Abstract
Reproductive strategies can have significant consequences for the viability of plant populations. Still, the effects of lower fruit set due to pollen limitation on plant demography and population persistence have rarely been explored. The objectives of this study were to assess the ecological factors determining female reproductive success and to study the impact of pollen limitation on population growth of Dracocephalum austriacum L. (Lamiaceae), a critically endangered species with a discontinuous distribution across Europe. Despite the significant background information gathered on the population dynamics and genetic diversity of D. austriacum, little is known about its reproductive strategy and the effect it has on population growth. Thus, the reproductive system, pollinator assemblage and pollen limitation were studied in natural populations and the impact of pollen-limited seed production on population growth was assessed using existing transition matrix models. The results revealed that D. austriacum is protandrous self-compatible species that produces very few seeds in the absence of pollinators. The flowers are visited by several insects, including legitimate pollinators (e.g., Bombus hortorum, Osmia spp.) and nectar robbers (other Bombus spp., O. aurulenta). Fruit and seed production was significantly pollen-limited in all populations studied. However, despite the positive effect of pollen supplementation on seed production, the resulting increase in seed number did not significantly increase population growth rates in any of the studied populations. Hence, we conclude that populations are demographically stable and current natural seed production is sufficient for the species’ persistence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss, fragmentation and overexploitation, biological invasions, and climate change are causing an ongoing decrease in the population numbers and sizes of many wild plant and animal species (Lande 1998; Dirzo and Raven 2003; Oostermeijer 2003). Frequently, changes in species diversity and abundance drive the disruption of mutualistic interactions, triggering a cascade of effects with major impacts on population persistence and ecosystem functioning (e.g., Bond 1994; Traveset and Richardson 2006). As a result, the number of threatened species continues to grow worldwide (IUCN 2012). Therefore, it is crucial to identify the biological traits and ecological and genetic processes that maintain (meta)population viability to ensure the survival of threatened species.
By its effects on demography and genetics, reproduction can have significant consequences for the viability of plant populations. In animal pollinated plants that rely on pollen vectors to set seeds, the abundance, efficiency and behaviour of flower visitors determine the quantitative and qualitative components of pollination and, consequently, the reproductive success of the plant. Pollen limitation, i.e., a quantity or quality of pollen inadequate for optimum seed set, has been described as a common feature of animal pollinated species (Ashman et al. 2004; Knight et al. 2005) and insufficient pollen receipt commonly reduces seed production in plant populations (e.g., Knight et al. 2005; Fernández et al. 2012). Pollen limitation can be a natural phenomenon, for example, many self-incompatible species have relatively low mean seed set, because not all pollen transferred by flower visitors is compatible. It can, however, also result from invasions by non-native species, reductions in plant or insect diversity, reduced population size and/or density, skewed sex or style morph ratios, and loss of S-alleles (Haig and Westoby 1988; Ashman et al. 2004; Eckert et al. 2010). Still, the consequences of pollen limitation are poorly understood (Larson and Barrett 2000; Ashman et al. 2004). Several studies have focused on the ecological and evolutionary causes of pollen limitation and its expected evolutionary consequences (Ashman et al. 2004; Eckert et al. 2010). However, few pollination studies have addressed if population growth is likely to suffer from a reduction in seed production caused by pollen limitation (Calvo and Horvitz 1990; Ehrlén and Eriksson 1995; Oostermeijer 2000). In particular in long-lived plants, seed production is only one component of the overall fitness, and generally has lower elasticity (i.e., contribution to the population growth rate) than adult survival (Franco and Silvertown 2004). To understand if changes in seed production due to pollen limitation affect the overall fitness, it is necessary to assess how variation in seed production affects the population growth rate of a population (Oostermeijer 2000; Münzbergová 2005). This can be achieved by estimation of vital rates of populations, such as fecundity, growth and survival, using transition matrix models (Caswell 2001). Studying the effect of pollen limitation on plant demography will help to understand the long-term consequences of pollen limitation for population persistence.
As a study system, we used Dracocephalum austriacum L. (Lamiaceae). This is a critically endangered species with a discontinuous distribution across Europe (Meusel et al. 1978; Council of European Communities 1992; Holub and Procházka 2000). Targeted on developing conservation measures, its population dynamics, genetic diversity and impacts of local habitat quality and climatic variation on population growth have recently been studied (Bonin et al. 2007; Dostálek et al. 2010; Nicolè et al. 2011; Andrello et al. 2012; Dostálek and Münzbergová 2013). Studies using transition matrix models performed in the Czech Republic and Slovakia showed that low seed production was a constant feature; seed production, together with seed germination and stasis and growth of small adult plants, was also the transition that contributed the most to variation in population growth rate (Dostálek and Münzbergová 2013). Furthermore, populations are relatively isolated and, consequently, genetically differentiated (Bonin et al. 2007; Dostálek et al. 2010). Despite the information gathered about this plant species so far, little is known about dependence on pollinator visitation and the effect of limited pollination services on seed production. The earlier demographic studies by Dostálek and Münzbergová (2013) enable a detailed evaluation of the effects of pollen limitation on population growth rates.
In this context, our objectives were (1) to study the reproductive system and pollination ecology of D. austriacum in natural populations to test whether or not female reproductive success, i.e., seed production, is reduced by limited pollination services, and (2) to assess whether lower female fitness due to pollen limitation affects long-term population growth. To accomplish (1), we performed controlled pollination experiments, assessed pollinator assemblage and determined female fitness under open and supplementary pollinations. To meet objective (2), we analysed the demographic effects of increased seed production through pollen supplementation on population growth rates using existing transition matrix models for the study populations (Dostálek and Münzbergová 2013).
