Abstract
Purpose
High-dose methotrexate (HD-MTX)-based chemotherapy regimen is the first-line option for primary central nervous system lymphoma (PCNSL). This prospective cohort study aimed to evaluate the efficacy and adverse effects of HD-MTX plus idarubicin (IDA) in patients with newly diagnosed immunocompetent PCNSL.
Methods
We recruited newly diagnosed PCNSL patients from January 2017 to August 2020. Patients were assigned into two groups: HD-MTX monotherapy and HD-MTX plus IDA (HD-MTX/IDA). In the HD-MTX monotherapy group, patients were treated with MTX 8 g/m2 alone on day 1, while the HD-MTX/IDA group received MTX 8 g/m2 on day 1 and IDA 10 mg/m2 on day 2. Treatments were repeated every 3 weeks for 8 cycles except for progression and/or unacceptable toxicity.
Results
We recruited 61 PCNSL patients, including 36 in the HD-MTX and 25 in the HD-MTX/IDA group. The CR rate was 68% in the HD-MTX/IDA group and 72.22% of patients in the HD-MTX monotherapy group (p = 0.7221), while the overall response rate was 72% vs. 77.78% (p = 0.6063). Median PFS in HD-MTX/IDA group and HD-MTX monotherapy group were 15.6 months and 18.5 months, respectively (p = 0.6374). Median OS was not reached in both groups. There were no significant differences in adverse effects between the two groups.
Conclusions
The combination of IDA with HD-MTX showed no obvious therapeutic advantage over HD-MTX monotherapy in newly diagnosed patients with PCNSL. HD-MTX dose of 8 g/m2 monotherapy can still provide better therapeutic benefits in patients with acceptable adverse effects. Future studies could explore HD-MTX in combination with other chemotherapeutic agents in the first-line treatment of PCNSL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive extranodal non-Hodgkin’s lymphoma (NHL) that restricted to the brain, leptomeninges, cerebrospinal fluid, eyes, or spinal cord without evidence of a systemic lymphoma involvement. PCNSL accounts for only 2–4% of malignant brain tumors and 4–6% of extranodal lymphomas in Western countries [1,2,3] Unfortunately, PCNSL shows highly aggressive biological behavior within its distinct sites of occurrence, and thus, clinical outcomes remain poor [4].
Surgery is mainly used to establish the histological diagnosis of PCNSL due to the high risk of postoperative neurological impairment and other adverse outcomes [5, 6]. Radiation therapy has also been used for the treatment of PCNSL for decades, but its role has been diminishing lately because of significant age-related neurotoxicity [7, 8].
MTX–based therapies (MTX dose of 3–8 g/m2) are the standard induction therapy for PCNSL, but promoted tumor response and survival outcome were also observed when MTX was combined with other chemotherapeutic agents [9,10,11,12]. Previous studies using MTX-based multi-drug chemotherapy regimens containing idarubicin (IDA) have demonstrated promising results [13,14,15,16].
IDA is a semi-synthetic daunorubicin analog. Characterized by its high lipophilicity, IDA exerts its anti-neoplastic effect by increasing the cellular uptake rate. Studies have shown that the IDA metabolite idarubicinol can be detected in the cerebrospinal fluid after intravenous administration. Furthermore, idarubicinol also has good penetrability to brain tumor tissues [17, 18]. Therefore, IDA is expected to be a potential drug in PCNSL patients, as the combination of MTX and IDA had shown better control of PCNSL in our retrospective study [19].
This prospective cohort study aimed to evaluate whether MTX plus IDA improves the CR and PFS in newly diagnosed PCNSL patients.
Patients and methods
Study design and participants
A prospective cohort single-center study was carried out in patients with newly diagnosed PCNSL from January 2017 to August 2020 and followed up until May 2021. The inclusion criteria for this study were as follows: (a) age ≤ 65 years; (b) pathological diagnosis of diffuse large B-cell lymphoma (DLBCL); (c) Confirmation of no involvement of sites outside the CNS by 18F-FDG-PET combined with computed tomography (CT) imaging (PET-CT) or contrast-enhanced CT; (d) and no HIV infection and no immunosuppression. Patients would be excluded if they had systemic lymphoma, immunodeficiency disease, or had received only one cycle of therapy. All patients underwent physical examination and neurological evaluation on admission, and if there were no contraindications, an immediate lumbar puncture was performed before the first cycle of chemotherapy. The Huashan Hospital Ethics Committee approved the study protocol, and informed consent was obtained from all patients.
