in 13 patients according to Hans algorithm using immunohistochemistry markers CD10, BCL6, MUM1 (Online Resource 1, Fig. 1). Subtyping could not be done in two patients probably because of administration of corticosteroids prior to biopsy. Immunohistochemistry for EBV-LMP1 was negative in all assessed (N = 19) patients.
All 22 (100%) patients received induction chemotherapy (RMPV and MPV regimens in 14 and 8 patients respectively). The median number of cycles of induction chemotherapy was 5 (range 2–5). In patients undergoing RMPV regimen, the median number of cycles of rituximab added to the induction chemotherapy was 5 (mean 4.6; range 2–5). Chemotherapy interruption/delay was noted in 15 (68.18%) patients. Out of 19 patients who completed HDMTX based induction chemotherapy, 10 (52.63%) patients achieved CR, 8 (42.11%) patients had PR and 1 patient had PD. Two patients on RMPV regimen died due to chemotherapy related toxicities (sepsis). Induction chemotherapy was otherwise well tolerated with severe (grade 3/4) toxicities being mostly haematological-anaemia in one patient, neutropenia in 8 (36.36%) patients and thrombocytopenia in one patient (Online Resource 1, Table 1). Only one patient developed complicated febrile neutropenia. The major non-haematological toxicity was infection (Online Resource 1, Table 2). Nine patients received reduced dose and nine received standard dose WBRT. In our study, WBRT was excellently tolerated with no reported grade 3/4 toxicity (Online Resource 1, Table 3). Consolidation chemotherapy with high dose cytarabine was given in 15 (68.18%) patients. Grade 3/4 neutropenia was observed in 3 (20%) patients during consolidation chemotherapy (Online Resource 1, Table 4). After a median follow-up period of 11.25 months (mean 12.41 months), four patients had disease progression and eight patients had died, the causes being disease progression in two, chemotherapy related toxicity in two, non-cancer related in three patients and unknown in one. The estimated median OS was 19 months. The median PFS had not been reached. The actuarial rates of PFS were 94.1 and 50.2%, disease specific survival were 86.4 and 61.4% and OS were 68.2 and 48.5%, respectively at 1 and 2 years (Fig. 2), (Online Resource 1, Table 5). Three patients in reduced dose WBRT arm had recurrence and two of them died of progressive disease, whereas there was no recurrence or disease related death in standard dose WBRT arm. Salvage treatment was given to all three patients with recurrence after reduced dose WBRT. A combination of rituximab and temozolomide was given to one patient, who died after one cycle due to progressive disease. Single agent temozolomide was given to one patient, who also died after one cycle (she was advised a combination of rituximab and temozolomide, but could not afford rituximab). One patient was re-challenged with MPV regimen and he had marked symptomatic improvement after two cycles and was undergoing the 3rd cycle while the database was locked. On univariate analysis of OS (Table 1), use of RT (p value < 0.0001), use of consolidation Ara-C (p value 0.026) and negative CSF cytology (p value 0.0076) led to significantly improved outcome. On multivariate analysis of OS, only CSF cytology retained prognostic significance with p value of 0.021 and hazard ratio (HR) of 6.71. On univariate analysis of PFS (Table 1), age ≤ 50 years and use of standard dose WBRT (45 Gy) led to significantly improved outcome (p value 0.03 and 0.02 respectively) (Fig. 3). The overall RRs to induction chemotherapy with and without rituximab were not significantly different (90.9 versus 100%; p value 1) (Online Resource 1, Table 6). There was no significant difference in treatment outcome according to the molecular subtype of PCNSL (Table 1). Serial neuropsychological assessments revealed marked improvement in general cognition and other domains (e.g. verbal fluency and motor speed) after induction chemotherapy, which persisted for 6 months after completion of primary treatment and then stabilised (Table 2). The mean EORTC global health status/Qol score declined from 58.3 at baseline to 41.67 after induction chemotherapy and then increased to 66.67 at 6 and 12 months after completion of treatment (p value 0.748) (Table 2).There was no statistically significant difference in short term neurocognitive outcome and quality of life between reduced and standard dose WBRT arms (Online Resource 1, Table 7).