Methods
Plant species
Dracocephalum austriacum is a perennial herb with erect or ascending stems up to 60 cm tall. The flowers are concentrated in inflorescences of one to several tens of flowers usually organized in whorls of six flowers each. They are attractive to flower-visiting insects, presenting inflorescences with several simultaneously open flowers. The flowers are large (44.9 ± 0.26 mm in length; mean ± SE) and zygomorphic, with a bilabiate corolla, as in most Lamiaceae. Nectar accumulates in the base of the long corolla tube (1.1 ± 0.04 μl with 36.1 ± 2.18 % of sugar, n = 26) and many flowers experience nectar robbing. The flowers have a relatively short lifespan (4 ± 0.2 days, n = 30). Flowering occurs from mid-May to mid-June. Typical for the family, the fruit is composed of four 1-seeded nutlets, which mature in June–July.
Dracocephalum austriacum grows on rocky steppes and rocky sunny slopes (Hrouda 2002) and it is a species of high conservation interest in Europe, listed in Annex II of the EU Habitat Directive (Council of European Communities 1992). In the Czech Republic it is considered critically endangered (Holub and Procházka 2000).
Study populations
The study was carried out in the spring of 2008 in the Protected Landscape Area of Czech Karst in the Czech Republic. This is an area with prevailing limestone bedrock covered with oak forests and with a rich biodiversity, in particular in the herbaceous layer. Eight populations of D. austriacum are located in this area, ranging in size from a few up to approximately 1100 adult individuals. Four populations were selected for this study: Císařská rokle (C1), a population composed of 210 adult individuals, situated on a rocky ridge on the slope of a ravine, 270 m a.s.l.; Haknovec (C2), the biggest population with approximately 1100 adult individuals, situated on the southern and south-eastern slope of Haknová mountain, 287 m a.s.l.; Kodská stěna (C4), a population comprising 180 adult individuals and situated on rocky steppes on the upper edge of Kodská stĕna, 350 m a.s.l.; and Velká hora (C8), a population composed of 630 adult individuals situated on rocky edges and ridges on the southern and south-eastern slope of Velká hora mountain, 320 m a.s.l. (for more details see Dostálek et al. 2010). The population C4 was used to investigate the breeding system of the species; all populations were used to study pollinators, quantify pollen limitation and construct transition matrix models except C8 for which no demographic information was available.
Breeding system
To determine the reproductive system and the consequences of insect exclusion and pollen source on the reproductive success of D. austriacum, the following treatments were applied in population C4: (1) spontaneous autogamy, flowers were bagged with mosquito net to exclude insects; (2) obligate autogamy, flowers were bagged and pollinated with their own pollen; (3) geitonogamy, emasculated flowers were bagged and pollinated with pollen sampled from other flowers on the same plant; (4) outcrossing within the population, emasculated flowers were bagged and pollinated with a fresh pollen mixture collected from 10 distinct plants from the same population; (5) outcrossing between populations, emasculated flowers were bagged and pollinated with a fresh pollen mixture collected from 10 distinct plants from the nearest population (population C1, 1 km apart from C4); (6) supplementary pollination, flowers were left open to natural levels of pollination and supplementary pollinated with a fresh pollen mixture collected from 10 distinct plants in the same population; (7) control, flowers without treatment left open to natural levels of pollination. For the experiment, 15 distinct plants were selected and one inflorescence bagged per plant to receive treatments 1–6 (2–3 flowers per treatment) and another left open to natural pollination (treatment 7). Treated flowers were followed daily until senescence and the corollas were collected in 70 % ethanol after flowers wilted and the corolla detached. Because the style detached from the ovary after fertilization or after the flower wilted, the styles of these flowers were subsequently collected and stored in 70 % ethanol to observe pollen tube development in the style. The styles were softened with 8 M sodium hydroxide for 30 min, stained with 0.05 % aniline blue for 2 h and squashed in a drop of 50 % glycerine (Dafni et al. 2005). Finally, they were observed through a Nikon Eclipse 80i epifluorescence microscope equipped with a UV-2A filter cube (330–380 nm excitation) and the pollen tubes in the style were counted. When nutlets were ripe, fruit set (number of flowers that matured at least one nutlet) and seed production (mean number of seeds produced per fruit) were recorded and seeds collected for seed weight measurement.
Finally, to assess the opportunities for selfing we studied the degree of separation between male and female structures (herkogamy) and the timing of pollen release and stigmatic receptivity (dichogamy). Herkogamy was assessed in corollas collected in the four populations and stored in 70 % ethanol, measuring the distance from the middle of the anthers to the base of the stigmatic lobes. To study pollen release, 30 flower buds from population C4 were marked and followed daily, recording availability of pollen in the anthers and stigmatic lobes opening from anthesis until senescence. Pollen availability was assessed by the presence of pollen grains in the anthers quantified after touching the anthers with a microscope slide, which was then observed under a microscope. The percentage of flowers with available pollen was calculated for each age class. To determine stigma receptivity over the course of the flower lifespan, fruit set was assessed in flowers pollinated with outcross pollen at different stages of anthesis. For this, prior to anthesis, flowers were emasculated, bagged and marked with the day of flower opening; all flowers were hand-pollinated with a fresh mixture of pollen collected from 10 distinct individuals; the hand-pollinations were made on flowers of 1 (n = 12), 2 (n = 17), 3 (n = 12), 4 (n = 12) and 5 days old (n = 12). Fruit set was recorded at maturity as the number of treated flowers that had at least one nutlet. The production of fruits was the indicator of stigma receptivity and the percentage of receptive stigmas was calculated for each age class.