Treatment protocols
Patients were separated into two groups based on the preferences of physicians, patients and guardians involved: the HD-MTX monotherapy cohort and HD-MTX/IDA cohort. Patients in the HD-MTX monotherapy group received MTX 8 g/m2 on day 1, while those in the HD-MTX/IDA group received HD-MTX 8 g/m2 on day 1 and IDA 10 mg/m2 on day 2. Both groups also received dexamethasone 15 mg on days 1–3. Each HD-MTX treatment was administered as a 3-h infusion. Prehydration and alkalinization were initiated at least 72 h before MTX administration. Diuresis was generally kept at 3500 ml/24 h. Standard leucovorin rescue was initiated 24-h after the start of HD-MTX infusion at a dose of 15 mg/m2 every 6 h for a total of eight times. If delayed elimination occurred, the leucovorin dose or rate of intravenous fluid hydration and alkalinization was increased. Every 3 weeks, 8 cycles were repeated until tumor progression or toxicity occurred. This study examined CR rate and PFS as primary endpoints and OS and safety as secondary endpoints.
The clinical features of all patients were collected from the medical records, including age, gender, height, weight, performance status, time of diagnosis, surgical resection, biopsy type, lesion site, number of lesions, HIV status, serum lactate dehydrogenase (LDH) level, etc. Magnetic resonance imaging (MRI) was used to assess the location and quantity of lesions in all patients. The International Extranodal Lymphoma Study Group (IELSG) defined involvement of the deep structures of the brain (periventricular regions, basal ganglia, brainstem, and/or cerebellum) [20].
Toxicity and outcome assessments
The continuity of treatment was evaluated by contrast-enhanced MRI scans every cycle and by PET-CT after 3 cycles of treatment and after all therapeutic procedures. CR was defined as the complete disappearance of all lesions; a partial response (PR) as a 50% reduction in tumor size; progressive disease (PD) as an increase of 25% in tumor size for all lesions and a new occurrence of lesions; stable disease (SD) was defined as an unclassifiable condition, which was not a CR, PR, or PD. Overall response (OR) was determined by CR plus PR. A toxicity assessment for each chemotherapy course was conducted using the Common Terminology Criteria for Adverse Events version 4.0. The worst toxicity per patient was considered for analysis. Within the first 2 years after treatment completion, patients were assessed every 3 months, then every 6 months in the 3rd year, and then annually.
Statistical analysis
Statistical Package for Social Science, version 26.0 (IBM SPSS Statistics, Armonk, NY: IBM Corp.) and Graphpad Prism version 8.0.1 (Graphpad Software) were used to perform all statistical analyses. All tests were two-sided, and p < 0.05 was considered statistically significant. Using chi-square and Fisher’s exact tests, patient characteristics of the two groups (HD-MTX and HD-MTX/IDA) were compared. Survival curves were plotted using the Kaplan–Meier method and analyzed using the log-rank test. PFS was calculated from the date of diagnosis to the date of tumor progression or recurrence, and OS was calculated from the date of diagnosis until death or the last follow-up. Cox proportional hazards regression was used in both the univariate and multivariate analyses.
Results
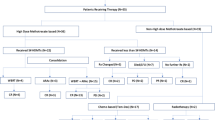
This study enrolled 61 newly diagnosed PCNSL patients, as shown in Fig. 1. Thirty-six patients received MTX monotherapy with a median age of 50, and 25 received HD-MTX/IDA with a median age of 52. In the HD-MTX/IDA group, 3 patients presented with elevated serum LDH, 6 with elevated WBC counts in CSF, and 14 with high CSF protein levels. In contrast, 1 patient showed elevated LDH, 14 with elevated WBC counts, and 25 elevated proteins in CSF in the MTX group. Besides, 15 patients (60%) had lymphoma that involved deep sites of the brain, and 9 patients (36%) had multiple lesions in the HD-MTX/IDA group, with 18 patients (50%) and 15 patients (41.67%) in the MTX group, respectively. These 2 cohorts demonstrated a balanced distribution of age, multiple lesions, involvement of deep structure, biopsy type, except for patients’ intracranial lesions greater than 2 cm in diameter. A considerably higher percentage of large intracranial lesions (greater than 2 cm) and lesion volumes were detected in the HD-MTX/IDA group over the MTX monotherapy group (Table 1).
