Abstract
The effects of habitat fragmentation on remnant plant populations have rarely been studied extensively using a single species. We have attempted to quantify the effects of forest fragmentation (primarily that of population size) on populations of Trillium camschatcense, a representative spring herb in the Tokachi plain of Hokkaido, Japan. In this region, intensive agricultural development over the past 100 years has divided once-large, continuous populations of this species into small, isolated fragments. Small populations generally produced fewer seeds than large populations, although this result differed between years. The level of seed production is unlikely to explain demographic structures based on life-history stages. Instead, the stage structure was better explained by population size, seedling recruitment being limited in smaller populations. This could be associated with edge effects because the stage structure in small populations corresponded well to that observed in forest edges, where altered microclimatic conditions strongly limit seedling recruitment. Small populations also experienced stochastic loss of rare alleles at allozyme loci as well as biparental inbreeding. Although one consequence of these changes is reduced fertility, the long-term effects on population growth can be controversial in long-lived forest herbs, since the negative effect on fertility may vary across years, and population growth rate may not be sensitive to changes in fertility. Further studies of long-term demography will reveal whether and how habitat fragmentation could limit population growth of remnant populations more than a century after fragmentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Destruction of natural habitat by humans leads to a reduction in total habitat area (i.e., habitat loss) as well as a change in habitat configuration (i.e., habitat fragmentation; Fahrig 2003). Whereas habitat loss undoubtedly has a large negative impact on biodiversity, many studies have suggested that habitat fragmentation can pose an important additional threat (e.g., Lindborg and Eriksson 2004; Verheyen et al. 2004). Fragmentation reduces the size of individual habitat remnants, increases their spatial isolation, thereby altering abiotic and biotic environmental conditions (Wilcove et al. 1986; Saunders et al. 1991), all of which can affect many fitness components of plants. Although interest in habitat fragmentation has increased over the past 15 years (citations of keywords “habitat fragmentation” in the ISI Web of Science database, 1980–1984, 1; 1985–1989, 6; 1990–1994, 99; 1995–1999, 471; 2000–2004, 1102), the effects of fragmentation have rarely been studied extensively using a single species.
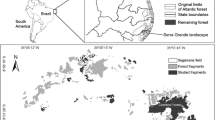
This paper provides an overview of how we have quantified the effects of habitat fragmentation in Trillium camschatcense Ker Gawler (Trilliaceae or Melanthiaceae; Table. 1), a representative forest herb in northern Japan. In the Tokachi plain of eastern Hokkaido, intensive land development since the 1880s has resulted in a highly fragmented landscape with a large number of forest remnants, many of which are smaller than 1 ha (Fig. 1). T. camschatcense is now often found in the understory of small remnant forests surrounded by pastureland or agricultural fields. Because this species commonly occurs in the region and originally forms large (>5 ha) populations, we can assume that once-large, continuous populations have been divided into small, isolated pieces due to forest fragmentation. Here we focus on three subjects related to our research: (1) reduced pollination and seed production, (2) edge effects on recruitment, and (3) loss of genetic diversity and inbreeding. In our research, we consider primarily the effect of remnant population size and also that of the condition of the surrounding landscape. In the final section of this paper, we summarize the research findings and their implications for conservation, and discuss the effects of fragmentation on the long-term persistence of remnant populations.
Forest fragmentation process on two typical landscapes in the Tokachi plain, Hokkaido, Japan, during the past 100 years. a Mainly residential area, b mainly agricultural area. Forest area (black) had decreased to <5% due to anthropogenic land conversion since the 1880s, when the Japanese government encouraged farmers to emigrate from the mainland. Figure courtesy of Yasuo Konno
Although T. camschatcense is not considered as endangered in the foreseeable future, there are several reasons why we have studied this still abundant species. First, common species should be primary contributors to ecosystem productivity. Changes in abundance of common species are likely to cause significant changes in the abundance or extinction of other ecologically linked species (Simberloff 1998). Second, it is increasingly recognized that remnant plant populations respond slowly to changes in landscape structure (Lindborg and Eriksson 2004; Helm et al. 2006), thus, under the deterministic effects of fragmentation, even still abundant species may decline to extinction long after fragmentation (“extinction debt”; sensu Tilman et al. 1994). Finally, a metapopulation model considering a community of species predicts that, after habitat destruction and fragmentation, common species are the first to become extinct (Hanski and Ovaskainen 2002). Although we are unaware of any study testing this prediction, this model motivated us to evaluate the consequences of habitat fragmentation for common species.