The effect of the pollination treatment on the number of pollen tubes in the style and on seed weight was analysed with a one-way ANOVA. Differences among pollination treatments on fruit set and seed production were analysed using Generalized Linear Models (GLM), with fruit set (presence/absence of developed fruit) adjusted to a binomial distribution a logit link function to model responses, and seed production (number of seeds per fruit) adjusted to a Poisson distribution with a log link function. The effect of flower lifespan on pollen availability and stigma receptivity was also analysed using GLM with the dependent variables adjusted to a binomial distribution and a logit link function. Likelihood ratio tests were also performed in each analysed enabling to compare the full model with a restricted model where the explanatory variables of interest are omitted and calculating P values using the χ2 distribution. When significant differences were found, differences between levels of each effect (i.e., pollination treatments or along flower lifespan) were analysed using multiple comparisons of means with Tukey contrasts. All statistical analyses were carried in R version 2.14.2 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria) with the packages pscl and multcomp.
Levels of inbreeding depression (δ) were determined for fruit set, seed production and seed weight by calculating the relationship between the output of manually self-pollinated (w s ) and cross-pollinated flowers (w x ) as: δ = 1 − (w s /w x ) (Charlesworth and Charlesworth 1987).
Assemblage and foraging behaviour of flower visitors
To determine the assemblage and behaviour of flower visitors, direct observations were made in every population. The observations were performed during the flowering peak in 6 patches (except in population C1 due its small area where only 4 patches were monitored) of 2 m2 that had similar plant density, distributed in different places within the population. Each patch was characterized for the number of D. austriacum plants and number of open flowers. Visits were recorded during series of 15 min censuses at different hours of the day (from 09:00 to 17:00 h, GMT + 1). In total, 170 censuses were performed across the four populations, corresponding to a net observation time of 42 h 30 min. During each census the following variables were registered: visitor species, type of visit (pollinator—when touching the anthers and style; nectar robber—when accessing the nectar by an incision at the base of the corolla, not touching the sexual organs; Inouye 1980), and number of flowers visited per plant and patch. Specimens of each flower visitor were collected and photographed for identification (voucher specimens deposited at the CFE, University of Coimbra).
The frequency of interaction was calculated for each visitor species and population as a measure of visitor efficiency (Herrera 1989; Fishbein and Venable 1996) by multiplying insect abundance (number of individuals per 15 min) and flower visitation rate (number of flowers visited per 15 min; Herrera 1989). The variation in abundance of flower visitors among populations were analysed using GLM, with the dependent variable (i.e., visitor abundance) adjusted to a Poisson distribution and a log link function for model responses. Differences in visitation rate among populations were assessed using one-way ANOVA after square root transformation to achieve normality and homoscedasticity.
Pollen limitation
Pollen limitation was investigated in all the study populations. One inflorescence on each of 15 distinct plants received the treatments (6) and (7) described above in section ‘Breeding system’. In population C4, these treatments were included in the pollination experiments described above. When mature, fruit set and seed production were recorded and seeds collected for seed weight measurement. From each plant and treatment, 10 seeds (if available) were placed in Petri dishes with wet filter paper and kept in a growth chamber (12/12 h light/dark regime, at 20/5 °C day/night temperature and with intensity of irradiation at 120 µmol m−2 s−1). The tip of each seed was cut to facilitate water uptake. Seed germination was recorded as percentage of germinated seeds after 3 months. Germinated seeds were replanted to small pots and kept in the growth chamber. Seedling mortality was recorded as percentage of seedlings that died after another 3 months.
Differences between supplementary pollination and control were tested using GLM with fruit set, seed production, seed weight, seed germination and seedling mortality as dependent variables. Fruit set, seed germination and seedling mortality were adjusted to a binomial distribution with a logit link function, seed production to a Poisson distribution with a log link function, and seed weight to a Gaussian distribution with identity link function. Population and individual were taken as a covariate in the tests of the whole dataset. Differences between treatments within each population were also tested separately using the same approach.
Effect of pollen supplementation on population dynamics
Our previous study on population dynamics of D. austriacum (Dostálek and Münzbergová 2013) showed that seed production substantially contributed to variation in population growth rate (seed production was responsible for 14 % of total variation in population growth rate, survival in the stage of small adults for 16 %, growth from small to large adults for 16 % and germination from seeds for 24 %). The effects of pollen supplementation on population dynamics were thus tested using previously developed transition matrix models which were constructed using demographic data collected in 2003–2006 in three of the studied populations of D. austriacum (populations C1, C2, and C4, for details see Dostálek and Münzbergová 2013). In each population we collected demography data in approximately 200 plant individuals. The life-cycle of D. austriacum was divided into four stages: seeds (a 1), seedlings (a 2; plants with one thin vegetative stem up to 10 cm high), small adult plants (a 3; plants with one stem higher than 10 cm or with 2–5 stems) and large adult plants (a 4; plants with more than 5 stems) (Fig. 1). To project the consequences of the changes in seed production that result from pollen supplementation, we compared the original transition matrices with matrices incorporating increased seed production transitions. Seeds produced in year t could become in year t + 1 either part of the soil seed bank, or seedlings if they germinate after dispersal. Hence, we specifically modified matrix elements of seed and seedling production by small and large adult plants (a 13, a 23 and a 14, a 24; a ij denote probabilities of transitions from stage j in year t to stage i in the next year, t + 1; Fig. 1) by multiplying them with index ΔS. ΔS = S PS/S C, where S PS is mean seed production per flowering stem treated with pollen supplementation and S C is mean seed production per flowering stem used as control (i.e., after natural pollination). Seed production was calculated per flowering stem, which enabled inclusion of the combined effect of pollen supplementation on both seed set and fruit set. We did not modify germination rate and seedling survival because we did not find significant consistent effect of pollen supplementation on these traits (see “Results” below).