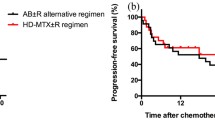
The median follow-up duration was 28.5 months (range, 9.3–53.3 months). Of the enrolled patients, 15 patients discontinued treatment due to disease progression, 8 in the MTX monotherapy group and 7 in the HD-MTX/IDA group. CR rates was 72.22% of patients in the MTX monotherapy and 68% in the HD-MTX/IDA group (p = 0.7221) after 3 courses of chemotherapy. OR rates were 77.78% and 72%, respectively (Table 2). Median PFS in the MTX group was 18.5 months (95% CI 11.084–25.916 months) compared to 15.6 months in the combination group (95% CI 5.36–25.84 months) (Fig. 2). Median OS was not reached for both groups (Fig. 3). In multivariate analysis, PFS showed no improvement in the HD-MTX/IDA group (p = 0.551, HR = 1.224 [95% CI, 0.630–2.380]) (Table 3).
Notably, the median PFS of patients with intracranial lesions ≥ 2 cm in diameter was 9.35 months, while the median PFS of patients with diameters less than 2 cm was 18.5 months.
Toxicity
Two patients in the HD-MTX/IDA group, and three in the MTX group suffered hematological toxicity, including anemia, neutropenia, and thrombocytopenia, but grade 3–4 toxicity was not frequent in either group. Grade 1–2 hepatotoxicity was experienced by 4 patients (16%) in the HD-MTX/IDA group and 8 patients (22.22%) in the MTX group (p = 0.7843), with 3 and 1 patient in the respective groups suffering grade 3 hepatoxicity. One patient in the HD-MTX/IDA group had grade 2 renal dysfunction, and febrile neutropenia occurred in one patient in the MTX group. All treatment-related toxicities were managed without severe adverse events or treatment-associated deaths in either group (Table 4).
Discussion
Given the rarity of PCNSL and the paucity of high-quality evidence regarding treatment is available. Only 2 randomized phase III studies and 5 randomized phase II studies have been performed [21,22,23,24,25]. No consensus exists on the optimal frontline regimens, chemotherapeutic agents in addition to HD-MTX, or consolidation therapy with WBRT versus high-dose chemotherapy and autologous stem cell transplant (ASCT) [11, 26, 27]. Chemoimmunotherapy of methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in the treatment of primary central nervous system lymphoma (IELSG32) has shown response rates in patients who received Methotrexate–cytarabine plus rituximab and thiotepa (MATRix, 87%) as compared to HD-MTX/cytarabine/rituximab without thiotepa (74%) and HD-MTX/cytarabine alone (53%) [23].
HD-MTX can penetrate the blood-brain barrier (BBB) and is PCNSL’s first-choice treatment. Doses of HD-MTX in the range of 3–8 g/m2 are frequently used [28, 29]. However, there is no agreement on the optimal MTX dose for PCNSL. Current National Comprehensive Cancer Network guidelines recommend doses of 3.5 g/m2 or higher for PCNSL [5].
MTX is also combined with other drugs to increase the therapeutic effect, and new agents that can cross the BBB effectively would be logical candidate drugs for addition to MTX. However, combining MTX with temozolomide, rituximab, or high-dose cytarabine, still lacks consensus [11, 30, 31].