Reduced pollination and seed production
Reduced reproduction is the best documented impact of fragmentation in animal-pollinated plants. Because small populations may be less attractive to pollinators than large populations (Sih and Baltus 1987), a reduction in population size results in decreased fruit and seed production due to insufficient pollen transfer. Moreover, in small populations, low genetic variation at the self-incompatibility (S) locus may limit the availability of compatible mates, thereby limiting seed production (Byers and Meagher 1992; Young et al. 2000). The availability of pollinators can also be affected by the landscape that surrounds a focal population. For example, because pollinators need flowering plant species continuously over their entire life-span, and often have specific nesting requirements (Kearns and Inouye 1997), a limited quantity of available habitat for pollinators may decrease pollinator abundance and activity, thus lowering reproductive output in plants. Jennersten (1988) showed that, due to decreased pollinator visitation, flowering individuals of maiden pink (Dianthus deltoides) set fewer seeds in a fragmented habitat than in a continuous habitat. Since that time, many investigations have tested these expectations based on a variety of fragmentation measures, such as population size and isolation. Hobbs and Yates (2003) reviewed studies of 60 species and found significant declines in reproductive success in 58% of cases (n=85; measurements were taken in more than 1 year for some species). Aizen et al. (2002) found that the incidence of negative effects on reproduction was independent of species breeding system and pollination specialization (i.e., the number of taxonomically similar animal species that plants depend on for pollination). A major criticism of previous research is that most studies have been conducted for only 1 year (Hobbs and Yates 2003), so that we still understand little about temporal variations in fragmentation effects.
Using 14 populations of T. camschatcense, we examined the effects of population size and surrounding landscape conditions on seed production for two consecutive years (Tomimatsu and Ohara 2002). Population size, defined as the estimated number of flowering plants, was determined by multiplying population area by flowering plant density (range =4–153,600). The surrounding landscape type was evaluated by classifying the study populations into two types: continuous and isolated. All the populations were distant from other T. camschatcense populations, so we did not consider the effect of population isolation. We found a significantly positive relationship between population size and seed production in 1999 but not in 1998 (Fig. 2). However, note that small populations of <50 flowering plants consistently produced fewer seeds in both years. Although the effect of landscape type was not statistically significant, “continuous” populations with abundant forest series nearby tended to produce more seeds than “isolated” populations located in small, isolated forests. These results indicate that small, fragmented populations produce fewer seeds than large, continuous populations, although the effect differed between the 2 years of study. The decrease in seed production could be attributed to pollen limitation, because the outcross pollen load was positively associated with seed production and hand-outcrossing increased seed production.
The number of seeds per flower was associated with the size of populations of Trillium camschatcense in 1999, but not in 1998. Two landscape types, continuous and isolated, were discriminated to describe the quantity and distribution of forests around populations; although not significant, “continuous” populations with nearby abundant forest series tended to produce more seeds than “isolated” populations located in small, isolated forests. These data are from 14 different populations: 12 in 1998 and 13 in 1999. A summary of results from analyses of covariance with population size (covariate) and landscape type (categorical, fixed effect) as main effects is also shown (*P<0.05). The solid line in 1999 indicates the regression line for all 13 populations where the regression coefficient was significantly different from zero. Redrawn from Tomimatsu and Ohara (2002)
The consequences of reduced seed production have rarely been examined (Ward and Johnson 2005). As this species is long-lived, we observed the stage structure (relative frequency of four growth stages) in six populations of different size (Fig. 3). The stage structure in small populations was characterized by a lower frequency of seedlings and a higher frequency of three-leaf plants. In populations of <220 flowering plants, almost no seedling recruitment was observed. However, these indices were highly correlated with population size (seedling, Kendall’s τ=0.97; three-leaf, τ=−1.00) but not with seed production, implying that seed production was unlikely to contribute much to subsequent demography. A possible explanation for the increased frequency of three-leaf plants is regression of flowering plants to the three-leaf stage. Flowering plants that suffer accidental damage to their stems or leaves sometimes return to the three-leaf stage the following year. Thus, regression may also have occurred due to environmental changes such as edge effects. This result is similar to the response of a related species, Trillium grandiflorum, to deer herbivory (Knight 2004).