Life-cycle of Dracocephalum austriacum, representing mean probabilities of reproduction (broken lines) and transitions of individuals between stages (solid lines). a ij denote probabilities of transitions from stage j in year t to stage i in the next year, t + 1. Probabilities were calculated as means over years 2003–2006 over three studied populations (C1—Císařská rokle; C2—Haknovec; C4—Kodská stěna). For further details see Dostálek and Münzbergová (2013)
To calculate the population growth rates and their confidence intervals of both the original matrices and the matrices with pollen supplementation we used a script developed by Münzbergová (2006, 2007) which works on statistic software MATLAB version 7.3.0.267 (The MathWorks, Inc., Natick, Massachusetts, USA). The MATLAB script bootstraps the single data points (individuals) used to create the transition matrices. These confidence intervals enable estimation of the variation in population growth rate and enable us to compare the differences between the two matrices (with and without pollen supplementation) (Caswell 2001). Stochastic population growth rates (λ S) were calculated for each of the studied populations by randomly selecting one of three annual matrices with equal probability for each of 105 model iterations and calculating the geometric mean of the acquired annual growth rates (Caswell 2001). Using this approach we incorporated temporal stochasticity into the model as well.
Results
Breeding system
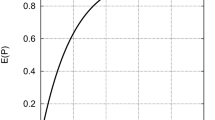
Flower functioning and sexual organ separation did not seem to prevent the occurrence of self-pollination. Pollen dehiscence occurred mainly on days 1–2 and decreased significantly with flower age (\( \chi_{4,150}^{2} \) = 75.13, P < 0.001), while stigma receptivity increased until it reached a peak at day 3 (\( \chi_{4,65}^{2} \) = 20.59, P < 0.001; Fig. 2). Despite the temporal separation of male and female phases (protandrous species), there is still a temporal overlap between both functions that may allow spontaneous and/or visitor-mediated self-pollination. Additionally, we observed a small extent of spatial separation between stigma and anthers (1.4 ± 0.07 mm, n = 139), which are displayed in the upper part of the corolla throat opening readily accessible to pollinators.
Pollen availability (open circles) and stigma receptivity (closed circles) along the lifespan of Dracocephalum austriacum flowers. Different letters reveal statistically significant differences at P < 0.05 after multiple comparisons of means with Tukey contrasts. Broken lines represent the trends of stigma receptivity and pollen availability along the flower lifespan
Pollination treatments significantly affected the number of developed pollen tubes in the style, fruit set, seed production and seed weight (Table 1). A reduced number of pollen tubes and low fruit set and seed production were observed after spontaneous autogamy (Table 1). Despite not significant, obligatory autogamy had lower outcomes for all the response variables than geitonogamy (Table 1). These results might constitute a real trend considering that the species is protandrous (Fig. 2), lowering the fitness of obligatory autogamy in comparison with geitonogamy due to a temporal separation of male and female functions (Fig. 2). Geitonogamous pollinations led to similar results as cross pollinations (for pollen tube development, fruit set and seed production) and indicate the absence of a self-incompatibility system.
The reproductive variables after cross pollination between individuals of the same population (outcrossing within population) and between individuals of different populations (outcrossing between population) were similar (Table 1). Inbreeding depression was low for all the reproductive variables (δ Fruit set = 0.26, δ Seed production = 0.38, δ Seed weight = 0.13). Still, the level of inbreeding depression varied greatly at the individual level (ranging from −0.50 to 1.00, −0.14 to 1.00 and −0.10 to 0.50 for fruit set, seed production and seed weight, respectively). Also, seed mass after geitonogamous pollination was less than half of seed mass after cross pollination within population (for example, 100 hypothetical flowers yield a seed mass of 208.8 mg after geitonogamy versus 521.7 mg after outcrossing). Under natural conditions (control treatment) fruit set was lower than in the supplementary pollination treatment (Table 1; see details below).
Assemblage and foraging behaviour of flower visitors
Despite some differences in the overall assemblage, the localities studied shared the main pollinators (Table 2). Dracocephalum austriacum was mainly visited by several species from the genera Bombus and Osmia (Table 2). Species of these two genera were the main pollinators in populations C4 and C1; in population C8 the main pollinators were Osmia spp.; and in C2, Bombus and Anthophora were the main pollinators (Table 2). The flower visitors were observed collecting nectar and/or pollen and showed different behaviours (Table 2); they collected pollen after landing on the lower lip and/or collected nectar either by a legitimate visit (i.e., insertion of the proboscis through the corolla throat) or by making incisions at the base of the corolla tube (nectar robbing; Inouye 1980). Only in the first two cases, the visitor contacted the sexual organs and pollinated the flower. Nectar robbing was frequently observed and undertaken mainly by Bombus pascuorum in population C2 and by Osmia aurulenta in the other populations (Table 2). No differences among populations were observed in the abundance of flower visitors (\( \chi_{ 3, 1 70}^{2} \) = 2.57, P = 0.463) and visitation rates (F 3,170 = 1.25, P = 0.293).