IDA is a semi-synthetic daunorubicin analog. Characterized by its high lipophilicity, IDA expresses its anti-neoplastic effect by increasing the cellular uptake rate, suggesting IDA could be an ideal candidate for the treatment of PCNSL. Previous retrospective single-center study found that MTX (3 g/m2) combined with IDA exerted a satisfactory control of PCNSL [19], whereas our previous research found that MTX doses of 8 g/m2 provided a higher CR rate and better PFS benefits over 3.5 g/m2 (68.29% vs. 43.75%, p = 0.03 and 17.7 vs. 9.05 months, p = 0.03, respectively), with acceptable adverse effects [32]. However, there is no consensus on HD-MTX 8 g/m2 alone or plus other chemotherapeutic agents delivered in patients with newly diagnosed PCNSL. So, this prospective cohort study aimed to further assess the effect of MTX (8 g/m2) together with IDA on the possible improvement of the CR and PFS in newly diagnosed PCNSL patients.
Compared with previous studies, our prospective cohort study found that the combination of IDA with MTX (8 g/m2) did not improve CR rates, OS, and PFS in PCNSL patients. The reason may be that MTX (8 g/m2) monotherapy can still provide a good curative effect. Besides, the characteristics of patients in both groups were not well-matched, with more patients receiving HD-MTX/IDA having intracranial tumor diameter greater than 2 cm (48% vs. 19.44%) and larger lesion volume than those in the single-agent group. This imbalance between the two groups may be another factor that may have influenced treatment outcomes.
This was the first prospective trial to examine the effects of combining MTX (8 g/m2) with an IDA regimen in patients with untreated PCNSL. Compared with MTX single-agent chemotherapy, IDA combined with MTX showed no apparent therapeutic advantage. Monotherapy with MTX at a dose of 8 g/m2 can still provide therapeutic benefits with acceptable side effects.
Conclusions
This study compared newly diagnosed PCNSL patients treated with HD-MTX (8 g/m2) monotherapy or combined with IDA and concluded that the combination therapy showed no apparent therapeutic advantages. Future studies could explore HD-MTX in combination with other chemotherapeutic agents in the first-line treatment of PCNSL.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Schaff LR, Grommes C (2021) Primary central nervous system lymphoma. Blood. https://doi.org/10.1182/blood.2020008377
Mendez JS, Ostrom QT, Gittleman H et al (2018) The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 20:687–694
Chukwueke U, Grommes C, Nayak L (2022) Primary central nervous system lymphomas. Hematol Oncol Clin North Am 36:147–159
Alcantara M, Fuentealba J, Soussain C (2021) Emerging landscape of immunotherapy for primary central nervous system lymphoma. Cancers (Basel) 13:5061
Network. NCC. NCCN clinical practice guidelines in oncology: central nervous system cancers (Version1.2021). Available at https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed on 4 Jun 2021
Sieg N, Naendrup JH, Gödel P et al (2021) Treatment patterns and disease course of previously untreated primary central nervous system lymphoma: feasibility of MTX-based regimens in clinical routine. Eur J Haematol 107:202–210
van der Meulen M, Dirven L, Habets EJJ et al (2018) Cognitive functioning and health-related quality of life in patients with newly diagnosed primary CNS lymphoma: a systematic review. Lancet Oncol 19:e407–e418
Correa DD, Braun E, Kryza-Lacombe M et al (2019) Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol 144:553–562
Yoon WS, Park JS, Kim YI et al (2021) High-dose methotrexate monotherapy for newly diagnosed primary central nervous system lymphoma: 15-year multicenter experience. Asia Pac J Clin Oncol 17:123–130
Liu Y, Yao Q, Zhang F (2021) Diagnosis, prognosis and treatment of primary central nervous system lymphoma in the elderly population (Review). Int J Oncol 58:371–387
Holdhoff M, Mrugala MM, Grommes C et al (2020) Challenges in the treatment of newly diagnosed and recurrent primary central nervous system lymphoma. J Natl Compr Canc Netw 18:1571–1578
Chihara D, Dunleavy K (2021) Primary central nervous system lymphoma: evolving biologic insights and recent therapeutic advances. Clin Lymphoma Myeloma Leuk 21:73–79