Stage structure (relative frequency of four growth stages) for six populations of T. camschatcense in 2000 is shown. Smaller populations were characterized by a lower proportion of seedlings and a higher proportion of three-leaf plants. Populations with 220 flowering plants or less showed almost no seedling recruitment. Neither of these indices were associated with seed production, implying that seed production was unlikely to contribute much to subsequent demography. Data are from Table 4 in Tomimatsu and Ohara (2002)
Edge effects on recruitment
Another important consequence of habitat fragmentation is a marked increase in edges (Saunders et al. 1991; Murcia 1995), where many physical conditions are often altered. For instance, forest edges are characterized by higher air and soil temperatures, and lower relative humidity and soil moisture than forest interiors (Kapos 1989; Matlack 1993; Chen et al. 1995). An altered environment around habitat edges could directly influence plant reproduction and recruitment (e.g., Ferreira and Laurance 1997; Laurance et al. 1998). Recruitment could also be affected indirectly through modification of biological interactions such as pollination (Powell and Powell 1987), seed predation (Jules and Rathcke 1999), and herbivory (Restrepo and Vargas 1999). Because small populations of T. camschatcense, which typically grow in small forest fragments, have high proportions of edges, edge-related changes in abiotic and biotic environments may provide better explanations for the low seedling recruitment observed in small populations (Fig. 3).
We evaluated edge effects on recruitment within a small fragment of ca. 0.8 ha (Tomimatsu and Ohara 2004). The size of this population was 10,200 flowering plants and the topography was generally gentle. Within this fragment we established six transects along which the density of plants at each of the four growth stages was investigated (Fig. 4). The density of seedlings and one-leaf plants was considerably lower in the forest edges than in the interiors, whereas the edge effects experienced by flowering plants were much more moderate. This result suggests that seedling recruitment is strongly limited around forest edges. In our study, seed production did not differ between forest edges and interiors; neither fruit set nor the number of seeds per flower showed any relationship with distance from the nearest forest edge. Also, seed dispersal distance by ants is likely to be short (0.6 m on average; Ohara and Higashi 1987) and we did not observe significant seed predation. We therefore suggest that microclimatic edge effects limit seed germination and subsequent survival. The density of seedlings was indeed related to some microclimatic changes (see Table 3 in Tomimatsu and Ohara 2004), which can affect the cues required for germination. Edge orientation also appears to play a significant role in the demography of T. camschatcense; edge effects on young stages were particularly strong in edges with a southerly orientation (Fig. 4). This is an expected result, because south-facing edges receive high solar radiation at mid- to high-latitudes in the northern hemisphere (Wales 1972; Matlack 1993; Chen et al. 1995). Moderate edge effects in flowering plants can be accounted by the long life span of this plant (>50 years) and its high survival rate. Because adult plants are often less sensitive to environmental changes than juveniles (Bach et al. 2005), it is possible that many flowering plants have survived fragmentation.
A strong edge effect on seedling recruitment was observed within a small, isolated population of T. camschatcense. The average plant density of each growth stage was investigated every 10 m along six transects (left, bold lines). Every sample point (n=55) is indexed by its spatial coordinates (x, y) and the results of quadratic regression analysis (z=a+bx+cx2+dy+ey2+fxy, where z is plant density) are shown for the seedling (R2=0.45), one-leaf (R2=0.25), and flowering (R2=0.29) stages. The result for the three-leaf stage is not shown because the fitted model did not differ from the null model. The interval between grids is 10 m and the point where plant density was predicted to be highest by the model is indicated by a cross (×). Shaded areas indicate the forest fragment. The density of seedlings and one-leaf plants was considerably lower in the forest edges than in the interiors, whereas the edge effects experienced by flowering plants were much more moderate. Modified from Tomimatsu and Ohara (2004)
Stage structure in the forest edges corresponded well with that observed in small populations, i.e., low frequency of seedlings and high frequency of three-leaf plants (Figs. 3, 5). Because smaller populations were expected to have high proportions of edges, the correspondence in the stage structures between small populations and forest edges points to the importance of edge effects in limiting recruitment. The magnitude of edge effects may be related to population size. The distance from the center of the population to the nearest forest edge was highly correlated with the size of the 14 study populations (Kendall’s τ=0.729, P<0.001). Although previous literature has emphasized disruption of pollination and seed production as particularly important for population persistence (Rathcke and Jules 1993; Olesen and Jain 1994), some recent studies, including ours, suggest that survival processes after seed production can also be crucial. For example, Bruna (1999, 2002) found that seed germination of the understory herb Heliconia acuminata was much lower in forest fragments than in continuous forest, probably because of microclimatic edge effects, leading to decreased seedling density. In western trillium (Trillium ovatum), populations within ∼65 m of forest edges showed almost no recruitment of young plants due to increased seed predation by deer mice (Peromyscus maniculatus; Jules and Rathcke 1999; Tallmon et al. 2003). Despite these concerns, edge effects have received relatively little attention in plant studies.