Pollen limitation
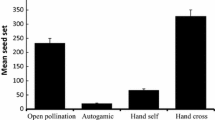
Strong evidence for pollen limitation affecting fruit and seed production was found in the populations studied (Fig. 3), i.e., there was significantly higher production of fruits and seeds in open pollinated flowers supplemented with pollen as compared to the open pollinated flowers used as controls (\( \chi_{1, 1 2 5 9}^{2} \) = 39.53, P < 0.001 for fruit set; Fig. 3a, and \( \chi_{1, 1 2 5 9}^{2} \) = 39.84, P < 0.001 for seed production; Fig. 3b). Still, fruit set after supplementary pollination was only 64.9 % in population C1 (while it was >90.0 % in the others) and for all populations seed production was low, ranging from 1.3 to 2.4 seeds per flower, indicating that more factors than pollen limitation are affecting female reproductive success.
Pollen limitation in the populations of Dracocephalum austriacum studied. Effect of pollen supplementation on: a fruit set (percentage of flowers that matured at least one nutlet); b seed production (mean number of seeds per fruit); c seed germination; and d seedling mortality. Pollination treatments: control—open pollinated flowers (white bars), pollen supplementation—open pollinated flowers supplemented with outcrossing pollen (grey bars); populations: C1—Císařská rokle; C2—Haknovec; C4—Kodská stěna; C8—Velká hora. Values are given as mean and standard error of the mean; sample sizes for both variables are provided inside the bars of fruit set; asterisks indicate significant differences between treatments within populations after GLM analyses: *P < 0.05; **P < 0.01; ***P < 0.001; n.s. non-significant
No significant differences between supplemented and open pollinated treatments were found for seed weight (\( \chi_{1, 5 7 4}^{2} \) = 0.47, P = 0.495; Fig. 3c). Similar results were obtained for seed germination (\( \chi_{1, 5 5 4}^{2} \) = 2.14, P = 0.144) when analysing the data altogether; however, there were significant differences in seed germination within single populations, with open pollinated treatment having significantly lower seed germination in populations C1 and C2 (and despite not significant also in population C4), but the opposite trend in population C8 (Fig. 3d). We also recorded significantly lower seedling mortality after pollen supplementation (\( \chi_{1,266}^{2} \) = 4.69, P = 0.030), however it was not significant when analysed within single populations (Fig. 3e).
Effect of pollen supplementation on population dynamics
The mean increase in seed production per flowering stem after pollen supplementation (ΔS) relative to controls was 1.96, 1.53 and 2.75 in populations C1, C2 and C4, respectively. All stochastic population growth rates tended to be higher for the pollen supplementation treatment. However, 95 % confidence intervals of λ S overlapped between matrices from pollen supplemented and control treatments in each population, which indicates that population growth rate did not increase much as a consequence of pollen supplementation (Fig. 4). None of the stochastic population growth rates significantly decreased below 1.
Expected effect of pollen supplementation on population growth rate in populations of Dracocephalum austriacum. Pollination treatments: control—open pollinated flowers (white bars), pollen supplementation—open pollinated flowers supplemented with outcrossing pollen (grey bars); populations: C1—Císařská rokle; C2—Haknovec; C4—Kodská stěna. Values are given as stochastic population growth rate (λ S) and its 95 % confidence interval
Discussion
Reproductive strategy
The pollination experiments revealed that D. austriacum is a self-compatible species with low ability to autonomously self-fertilize, thus, depends on pollen vectors to mediate most of the pollen transfer. This strategy is similar to the congeneric D. ruyschiana (Milberg and Bertilsson 1997), but contrasts with other species from Lamiaceae, in which delayed selfing is a common mechanism for reproductive assurance (Navarro 1997; Sales et al. 2010). The low ability to self-fertilise autonomously is most probably due to the combination of different levels of herkogamy (i.e., spatial separation of male and female organs) and temporal separation of male and female functions (protandry). The ability to autonomously self-fertilise, albeit reduced, might be advantageous under certain scenarios such as unpredictable pollination services in fragmented landscapes (Navarro 1997; Lennartsson 2002), but will also bear its costs on future survivorship and reproduction due to inbreeding depression (Morgan et al. 1997; Lennartsson 2002). The relative contribution of selfing and outcrossing will depend on the relative positions of anthers and stigma (Classen-Bockhoff 2007), the maturation schedules of male and female phases (Navarro 1997; Zhang et al. 2011), the number of flowers simultaneously open in an individual and on the behaviour of pollinators (mixed mating systems; Goodwillie et al. 2005).
Cross pollination within and between populations also shed some light on the inbreeding and outbreeding depression. First generation crosses between inbred populations may lead to higher reproductive output as a result of heterosis (Leimu et al. 2006). They can also lead to lower seed set if there are genetic incompatibilities between the populations, e.g., resulting from differences in paternal and maternal environment (Dudash and Fenster 2000; Fenster and Galloway 2001; Galloway 2001). Reproductive success of crosses between populations was not significantly different from crosses within populations, suggesting that there is neither early-acting outbreeding depression nor heterosis. Considering the genetic differentiation found among the populations of this species (Bonin et al. 2007; Dostálek et al. 2010, including also the populations of the present study), future studies need to follow the progeny of these crosses in subsequent generations in the parental populations to fully understand the degree of outbreeding and, thus gain further insights on local adaptation of the populations.