Discussion
The treatment of primary central nervous system lymphoma has evolved over the decades from radical radiotherapy to combined modality treatment with HDMTX based induction chemotherapy followed by WBRT and consolidation chemotherapy. The current trend is to decrease the dose of WBRT or to avoid it altogether, with a view to decreasing the long term neurocognitive sequel of treatment [4, 14, 16, 17, 19]. The aim of our study was to assess the feasibility of response adapted WBRT after HDMTX based chemotherapy in newly diagnosed patients with PCNSL in the Indian scenario. We gave reduced dose WBRT (23.4 Gy) in patients with CR and standard dose WBRT (45 Gy) in patients with PR, SD or PD after HDMTX based induction chemotherapy. A phase II study with a similar design has already been published from Memorial Sloan Kettering Cancer Center(MSKCC) [16, 17]. In the initial report of the MSKCC trial by Shah et al. (N = 37), after a median follow-up of 37 months, the estimated 2-year OS and PFS rates were 67 and 57%, respectively [16]. The estimated median PFS was 40 months. For the 19 patients who received reduced-dose WBRT (23.4 Gy), the estimated 2-year OS and PFS rates were 89% and 79%, respectively. The relapse rate after CR was 26%. In the updated report of the MSKCC trial by Morris et al. (N = 52), the median PFS and OS were 3.3 and 6.6 years respectively [17]. Thirty-one patients (60%) achieved CR to RMPV regimen and received reduced dose WBRT. The 2-year PFS rate in this group was 77%. The median PFS was 7.7 years and the median OS was not reached.
In our trial, we enrolled 22 newly diagnosed immunocompetent patients with PCNSL. In the evaluable patients (N = 19), after induction chemotherapy, 10 (52.63%) and 8 (42.11%) patients had CR and PR respectively in our study, leading to an overall RR of 94.74%. This is in accordance with the published medical literature [16, 17]. The first three patients who attained CR to induction chemotherapy and subsequently received dose reduced WBRT (23.4 Gy), progressed in brain with a median time to progression of 17.5 months. Two of these three patients died due to progressive disease soon after receiving the 1st cycle of salvage chemotherapy. It should be noted that one patient with intraocular B cell NHL, progressed in brain after completing five cycles of induction chemotherapy (before RT). No patient in the standard WBRT arm had disease progression after receiving RT. There was no significant difference in the patient related, tumour related and treatment related factors in these two arms with the following exceptions: deep seated tumours were more common in the reduced dose WBRT arm (100 versus 33.3%, p value 0.019), inadvertent excision of tumour was more common in the reduced dose WBRT arm (66.7 versus 11.1%, p value 0.05) and chemotherapy interruptions/delays were more frequent in the reduced dose WBRT arm (88.9 versus 33.3%, p value 0.05) (Online Resource 1, Table 8). In addition, 5 out of 9 (55.6%) patients in reduced dose WBRT arm and 2 out of 9 (22.2%) patients in standard dose WBRT arm had MSKCC prognostic class 3 (p value 0.19). In the entire cohort, the median PFS was not reached and the actuarial rates of PFS were 94.1% at 1 year and 50.2% at 2 years. On univariate analysis of PFS, younger patients (age ≤ 50 years) and use of standard dose WBRT (45 Gy) led to significantly improved outcome. Also, it is interesting to note that pertaining to PFS, the best results were achieved in patients with PR, followed by patients with CR, followed by patients with PD to induction chemotherapy (p value 0.0001). This may be explained by the fact, that patients with PR received standard dose WBRT (45 Gy) in contradistinction to patients with CR who received reduced dose WBRT (23.4 Gy). This study is giving an early signal that WBRT dose reduction from 45 to 23.4 Gy in patients with PCNSL, who attain CR to HDMTX based chemotherapy, may engender disease progression and eventual death in the absence of effective salvage treatment. The median OS in our study was noted to be 19 months and the actuarial rates of OS were 68.2% at 1 year and 48.5% at 2 years. This compares poorly with the published medical literature [4, 15,16,17]. We believe that the OS outcome in our study has been marred by the relatively high incidence of toxic deaths (9.09%) and non-cancer related deaths (13.64%). Also, it should be noted that patients in our study had a high burden of poor prognostic factors e.g. ECOG PS 2 and 3 in 22.73 and 59.09% patients respectively, increased CSF protein in 90.48% patients, increased serum LDH in 45.45% patients and deep seated lesion in 75% patients. Pertaining to the MSKCC prognostic class, 11 out of 22 patients (50%) belonged to class 2/3. Out of 16 patients with DLBCL, 13 (81.25%) patients had activated B cell subtype, which portends a poor prognosis.