Stage structure (relative frequency of four growth stages; mean of sample points +1 SD) within a small, isolated population of T. camschatcense is shown. All sample points (n=55) were placed into four discrete categories based on the distance from the nearest forest edge (0≤x<10, 10≤x<20, 20≤x<30, x≥30 m). Stage structure in the forest edges was characterized by a lower frequency of seedlings and a higher frequency of three-leaf plants than in the interiors. Log-linear analysis shows that theses changes in stage structure with distance from the edge are statistically significant (P<0.001). A similar effect was also observed in two other small populations (see Fig. 5 in Tomimatsu and Ohara 2004), emphasizing the role of edge-related decreases in seed germination and subsequent survival in limiting recruitment. Redrawn from Tomimatsu and Ohara (2004)
Genetic diversity and inbreeding
In summary, small and fragmented populations face three genetic threats. First, loss of allelic diversity erodes the ability of populations to evolve with long-term environmental changes (Frankel et al. 1995). Fragmented populations are predicted to experience stochastic loss of rare alleles first, because only a small portion of the original gene pool remains after fragmentation (reviewed by Ellstrand and Elam 1993; Young et al. 1996). Subsequently, random genetic drift will reduce allelic richness, although it takes several generations for drift to have significant impact. Second, fragmentation may elevate inbreeding by restricting potential mates and/or changing pollination systems (Rajimann et al. 1994; Frankham 2005). In contrast to the loss of genetic diversity, inbreeding can act rapidly and results in the reduction of fitness (i.e., inbreeding depression). Finally, deleterious mutations will accumulate and reduce fitness because selection is less effective in small populations (Lynch et al. 1995). The latter impact appears to be less important than the other factors (reviewed by Frankham 2005), so we will not consider this issue here.
As with many studies (e.g., van Treuren et al. 1991; Young et al. 1999), small populations of T. camschatcense have lost rare alleles that were expected to initially be present at low frequencies (Tomimatsu and Ohara 2003a). Using 11 allozyme loci, we found a significant relationship between population size and the mean number of alleles per locus (Fig. 6a). When common (frequency of p≥0.1) and rare (p<0.1) alleles are analyzed separately, all alleles that were not observed in small populations were rare, and the mean number of common alleles was the same (1.18) for all populations (Fig. 6b). Because only one or two generations have passed since fragmentation, the effect of drift should be minor. Thus, the decrease in allelic richness in small populations was attributable to genetic bottlenecks at the time of fragmentation. Although heterozygosity and inbreeding coefficient had no relationship with population size (see Fig. 3 in Tomimatsu and Ohara 2003a), we found significant deficits of heterozygotes relative to Hardy–Weinberg expectations in two small populations, probably due to biparental inbreeding. Considering that biparental inbreeding occurs as a result of localized pollen transfer combined with the fine-scale spatial genetic structure of flowering plants, fragmentation may have affected pollinator fauna and their behavior in these populations. This could hardly be tested, however, because flowers are visited by a wide range of insects, the visitation frequencies of which being too low to be quantified (Tomimatsu and Ohara 2003b). Whereas the loss of allelic diversity typically impacts over the long-term, inbreeding depression may further reduce seed germination and subsequent survival (e.g., Oostermeijer et al. 2003) in addition to reduced seed production and microclimatic edge effects. However, the impacts of fragmentation on plant mating systems are relatively poorly understood, and even greater gene flow among populations has been reported for some tree species (e.g., White et al. 2002).