Floral visitors and reproductive success
Regardless of the mating system, D. austriacum largely relies on pollen vectors to achieve pollination. The main legitimate pollinators of D. austriacum were Bombus hortorum and Osmia species but up to 16 different taxa were observed visiting this plant, a broader spectrum than D. ruyschiana (Milberg and Bertilsson 1997). Some differences were observed in the pollinator assemblage but, overall, populations shared the main flower visitors, each species varying in their relative abundance within the population. These differences in insect composition and abundance are common in nature and can be due to micro-habitat preferences by pollinators, including food resource composition, microclimatic variables, nesting places among others (Herrera et al. 2002; Moeller 2005). Still, the overall insect abundance and visitation rates did not differ significantly between populations, despite the significant differences in reproductive success among populations. The latter may result from differences in the efficiency or behaviour of the main pollinators (abundances of which varies among populations) (Herrera 1989; Fishbein and Venable 1996). Indeed, among the floral visitors observed, nectar robbers were a steady feature in all the populations studied. The variable behaviour of nectar robbers will result in different contributions to pollination and, possibly, in different impacts in the behaviour of efficient pollinators and in the reproductive success of the plant (Castro et al. 2008; Irwin et al. 2010).
Pollen availability and quality is one of the main determinants of female reproductive success (Haig and Westoby 1988; Griffin and Barrett 2002). Indeed, pollen limitation has been shown to be a widespread phenomenon among animal-pollinated species (Burd 1994; Ashman et al. 2004; Knight et al. 2005). Our pollen supplementation experiments revealed that in all populations, pollen limitation reduced the amount of fruits and seeds and that the degree of pollen limitation varied among populations. These results reflect limited pollination services by floral visitors and differences in pollinator assemblages, relative abundances and/or behaviour (and consequently, pollination efficiency) among populations described above. Differences in insect composition and abundance are common in nature and can be due to differences in micro-habitat preferences by pollinators, as described above (Herrera et al. 2002; Moeller 2005). However, the final reproductive success might also be determined by other factors, such as resource availability and resource allocation (Griffin and Barrett 2002; Wesselingh 2007). This was most probable affecting the production of fruits in population C1, but also the production of seeds in all populations, which remained below 2.4 seeds per fruit (out of 4 ovules) even after supplementary pollination.
Alternatively, the low reproductive success after pollen supplementation (e.g., in population C1) could also be associated with a high inbreeding coefficient (Dostálek et al. 2010; but see Nicolè et al. 2011). However, low inbreeding depression was observed in the present study. The low inbreeding depression observed could be due to the fact that (i) seedlings were grown in a growth chamber, and (ii) only performance in the first stages of the life-cycle was measured, stages that could be free of inbreeding depression. Inbreeding depression is generally expressed most in the more stressful natural environment and/or in later developmental stages (e.g., Dudash 1990; Fenster and Galloway 2001; Willi et al. 2007; Raabová et al. 2009). In addition, the populations of D. austriacum studied were relatively big, whereas inbreeding depression might have started to play a major role in smaller populations (Dostálek et al. 2010). Furthermore, when analysing the data at the individual level, plants revealed variable degrees of inbreeding, most probably resulting from the mixed mating strategy observed in this species.
Pollen limitation and population performance
The question that remains is whether the lower fruit set observed in all population studied has consequences for population persistence. Seed production is definitely one of the life-cycle transitions with substantial contribution to the variation in population growth in D. austriacum (Dostálek and Münzbergová 2013). Consequently, lower fruit-to-flower ratios due to pollen limitation are expected to have effects on plant demography and population persistence. Still, only a small set of studies used a demographic approach in pollination studies to understand the long-term consequences of pollen limitation in endangered species (Bierzychudek 1981; Calvo and Horvitz 1990; Calvo 1993; Ehrlén and Eriksson 1995; Parker 1997; García and Ehrlén 2002; Knight 2004; Price et al. 2008; Horvitz et al. 2010; Law et al. 2010). In the current study, we observed that pollen supplementation tended to have positive effects on population growth rates. However, because of the sampling variance in lambda, the growth rates after natural pollination and pollen supplementation were similar for the three studied populations. Furthermore, all studied populations were demographically stable (Dostálek and Münzbergová 2013), which means that natural seed production was already high enough for population persistence. Non-significant differences in population growth rates after pollen supplementation were also reported, for example in Lathryrus vernus (Ehrlén and Eriksson 1995; but see also Horvitz et al. 2010), Primula veris (García and Ehrlén, 2002) and Trillium grandiflorum (Knight 2004). Pollen supplementation may have no impact on population growth rates either because of trade-offs between amount and quality of the offspring or because it constitutes a significant cost of reproduction for the plants. For example, several studies revealed positive effects of pollen supplementation on seed production and subsequently on population growth but, at the same time, pollen supplementation significantly reduced flower, fruit and seed production in subsequent years, and thus had no long-term effects on population growth (Bierzychudek 1982; Calvo 1993; Ehrlén and Eriksson 1995). Indeed, despite the initial assumptions, reduced seed production due to pollen limitation does not necessarily have to result in demographic consequences for the population. This is the case of long-lived species with multiple reproductive episodes, where the sensitivity of the population to increased seed set might be low in comparison with other vital rates of the life-cycle, such as, adult survival (Ashman et al. 2004; Feldman and Morris 2011). On the contrary, in monocarpic species the elasticity values for fecundity are high and lower seed production due to limited pollination services, even if low, is expected to have significant consequences on population growth and persistence (Franco and Silvertown 2004; Ramula 2008; Law et al. 2010). All the above emphasises the importance of using demographic approaches to assess the real impact of some events that are assumed to affect population growth and persistence, such as pollen limitation or herbivory, but also reveal the importance to go further beyond the results obtained in this manuscript and include the impacts of such events in future performances of the plant.