In our study, 14 (63.64%) patients received RMPV and 8 (36.36%) patients received MPV regimen. 7 out of 14 (50%) patients on RMPV regimen and 2 out of 8 (25%) patients on MPV regimen had MSKCC prognostic class 3 (p value 0.445). There was no significant difference in the other patient related, tumour related and treatment related factors in these 2 arms (Online Resource 1, Table 9). There was no significant difference in the complete RR-54.5 versus 50% (p value 1) and overall RR-90.9 versus 100% (p value 1) in patients receiving RMPV and MPV regimens respectively. It is notable that addition of rituximab to MPV regimen had no significant impact on PFS and OS on univariate analysis. There is an existing concern amongst the oncologists regarding the penetration of the large molecule of rituximab across the blood brain barrier and its therapeutic concentration in the brain parenchyma. However, extrapolating from the impact of rituximab on survival outcome in systemic B cell NHL, a multitude of single arm and randomized phase II trials have explored the addition of rituximab to HDMTX based induction chemotherapy in patients with PCNSL and most of them have reported favourable outcome with this chemo-immunotherapy approach [11, 16, 17, 20]. In the recently published multi-centre randomized phase 2 IELSG-32 trial, 227 patients with newly diagnosed PCNSL were randomly assigned (1:1:1) to receive four 3 weekly courses of HDMTX and cytarabine (group A); or the same combination along with rituximab (group B); or the same methotrexate–cytarabine–rituximab combination along with thiotepa (group C) [20]. Patients with responsive or stable disease after induction chemotherapy were randomly allocated between WBRT and high dose chemotherapy followed by autologous stem cell transplantation (ASCT) for consolidation. At a median follow-up of 30 months, the CR rates in patients in group A, B and C were 23, 30 and 49% respectively. Grade 4 haematological toxicity was more frequent in patients treated with methotrexate, cytarabine, thiotepa and rituximab (MATRix regimen), but infective complications were similar in the three groups. The incidence of toxic death was 6% in this study. Based on the study results, the authors have proposed MATRix combination as the new standard of chemo-immunotherapy for patients with newly diagnosed PCNSL, aged up to 70 years. In a recently presented multicentre randomized phase III trial, 200 patients with newly diagnosed PCNSL were randomized to induction with two cycles of MBVP (HDMTX, carmustine, teniposide, prednisone) chemotherapy with (arm B) or without (arm A) rituximab [21]. Responding patients received consolidation chemotherapy with high dose cytarabine. Patients aged more than 60 years were not irradiated whereas younger patients received WBRT 30 Gy with an additional boost of 10 Gy in patients with PR. In this trial, the addition of rituximab to HDMTX-based chemotherapy did not improve RRs (CR/CR unconfirmed rates-66% in arm A and 68% in arm B & overall RR-87% in both arms), EFS or PFS. The incidence of treatment related mortality was 7% in arm A and 3% in arm B. In our study, the incidence of treatment related mortality was 9.09% (2/22 patients). Both these patients were on RMPV regimen. The cause of deaths were complicated febrile neutropenia leading to sepsis, disseminated intravascular coagulation and neutropenic enterocolitis after 3rd cycle in one patient and sepsis (E. coli) after 4th cycle in one patient. In future, we will endeavour to bring down the incidence of treatment related mortality in our patients. In this regard, the use of primary prophylaxis with G-CSF after RMPV regimen will be crucial in preventing fatal febrile neutropenia [16]. The issue of addition of rituximab to HDMTX based regimen in patients with PCNSL needs to critically evaluated in the Indian setting because of its investigational role, increased toxicity and higher cost.