Small populations of T. camschatcense have lost rare alleles that were expected to initially be present at low frequencies. a Allelic diversity at 11 allozyme loci was significantly related to the log of population size. b When common (p≥0.1) and rare (p<0.1) alleles are analyzed separately, all alleles that were not observed in small populations were rare. A rarefaction simulation analysis indicates that this relationship was not an artifact produced by the variation in sample sizes. Redrawn from Tomimatsu and Ohara (2003a)
Conclusions and future prospects
Forest fragmentation affects the demographic and genetic processes of remnant populations of T. camschatcense. Small, fragmented populations generally experienced reduced seed production (Fig. 2), edge effects on recruitment (Figs. 4, 5), and loss of genetic diversity (Fig. 6). Biparental inbreeding also appears to occur in several small populations. Consequently, fertility was more limited in smaller populations, in which the stage structure was skewed toward more mature stages (Fig. 3). The stage structure was better explained by population size rather than the level of seed production, emphasizing the significance of edge effects. Our research is based entirely on the premise that current population sizes reflect the history of forest fragmentation. This is highly likely because, prior to Japanese settlement, the study area was mostly forested (Fig. 1b) and at present the species usually occurs across forest fragments that are fully surrounded by agricultural fields (Fig. 4).
Implications for conservation
Our study provides some implications for conservation of habitats of T. camschatcense (see Tomimatsu and Ohara 2002, 2003a, 2004 for details). In short, populations of >1,000 flowering plants were successful in constantly producing high seed output (Fig. 2), whereas at least 550 flowering plants are required to retain high allelic diversity (Fig. 6). At population sizes below that number, some elevation of the inbreeding coefficient also occurrs. In contrast, much larger sizes are needed to avoid edge effects on recruitment. Because edge effects occurred >70 m from edges into small fragments (Fig. 4), forests of at least 2 ha (corresponding to ca. 20,000 flowering plants) may be needed to avoid any of the effects examined here. Although these implications also indicate the importance of edge effects, they depend on both the shape of forest reserves and population sizes. On the northern side of the forest where we studied edge effects, the density of seedlings and one-leaf plants was high even at 10–20 m from the forest edges (Fig. 4). Thus, the edge effects can be greatly ameliorated if the shape and orientation of remnant forests are taken into consideration.
Remnant population dynamics
The reduction in fertility is a typical consequence of habitat fragmentation (e.g., Bruna 2002; Oostermeijer et al. 2003; Tallmon et al. 2003; Ward and Johnson 2005). However, the effects of reduced fertility on remnant population growth can be controversial in forest herbs for two interrelated reasons. First, given the stochastic nature of pollinator-mediated pollen transfer and microclimatic environment, the negative effect of fragmentation on fertility may vary among years. Figure 7 shows the temporal variation in per-plant fertility for small and large populations of T. camschatcense over five census periods. It should be emphasized that the fertility in the small population varied among years and was exceptionally high in the second year. Forest herbs are typically long-lived (Ehrlén and Lehtilä 2002), and are capable of storage of reproductive potential over extended time periods due to their long-lived reproductive plants (Eriksson 2000; Honnay et al. 2005). Therefore, the negative effect of low fertility in a sequence of years on remnant population growth may be compensated by that of high fertility in occasional years (“storage effect”; Higgins et al. 2000). Nevertheless, the effect on fertility has rarely been quantified thus far (Hobbs and Yates 2003).
Per-plant fertility in a small population of T. camschatcense was generally limited compared to that in a large population, but it varied greatly across five census periods (0 1999–2001, 1 2000–2002, 2 2001–2003, 3 2002–2004, 4 2003–2005). Mean and SD of the number of seedlings per flowering plant are shown for small (population size, 220) and large (126,000) populations. More precisely, per-plant fertility is determined by the number of seedlings divided by the number of flowering plants observed 2 years before, because the seeds require two winter seasons to become seedlings (“double dormancy”; Samejima and Samejima 1962; Baskin and Baskin 1998)
Second, population growth rate may not be sensitive to changes in fertility. The dynamics of a remnant population are determined by its vital rates such as survival, growth, and fertility, which can be analyzed with matrix population models. In this kind of model—assuming no covariance among vital rates—the effect of changes in fertility (ΔF) on population growth rate (λ) depends on the sensitivity of λ to changes in fertility (∂λ/∂F) and can be approximated by (Caswell 2001):
The sensitivity quantifies the absolute effect of infinitesimal changes in F on λ, holding all the other vital rates constant. For interspecific comparison, we consider the elasticity, or proportional sensitivity, of λ, which is given by F/λ·∂λ/∂F (see de Kroon et al. 2000 for details). Comparative analysis of plant demography shows that the elasticity of fertility generally decreases in plants with increasing life span (Silvertown et al. 1996; Franco and Silvertown 2004). Therefore, given the long life history of forest herbs, even a large reduction in fertility may have only a negligible effect on λ. In our species, the growth rate is also relatively insensitive to changes in fertility (eF∼0.05; H. Tomimatsu and M. Ohara, unpublished data). This could reflect their ability to store reproductive potential over a long period.