Conclusion
The present study provides insight into the reproductive biology and pollination ecology of the critically endangered D. austriacum, and showed that a relatively low level of seed production might be fully natural and does not need to be detrimental to population viability (see also, for example, Ashman et al. 2004; Tomimatsu and Ohara 2006). Because of this, studies on reproduction and pollen limitation should preferably include demographic monitoring and modelling to evaluate the effects of observed changes on population growth rates. In addition, experiments with in- and outbred seeds should preferably be included to study the demographic consequences of the synergistic interaction between the Allee-effect and inbreeding depression (Oostermeijer 2000).
References
Andrello M, Nicolè F, Till-Bottraud I, Gaggiotti OE (2012) Effect of stage-specific vital rates on population growth rates and effective population sizes in an endangered iteroparous plant. Conserv Biol 26:208–217
Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DR, Dudash MR, Johnston MO, Mazer SJ, Mitchell RJ, Morgan MT, Wilson WG (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421
Bierzychudek P (1981) Pollinator limitation of plant reproductive effort. Am Nat 117:838–840
Bierzychudek P (1982) The demography of jack-in-the-pulpit, a forest perennial that changes sex. Ecol Monogr 52:335–351
Bond WJ (1994) Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philos Trans R Soc B Biol Sci 344:83–90
Bonin A, Nicolè F, Pompanon F, Miaud C, Taberlet P (2007) Population adaptive index: a new method to help measure intraspecific genetic diversity and prioritize populations for conservation. Conserv Biol 21:697–708
Burd M (1994) Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83–139
Calvo RN (1993) Evolutionary demography of orchids: intensity and frequency of pollination and the cost of fruiting. Ecology 74:1033–1042
Calvo RN, Horvitz CC (1990) Pollinator limitation, cost of reproduction, and fitness in plants: a transition matrix demographic approach. Am Nat 136:499–516
Castro S, Silveira P, Navarro L (2008) Consequences of nectar robbing for the fitness of a threatened plant species. Plant Ecol 199:201–208
Caswell H (2001) Matrix population models: construction, analysis, and interpretation. Sinauer Associates, Sunderland
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst 18:237–268
Classen-Bockhoff R (2007) Floral construction and pollination biology in the Lamiaceae. Ann Bot Lond 100:359–360
Council of European Communities (1992) Council Directive 92/43/EEC of 21 May on the conservation of natural habitats and of wild fauna and flora. Off J Eur Commun 35:7–50
Dafni A, Kevan P, Husband BC (2005) Practical pollination biology. Enviroquest Ltr, Cambridge
Dirzo R, Raven PH (2003) Global state of biodiversity and loss. Annu Rev Environ Res 28:137–167
Dostálek T, Münzbergová Z (2013) Comparative population biology of critically endangered Dracocephalum austriacum L. in two distant regions. Folia Geobot 48:75–93
Dostálek T, Münzbergová Z, Plačková I (2010) Genetic diversity and its effect on fitness in an endangered plant species, Dracocephalum austriacum L. Conserv Genet 11:773–783
Dudash M (1990) Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution 44:1129–1139
Dudash M, Fenster C (2000) Inbreeding and outbreeding depression in fragmented populations. In: Young A, Clarke G (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge, pp 35–53
Eckert CG, Kalisz S, Geber MA, Sargent R, Elle E, Cheptou P-O, Goodwillie C, Johnston MO, Kelly JK, Moeller DA, Porcher E, Ree RH, Vallejo-Marin M, Winn AA (2010) Plant mating systems in a changing world. Trends Ecol Evol 25:35–43
Ehrlén J, Eriksson O (1995) Pollen limitation and population growth in a herbaceous perennial legume. Ecology 76:652–656
Feldman TS, Morris WF (2011) Higher survival at low density counteracts lower fecundity to obviate Allee effects in a perennial plant. J Ecol 99:1162–1170
Fenster C, Galloway L (2001) Inbreeding and outbreeding depression in natural populations of Chamaecrista fasciculata (Fabaceae). Conserv Biol 14:1406–1412
Fernández JD, Bosch J, Nieto-Ariza B, Gómez JM (2012) Pollen limitation in a narrow endemic plant: geographical variation and driving factors. Oecologia 170:421–431
Fishbein M, Venable DL (1996) Diversity and temporal change in the effective pollinators of Asclepias tuberosa. Ecology 77:1061–1073
Franco M, Silvertown J (2004) A comparative demography of plants based upon elasticities of vital rates. Ecology 85:531–538
Galloway LF (2001) The effect of maternal and paternal environments on seed characters in the herbaceous plant Campanula americana (Campanulaceae). Am J Bot 88:832–840
García MB, Ehrlén J (2002) Reproductive effort and herbivory timing in a perennial herb: fitness components at the individual and population levels. Am J Bot 89:1295–1302
Goodwillie C, Kalisz S, Eckert CG (2005) The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annu Rev Ecol Evol Syst 36:47–79
Griffin SR, Barrett SCH (2002) Factors affecting low seed: ovule ratios in a spring woodland herb, Trillium grandiflorum (Melanthiaceae). Int J Plant Sci 163:581–590
Haig D, Westoby M (1988) On limits to seed production. Am Nat 131:757–759
Herrera CM (1989) Pollinator abundance, morphology, and flower visitation rate: analysis of the quantity component in a plant–pollinator system. Oecologia 80:241–248
Herrera CM, Cerdá X, García MB, Guitián J, Medrano M, Rey PJ, Sánchez-Lafuente AM (2002) Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. J Evol Biol 15:108–121