Ferreri AJ, Ciceri F, Brandes AA et al (2014) MATILDE chemotherapy regimen for primary CNS lymphoma: results at a median follow-up of 12 years. Neurology 82:1370–1373
Qian L, Zhou C, Shen J et al (2016) Treatment of newly diagnosed B-cell origin primary CNS lymphoma with systemic R-IDARAM chemotherapy and intrathecal immunochemotherapy. Oncotarget 7:25783–25790
Olivier G, Clavert A, Lacotte-Thierry L et al (2014) A phase 1 dose escalation study of idarubicin combined with methotrexate, vindesine, and prednisolone for untreated elderly patients with primary central nervous system lymphoma. The GOELAMS LCP 99 trial. Am J Hematol 89:1024–1029
Zhao D, Qian L, Shen J et al (2014) Combined treatment of rituximab, idarubicin, dexamethasone, cytarabine, methotrexate with radiotherapy for primary central nervous system lymphoma. J Cell Mol Med 18:1081–1086
Crivellari D, Lombardi D, Spazzapan S et al (2004) New oral drugs in older patients: a review of idarubicin in elderly patients. Crit Rev Oncol Hematol 49:153–163
Reid JM, Pendergrass TW, Krailo MD et al (1990) Plasma pharmacokinetics and cerebrospinal fluid concentrations of idarubicin and idarubicinol in pediatric leukemia patients: a Childrens Cancer Study Group report. Cancer Res 50:6525–6528
Fan N, Zhang L, Xu X et al (2017) Methotrexate plus idarubicin improves outcome of patients with primary central nervous system lymphoma. Oncotarget 8(32):53701–53713
Ferreri AJM, Blay J-Y, Reni M et al (2003) Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 21:266–272
Thiel E, Korfel A, Martus P et al (2010) High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 11:1036–1047
Bromberg JEC, Issa S, Bakunina K et al (2019) Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 20:216–228
Ferreri AJM, Cwynarski K, Pulczynski E et al (2016) Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 3:e217–e227
Ferreri AJ, Reni M, Foppoli M et al (2009) High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 374:1512–1520
Omuro A, Chinot O, Taillandier L et al (2015) Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol 2:e251–e259
Brezina T, von Dewitz H, Schroeder T et al (2022) First-line high-dose therapy and autologous blood stem cell transplantation in patients with primary central nervous system non-Hodgkin lymphomas-a single-centre experience in 61 patients. Ann Hematol 101:607–616
Gritsch D, Mrugala MM, Marks LA et al (2021) Is autologous stem cell transplantation a safe and effective alternative to whole brain radiation as consolidation therapy in patients with primary central nervous system lymphoma?: A critically appraised topic. Neurologist 26:137–142
Choi YS (2020) Recent advances in the management of primary central nervous system lymphoma. Blood Res 55:S58-s62
Seidel S, Margold M, Kowalski T et al (2021) Patients with primary central nervous system lymphoma not eligible for clinical trials: prognostic factors, treatment and outcome. Cancers (Basel) 13:2934
Fox CP, Phillips EH, Smith J et al (2019) Guidelines for the diagnosis and management of primary central nervous system diffuse large B-cell lymphoma. Br J Haematol 184:348–363
Yuan Y, Ding T, Wang S et al (2021) Current and emerging therapies for primary central nervous system lymphoma. Biomark Res 9:32
Li Q, Ma J, Ma Y et al (2021) Improvement of outcomes of an escalated high-dose methotrexate-based regimen for patients with newly diagnosed primary central nervous system lymphoma: a real-world cohort study. Cancer Manag Res 13:6115–6122
Acknowledgements
Not applicable.
Funding
Shanghai Shenkang Clinical Innovation Project (Project No. SHDC12020112). Beijing Medical and Health Foundation (Project No. YWJKJJHKB175B). Clinical Research Plan of SHDC (SHDC 2020CR6005-002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Data collection and data analysis were performed by QL and YM. The first draft of the manuscript was written by QL and BC, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing Li and Yan Ma are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Q., Ma, Y., Lin, Z. et al. A prospective cohort study of methotrexate plus idarubicin in newly diagnosed primary CNS lymphoma. J Neurooncol 163, 39–46 (2023). https://doi.org/10.1007/s11060-022-04062-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04062-z