Sensitivity or elasticity is based on a derivative and thus is a local analysis (Caswell 2000). If large changes in fertility are involved, Eq. 1 may not provide good predictions of Δλ. In addition, predictions may differ when covariances among vital rates resulting from life-history trade-offs and density-dependent processes are considered. Thus, an effective approach to evaluating the consequences of fragmentation would be to analyze long-term demographic data using matrix projections. If λ<1, the population will decline (Fig. 8a). As an alternative, there is also a possibility that the population has already reached a new equilibrium after fragmentation (i.e., λ=1; Fig. 8b). Considering the slow response of plant populations to fragmentation, it is also important to determine how long it takes before the populations reach the new equilibrium. A few studies have addressed this problem in grassland communities. Lindborg and Eriksson (2004) recently demonstrated that the current species richness of Swedish seminatural grasslands depends on the connectivity of habitats 50–100 years ago but not on current areas and connectivity. Similarly, Helm et al. (2006) found that the current species richness of Estonian calcareous grasslands depends on the areas and connectivity of habitats 70 years ago but not on current conditions. The take-home message from these studies is that a period of several decades or even a century may be too short for plant communities to respond to changes in landscape conditions and that fragmentation may eventually lead to further substantial loss of biodiversity in the future. Because no such study has been conducted in forest ecosystems, it is an interesting question whether the remnant populations of T. camschatcense are still declining over a century after fragmentation. In our populations, projection matrix analysis including stochastic simulations is currently underway and will be published elsewhere.
Hypothetical dynamics of a local population following habitat fragmentation. The population can either continue to decline to extinction (a) or reach a new equilibrium (b) so that, after sufficient time, the population may not necessarily show negative growth (λ<1) even if it experiences negative effects on fertility
The goal of our research in the Tokachi plain is to seek the key mechanisms of change in species diversity and composition of the forest herb community following habitat fragmentation. Forest herbs comprise most of the vascular plant species diversity in temperate deciduous forests (Whigham 2004). Because the understory community also includes many species on the national and/or regional Red List, any deleterious effects on the persistence of these species would be a cause for concern. Species invasion can be another key mechanism. Although exotic species in the community are few and seem to be nonaggressive invaders, a dwarf bamboo (Sasa nipponica) native to this area seems to have expanded its range into forest interiors (H. Tomimatsu, personal observation). Where it does occur, the dwarf bamboo tends to form dense stands (up to 160 culms m−2) that seem to result in the exclusion of native understory species. The role of the dwarf bamboo in changing plant communities also deserves further study.
References
Aizen MA, Ashworth L, Galetto L (2002) Reproductive success in fragmented habitats: do compatibility systems and pollination specialization matter? J Veg Sci 13:885–892
Bach CE, Kelly D, Hazlett BA (2005) Forest edges benefit adults, but not seedlings, of the mistletoe Alepis flavida (Loranthaceae). J Ecol 93:79–86
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego, CA
Bruna EM (1999) Seed germination in rainforest fragments. Nature 402:139
Bruna EM (2002) Effects of forest fragmentation on Heliconia acuminata seedling recruitment in central Amazonia. Oecologia 132:235–243
Byers DL, Meagher TR (1992) Mate availability in small populations of plant species with homomorphic sporophytic self-incompatibility. Heredity 68:353–359
Caswell H (2000) Prospective and retrospective perturbation analyses: their roles in conservation biology. Ecology 81:619–627
Caswell H (2001) Matrix population models: construction, analysis, and interpretation, 2nd edn. Sinauer, Sunderland, MA
Chen J, Franklin JF, Spies TA (1995) Growing-season microclimatic gradients from clearcut edges into old-growth Douglas-fir forests. Ecol Appl 5:74–86