Apart from the issue of toxic death in two patients, HDMTX based induction chemotherapy was well tolerated with severe (grade 3/4) toxicities being mostly haematological. We limited ourselves to five cycles of HDMTX based chemotherapy in all patients instead of additional two cycles in partial responders chiefly out of concern about chemotherapy related toxicity and tolerance issues. During consolidation chemotherapy with high dose Ara-C, grade 3/4 neutropenia was observed in 3 (20%) patients.
Whole brain RT was excellently tolerated and the side effects were mostly mild (grade 1/2) dermatitis and conjunctivitis. With a median follow up of only 11.25 months, no treatment related late effect has been discerned. Undoubtedly, with the passage of time, the late effects of radiotherapy and chemotherapy will manifest and they need to be meticulously documented and addressed.
Serial neuropsychological assessments revealed that there was marked improvement in general cognition and other domains (e.g. verbal fluency and motor speed) after induction chemotherapy, which persisted for 6 months after completion of primary treatment and then stabilised. This initial improvement was most likely due to response of tumour to chemotherapy and possibly WBRT. Although there was some improvement in quality of life parameters with the passage of time, most of them were statistically non-significant except for future uncertainty and weakness of legs. The mean EORTC global health status/Qol score declined from 58.3 at baseline to 41.67 after induction chemotherapy and then increased to 66.67 at 6 and 12 months after completion of treatment. The initial decline in QoL score could be due to induction chemotherapy related acute toxicities. There were no significant difference in any cognitive or quality of life domain between reduced dose and standard dose WBRT arms at baseline and subsequent follow up visits. However long term follow up is required to detect late neurological toxicities, particularly neurocognitive decline. The various approaches to mitigate this issue are avoidance of WBRT from the frontline management of PCNSL [4, 11], use of reduced dose WBRT [16, 17] and partial brain radiation instead of WBRT for consolidation [13, 22]. In the recently closed phase II RTOG 11–14 trial, newly diagnosed patients with PCNSL were randomized to receive R-MPV and cytarabine with or without reduced dose WBRT (23.4 Gy). The primary objective of the trial was to determine the median PFS in the two arms on an intent-to-treat basis and the results are eagerly awaited. In the era of combined modality treatment, researchers from Japan have shown interest in focal radiation, particularly in patients of PCNSL with unifocal disease [13]. In a retrospective report of 43 patients with PCNSL (74.4%-unifocal disease) treated with focal RT (median dose-50 Gy) from Japan, cumulative rates of in-field and out-of-field (in brain) recurrence at 5 years were 57 and 49%, respectively. The out-of field recurrence rates were 22 versus 83% for patients treated with safety margins of ≥ 4 versus < 4 cm (p = 0.0079) and 45 versus 67% in patients with a single lesion versus those with multiple lesions (p = 0.79). In a comprehensive report on radiotherapy parameters in 1054 patients with PCNSL treated from 1985 to 2009 in Japan, 92 and 8% of the patients received WBRT and partial brain RT [22]. The 5 year OS rates were 25 and 29% for patients treated with WBRT and partial brain RT respectively (p = 0.8). These studies have led to the generation of a hypothesis that in patients of PCNSL with unicentric disease receiving HDMTX based induction chemotherapy, partial brain RT with adequate safety margin may be considered instead of WBRT. However due to high risk of out-of-field recurrence in brain, further well designed prospective studies are warranted to test this hypothesis. There is also burgeoning interest in the use of stereotactic radiotherapy and radiosurgery in newly diagnosed patients with PCNSL. In a prospective, observational cohort study, 73 patients received single agent HDMTX (8 gm/m2) chemotherapy and 55 patients received the same chemotherapy regimen followed by gamma knife radiosurgery (GKRS) with a median dose of 11 Gy (range 11–16 Gy) to 50% isodose line [23]. After a mean follow-up of 36.9 months, the median survival was 26.8 months in the chemotherapy group and 47.6 months in the chemotherapy plus GKRS group (p value 0.0034). All lesions treated with GKRS showed CR on follow-up MRI. In future, we intend to conduct prospective studies in our institute on the omission of WBRT from the frontline management of PCNSL in patients > 60 years, who have achieved CR to HDMTX based induction chemotherapy and the use of WBRT-36 Gy/20 fractions/4 weeks followed by a boost of 9 Gy/5 fractions/1 week for consolidation in younger patients. Stereotactic RT could be a useful modality for the focal boost to minimize collateral irradiation of the uninvolved brain parenchyma.