Holub J, Procházka F (2000) Red list of vascular plants of the Czech Republic. Preslia 72:187–230
Horvitz CC, Ehrlén J, Matlaga D (2010) Context-dependent pollinator limitation in stochastic environments: can increased seed set overpower the cost of reproduction in an understorey herb? J Ecol 98:268–278
Hrouda L (2002) Dracocephalum austriacum L. In: Kubát K, Hrouda L, Chrtek JJ, Kaplan Z, Kirschner J, Štěpánek J (eds) Klíč ke květeně České republiky (Key to the flora of the Czech Republic). Academia, Prague, p 592
Inouye DW (1980) The terminology of floral larceny. Ecology 61:1251–1253
Irwin RE, Bronstein JL, Manson JS, Richardson L (2010) Nectar Robbing: ecological and evolutionary perspectives. Annu Rev Ecol Evol Syst 41:271–292
IUCN (2012) IUCN red list of threatened species, version 2012.2. http://www.iucnredlist.org. Accessed September 2012
Knight TM (2004) The effect of herbivory and pollen limitation on a declining population of Trillium grandiflorum. Ecol Appl 14:915–928
Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman TL (2005) Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497
Lande R (1998) Anthropogenic, ecological and genetic factors in extinction and conservation. Res Popul Ecol 40:259–269
Larson BMH, Barrett SCH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linnean Soc 69:503–520
Law W, Salick J, Knight TM (2010) The effects of pollen limitation on population dynamics of snow lotus (Saussurea medusa and S. laniceps, Asteraceae): threatened Tibetan medicinal plants of the eastern Himalayas. Plant Ecol 210:343–357
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? J Ecol 94:942–952
Lennartsson T (2002) Extinction thresholds and disrupted plant–pollinator interactions in fragmented plant populations. Ecology 83:3060–3072
Meusel H, Jäger E, Rauschert S, Weinert E (1978) Vergleichende Chorologie der zentraleuropäischen Flora—Karten. Gustav Fischer Verlag, Jena (in German)
Milberg P, Bertilsson A (1997) What determines seed set in Dracocephalum ryuschiana L. an endangered grassland plant. Flora 192:361–367
Moeller DA (2005) Pollinator community structure and sources of spatial variation in plant–pollinator interactions in Clarkia xantiana ssp. xantiana. Oecologia 142:28–37
Morgan MT, Schoen DJ, Bataillon TM (1997) The evolution of self-fertilization in perennials. Am Nat 150:618–638
Münzbergová Z (2005) Determinants of species rarity: population growth rates of species sharing the same habitat. Am J Bot 92:1987–1994
Münzbergová Z (2006) Effect of population size on the prospect of species survival. Folia Geobot 41:137–150
Münzbergová Z (2007) Population dynamics of diploid and hexaploid populations of a perennial herb. Ann Bot Lond 100:1259–1270
Navarro L (1997) Is the dichogamy of Salvia verbenaca (Lamiaceae) an effective barrier to self-fertilization? Plant Syst Evol 207:111–117
Nicolè F, Dahlgren JP, Vivat A, Till-Bottraud I, Ehrlén J (2011) Interdependent effects of habitat quality and climate on population growth of an endangered plant. J Ecol 99:1211–1218
Oostermeijer JGB (2000) Population viability analysis of the rare Gentiana pneumonanthe: the importance of genetics, demography and reproductive biology. In: Young A, Clarke G (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge, pp 313–334
Oostermeijer JGB (2003) Threats to rare plant persistence. In: Brigham C, Schwartz M (eds) Population viability in plants: conservation, management, and modeling of rare plants. Springer-Verlag, Heidelberg, pp 17–58
Parker IM (1997) Pollinator limitation of Cytisus scoparius (Scotch broom), an invasive exotic shrub. Ecology 78:1457–1470
Price MV, Campbell DR, Waser NM, Brody AK (2008) Bridging the generation gap in plants: pollination, parental fecundity, and offspring demography. Ecology 89:1596–1604
Raabová J, Münzbergová Z, Fischer M (2009) Consequences of near and far between-population crosses for offspring fitness in a rare herb. Plant Biol 11:829–836
Ramula S (2008) Population dynamics of a monocarpic thistle: simulated effects of reproductive timing and grazing of flowering plants. Acta Oecol 33:231–239
Sales F, Hedge IC, Christie F (2010) Salvia plebeia R. Br.: taxonomy, phytogeography, autogamy and myxospermy. Pak J Bot 42:99–110
Tomimatsu H, Ohara M (2006) Evaluating the consequences of habitat fragmentation: a case study in the common forest herb Trillium camschatcense. Popul Ecol 48:189–198
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216
Wesselingh RA (2007) Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytol 174:26–37
Willi Y, van Kleunen M, Dietrich S, Fischer M (2007) Genetic rescue persists beyond first-generation outbreeding in small populations of a rare plant. Proc R Soc B Biol Sci 274:2357–2364
Zhang Y-W, Zhao J-M, Wang Y (2011) The dynamics of pollen removal and deposition, and its effects on sexual phases in a protandrous plant: Glechoma longituba (Lamiaceae). Nord J Bot 29:105–111
Acknowledgments
This study was supported by GAČR P505/10/0593, postdoc GAČR project 13-10850P, by a long-term research development project no. RVO 67985939, institutional project MŠMT and by FCT and European Social Fund with the fellowship FCT/BPD/41200/2007 and IF/01267/2013 Starting grant to SC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, S., Dostálek, T., van der Meer, S. et al. Does pollen limitation affect population growth of the endangered Dracocephalum austriacum L.?. Popul Ecol 57, 105–116 (2015). https://doi.org/10.1007/s10144-014-0458-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-014-0458-x