Cochran ME, Ellner S (1992) Simple methods for calculating age-based life history parameters for stage-structured populations. Ecol Monogr 62:345–364
Ehrlén J, Lehtilä K (2002) How perennial are perennial plants? Oikos 98:308–322
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–242
Eriksson O (2000) Functional roles of remnant plant populations in communities and ecosystems. Glob Ecol Biogeogr 9:443–449
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Ferreira LV, Laurance WF (1997) Effects of forest fragmentation on mortality and damage of selected trees in central Amazonia. Conserv Biol 11:797–801
Franco M, Silvertown J (2004) A comparative demography of plants based upon elasticities of vital rates. Ecology 85:531–538
Frankel OH, Brown AHD, Burdon JJ (1995) The conservation of plant biodiversity. Cambridge University Press, Cambridge
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Hanski I, Ovaskainen O (2002) Extinction debt at extinction threshold. Conserv Biol 16:666–673
Helm A, Hanski I, Pärtel M (2006) Slow response of plant species richness to habitat loss and fragmentation. Ecol Lett 9:72–77
Higgins SI, Pickett STA, Bond WJ (2000) Predicting extinction risks for plants: environmental stochasticity can save declining populations. Trends Ecol Evol 15:516–520
Hobbs RJ, Yates CJ (2003) Impacts of ecosystem fragmentation on plant populations: generalizing the idiosyncratic. Aust J Bot 51:471–488
Honnay O, Jacquemyn H, Bossuyt B, Hermy M (2005) Forest fragmentation effects on patch occupancy and population viability of herbaceous plant species. New Phytol 166:723–736
Jennersten O (1988) Pollination in Dianthus deltoides (Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conserv Biol 2:359–366
Jules ES, Rathcke BJ (1999) Mechanisms of reduced trillium recruitment along edges of old-growth forest fragments. Conserv Biol 13:784–793
Kapos V (1989) Effects of isolation on the water status of forest patches in the Brazilian Amazon. J Trop Ecol 5:173–185
Kearns CA, Inouye DW (1997) Pollinators, flowering plants, and conservation biology. BioScience 47:297–307
Knight TM (2004) The effects of herbivory and pollen limitation on a declining population of Trillium grandiflorum. Ecol Appl 14:915–928
Kroon H de, van Groenendael J, Ehlén J (2000) Elasticities: a review of methods and model limitations. Ecology 81:607–618
Laurance WF, Ferreira LV, Rankin-de Merona JM, Laurance SG (1998) Rain forest fragmentation and the dynamics of Amazonian tree communities. Conserv Biol 12:460–464
Lindborg R, Eriksson O (2004) Historical landscape connectivity affects present plant species diversity. Ecology 85:1840–1845
Lynch M, Conery J, Bürger R (1995) Mutational meltdowns in sexual populations. Evolution 49:1067–1080
Matlack GR (1993) Microenvironment variation within and among forest edge sites in the eastern United States. Biol Conserv 66:185–194
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Ohara M, Kawano S (1986) Life history studies on the genus Trillium (Liliaceae) I. Reproductive biology of four Japanese species. Plant Species Biol 1:35–45
Ohara M, Higashi S (1987) Interference by ground beetles with the dispersal by ants of seeds of Trillium species (Liliaceae). J Ecol 75:1091–1098
Ohara M, Kawano S (2005) Life-history monographs of Japanese plants. 2: Trillium camschatcense Ker-Gawl. (Trilliaceae). Plant Species Biol 20:75–82
Ohara M, Takeda H, Ohno Y, Shimamoto Y (1996) Variations in the breeding system and the population genetic structure of Trillium kamtschaticum (Liliaceae). Heredity 76:476–484
Olesen JM, Jain SK (1994) Fragmented plant populations and their lost interactions. In: Loeschcke V, Tomiuk J, Jain SK (eds) Conservation genetics. Birkhäuser, Basel, pp 417–426
Oostermeijer JGB, Luijten SH, den Nijs JCM (2003) Integrating demographic and genetic approaches in plant conservation. Biol Conserv 113:389–398
Powell AH, Powell GVN (1987) Population dynamics of male euglossine bees in Amazonian forest fragments. Biotropica 19:176–179
Rajimann LEL, van Leeuwen NC, Kersten R, Oostermeijer JGB, den Nijs HCM, Menken SBJ (1994) Genetic variation and outcrossing rate in relation to population size in Gentiana pneumonanthe L. Conserv Biol 8:1014–1026
Rathcke BJ, Jules ES (1993) Habitat fragmentation and plant-pollinator interaction. Curr Sci 65:273–277
Restrepo C, Vargas A (1999) Seeds and seedlings of two neotropical montane understory shrubs respond differently to anthropogenic edges and treefall gaps. Oecologia 119:419–426