To summarize the strengths of the present study, it is the first and only prospective study looking at the feasibility of response adapted WBRT after HDMTX based chemotherapy in patients with newly diagnosed PCNSL in the Indian subcontinent using serial comprehensive neuropsychological and quality of life assessments. The limitations of the study include small sample size (N = 22), short follow up period (median: 11.25 months; mean: 12.41 months; follow-up is ongoing and this is the report on acute toxicity profile and early clinical outcome) and the fact that rituximab was added to MPV regimen according to patient preference and affordability and not in a randomized manner.
Conclusions
In patients with newly diagnosed primary central nervous system lymphoma, reduced dose WBRT after CR to HDMTX based chemotherapy may lead to suboptimal clinical outcome due to higher risk of recurrence, progression and early death. Positive CSF cytology (for malignant cells) at presentation is an independent poor prognostic factor determining OS. Whole brain radiotherapy in both reduced and standard dose, does not appear to have short term neuropsychological and quality of life detriment. However longer follow up is required to make definitive conclusions.
Data availability
Data generated or analysed during this study are included in this published article [and its supplementary information files] and remaining datasets are available from the corresponding author on reasonable request.
References
Ricard D, Idbaih A, Ducray F et al (2012) Primary brain tumours in adults. Lancet 379:1984–1996. https://doi.org/10.1016/S0140-6736(11)61346-9
Morris PG, Abrey LE (2009) Therapeutic challenges in primary CNS lymphoma. Lancet Neurol 8:581–592. https://doi.org/10.1016/S1474-4422(09)70091-2
Ferreri AJM, DeAngelis L, Illerhaus G et al (2011) Whole-brain radiotherapy in primary CNS lymphoma. Lancet Oncol 12:118–119. https://doi.org/10.1016/S1470-2045(11)70018-3 (author reply 119–120).
Thiel E, Korfel A, Martus P et al (2010) High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 11:1036–1047. https://doi.org/10.1016/S1470-2045(10)70229-1
Omuro A, Taillandier L, Chinot O et al (2011) Primary CNS lymphoma in patients younger than 60: can whole-brain radiotherapy be deferred? J Neurooncol 104:323–330. https://doi.org/10.1007/s11060-010-0497-x
Batchelor T, Carson K, O’Neill A et al (2003) Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol 21:1044–1049
Abrey LE, Yahalom J, DeAngelis LM (2000) Treatment for primary CNS lymphoma: the next step. J Clin Oncol 18:3144–3150
Omuro AMP, Ben-Porat LS, Panageas KS et al (2005) Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol 62:1595–1600. https://doi.org/10.1001/archneur.62.10.1595
Abrey LE, DeAngelis LM, Yahalom J (1998) Long-term survival in primary CNS lymphoma. J Clin Oncol 16:859–863
Ferreri AJM, Reni M, Pasini F et al (2002) A multicenter study of treatment of primary CNS lymphoma. Neurology 58:1513–1520
Rubenstein JL, Hsi ED, Johnson JL et al (2013) Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 31:3061–3068. https://doi.org/10.1200/JCO.2012.46.9957
Ekenel M, Iwamoto FM, Ben-Porat LS et al (2008) Primary central nervous system lymphoma: the role of consolidation treatment after a complete response to high-dose methotrexate-based chemotherapy. Cancer 113:1025–1031. https://doi.org/10.1002/cncr.23670
Shibamoto Y, Hayabuchi N, Hiratsuka J et al (2003) Is whole-brain irradiation necessary for primary central nervous system lymphoma? Patterns of recurrence after partial-brain irradiation. Cancer 97:128–133
Bessell EM, López-Guillermo A, Villá S et al (2002) Importance of radiotherapy in the outcome of patients with primary CNS lymphoma: an analysis of the CHOD/BVAM regimen followed by two different radiotherapy treatments. J Clin Oncol 20:231–236
DeAngelis LM, Seiferheld W, Schold SC et al (2002) Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: radiation therapy oncology group study 93-10. J Clin Oncol 20:4643–4648
Shah GD, Yahalom J, Correa DD et al (2007) Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 25:4730–4735. https://doi.org/10.1200/JCO.2007.12.5062
Morris PG, Correa DD, Yahalom J et al (2013) Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 31:3971–3979. https://doi.org/10.1200/JCO.2013.50.4910
Abrey LE, Batchelor TT, Ferreri AJM et al (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23:5034–5043. https://doi.org/10.1200/JCO.2005.13.524
Ferreri AJM, Verona C, Politi LS et al (2011) Consolidation radiotherapy in primary central nervous system lymphomas: impact on outcome of different fields and doses in patients in complete remission after upfront chemotherapy. Int J Radiat Oncol Biol Phys 80:169–175. https://doi.org/10.1016/j.ijrobp.2010.01.066
Ferreri AJ, Cwynarski K, Pulczynski E et al (2016) Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International extranodal lymphoma study group-32 (IELSG32) phase 2 trial. Lancet Haematol 3:e217–e227. https://doi.org/10.1016/S2352-3026(16)00036-3
Bromberg J, Issa S, Bukanina K et al (2017) Effect of rituximab in primary central nervous system lymphoma: results of the randomized phase III HOVON 105/ALLG NHL 24 Study. Blood 130:582
Shibamoto Y, Sumi M, Takemoto M et al (2014) Analysis of radiotherapy in 1054 patients with primary central nervous system lymphoma treated from 1985 to 2009. Clin Oncol 26:653–660. https://doi.org/10.1016/j.clon.2014.06.011
Alvarez-pinzon AM, Wolf AL, Swedberg H et al (2016) Primary central nervous system lymphoma (PCNSL): analysis of treatment by gamma knife radiosurgery and chemotherapy in a prospective, observational study. Cureus 8:e697. https://doi.org/10.7759/cureus.697
Acknowledgements
Financial assistance for research received from Indian Council of Medical Research (ICMR).
Funding
Dr. N. Adhikari received financial assistance for research from Indian Council of Medical Research (ICMR). This was an investigator initiated prospective phase II trial. The cost of imaging(MRI) and other investigations, chemotherapy (but not Rituximab) and radiotherapy in patients in this clinical trial was exempted by the competent authority at All India Institute of Medical Sciences, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human and animal participants
This article does not contain any study with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adhikari, N., Biswas, A., Gogia, A. et al. A prospective phase II trial of response adapted whole brain radiotherapy after high dose methotrexate based chemotherapy in patients with newly diagnosed primary central nervous system lymphoma-analysis of acute toxicity profile and early clinical outcome. J Neurooncol 139, 153–166 (2018). https://doi.org/10.1007/s11060-018-2856-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2856-y