Samejima J, Samejima K (1962) Studies on the eastern Asiatic Trillium (Liliaceae). Acta Hortic Gotob 25:157–257
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Sih A, Baltus M-S (1987) Patch size, pollinator behavior, and pollinator limitation in catnip. Ecology 68:1679–1690
Silvertown J, Franco M, Menges E (1996) Interpretation of elasticity matrices as an aid to the management of plant populations for conservation. Conserv Biol 10:591–597
Simberloff D (1998) Flagships, umbrellas, and keystones: is single-species management passé in the landscape era? Biol Conserv 83:247–257
Tallmon DA, Jules ES, Radke NJ, Mills LS (2003) Of mice and men and trillium: cascading effects of forest fragmentation. Ecol Appl 13:1193–1203
Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371:65–66
Tomimatsu H, Ohara M (2002) Effects of forest fragmentation on seed production of the understory herb Trillium camschatcense. Conserv Biol 12:1277–1285
Tomimatsu H, Ohara M (2003a) Genetic diversity and local population structure of fragmented populations of Trillium camschatcense (Trilliaceae). Biol Conserv 109:249–258
Tomimatsu H, Ohara M (2003b) Floral visitors of Trillium camschatcense (Trilliaceae) in fragmented forests. Plant Species Biol 18:123–127
Tomimatsu H, Ohara M (2004) Edge effects on recruitment of Trillium camschatcense in small forest fragments. Biol Conserv 117:509–519
Tomimatsu H, Ohara M (2006) Evolution of hierarchical floral resource allocation associated with mating system in an animal-pollinated hermaphroditic herb, Trillium camschatcense (Trilliaceae). Am J Bot 93:134–141
van Trenren R, Bijlsma R, van Delden W, Ouborg NJ (1991) The significance of genetic erosion in the process of extinction. I. Genetic differentiation in Salvia pratensis and Scabiosa columbaria in relation to population size. Heredity 66:181–189
Verheyen K, Vellend M, Calster HV, Peterken G, Hermy M (2004) Metapopulation dynamics in changing landscapes: a new spatially realistic model for forest plants. Ecology 85:3302–3312
Wales BA (1972) Vegetation analysis of north and south edges in a mature oak-hickory forest. Ecol Monogr 42:451–471
Ward M, Johnson SD (2005) Pollen limitation and demographic structure in small fragmented populations of Brunsvigia radulosa (Amaryllidaceae). Oikos 108:253–262
Whigham DF (2004) Ecology of woodland herbs in temperate deciduous forests. Annu Rev Ecol Evol Syst 35:583–621
White GM, Boshier DH, Powell W (2002) Increased pollen flow counteracts fragmentation in a tropical dry forest: an example from Swietenia humilis Zuccarini. Proc Natl Acad Sci USA 99:2038–2042
Wilcove DS, McLellan CH, Dobson AP (1986) Habitat fragmentation in the temperate zone. In: Soulé ME (eds) Conservation biology: the science of scarcity and diversity. Sinauer Associates, Sunderland, MA
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Young AG, Merriam HG, Brown AHD, Zich FA (1999) Genetic structure of fragmented populations of the endangered daisy Rutidosis leptorrhynchoides. Conserv Biol 13:256–265
Young AG, Brown AHD, Murray BG, Thrall PH, Miller CH (2000) Genetic erosion, restricted mating and reduced viability in fragmented populations of the endangered grassland herb Rutidosis leptorrhynchoides. In: Young AG, Clarke GM (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge, pp 335–359
Acknowledgments
We greatly thank Koji Nakamura, Tetsuya Kasagi, and the rest of the organizers for inviting us to the 22nd Symposium of the Society of Population Ecology in October 2005. We also acknowledge Yasuo Konno who kindly provided the nice Fig. 1; Elsevier and Blackwell Publishing for permission to reproduce Figs. 2–6; and two anonymous reviewers for improving the manuscript. The research was funded by grants from the Japan Science Society, the Hokscitec Foundation, the Suhara Memorial Foundation, the Ministry of Education, Culture, Sports, Science and Technology (21st Century COE Program, E-01), and the Japan Society for the Promotion of Science (15370006, 15570020, and 16370007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomimatsu, H., Ohara, M. Evaluating the consequences of habitat fragmentation: a case study in the common forest herb Trillium camschatcense. Popul Ecol 48, 189–198 (2006). https://doi.org/10.1007/s10144-006-0269-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-006-0269-9