Abstract
The aim of this study was to estimate genetic diversity and assess its importance for plant fitness in a species belonging to the most endangered species in Europe, Dracocephalum austriacum L., and to select the most valuable populations for conservation of genetic diversity within the species in the studied regions. We analyzed allozyme variation of 12 populations in three distinct regions (Czech Karst, Moravia and Slovak Karst) in Central Europe. The results showed high genetic diversity within populations (80.14%) and relatively low differentiation among populations within regions (9.42%) and between regions (10.45%). Seed production was significantly higher in larger, genetically more diverse and less inbred populations. The results suggest that genetic diversity has important effect on seed production in this species and thus can be expected to have strong direct consequences for plant fitness and vitality of the whole populations. They also show large variation in genetic diversity between populations and indicate which populations should get a priority in attempts to conserve all the genetic diversity within the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An important aspect in rare species conservation is not only maintaining sufficient number of individuals at a locality but also high genetic diversity (López-Pujol et al. 2003; Oostermeijer et al. 2003). Although this concept is generally accepted, the information about genetic diversity of rare species is still rather limited in many areas. Within Europe these data are missing even for many high priority species (Habitats Directive of NATURA 2000—Web 1: http://www.nature.cz/publik_syst2/files08/habitats%20directive_official%20text.pdf, accessed 1 January 2008) that are considered to be the most endangered plant species throughout Europe (but see Colas et al. 1997; Gaudeul et al. 2000; Brzosko et al. 2002; Glémin et al. 2005).
The importance of studying genetic diversity in rare species is illustrated by the fact that populations of rare and endangered species are often small and isolated. Genetic diversity in such populations is subjected to strong random changes in allele frequencies called genetic drift. In its most extreme case, genetic drift can lead to loss of alleles from the population and thus the loss of polymorphism such that a locus becomes fixed for a single allele (Lowe et al. 2004). In such a case the populations lose the ability to adapt to changing conditions and are susceptible to extinction even after small alterations in the environment (Newman and Pilson 1997; Frankham and Ralls 1998; Saccheri et al. 1998). A similar decrease of genetic diversity can also be caused by increased crossing among closely related individuals, which in turn leads to the increased homozygosity of individuals (Lowe et al. 2004). Crossing between closely related individuals may also lead to reduced fitness of populations—called inbreeding depression (DeMauro 1993; Anderson and Waldmann 2002; Ishihama et al. 2005). The two main causes of inbreeding depression are: (1) higher probability of expression of deleterious alleles and (2) reduced ability to adapt to environmental changes (see Reed and Frankham 2003). Both genetic drift and inbreeding may thus lead to the reduced fitness of the plants. Early detection of these negative effects by estimating the relationship between genetic diversity of the populations and plant fitness may help to reduce the risks of population extinction.
In spite of the potential importance of genetic diversity for plant fitness, Oostermeijer et al. (2003) concluded in a review of papers published between the years 1979 and 2000 on the conservation biology of wild plants, that there were only a few studies assessing the relationship between plant fitness and genetic diversity. The combination of studies on fitness and genetics, compared to studying them separately, can give us a much more reliable picture of the future prospects of the populations (Colas et al. 1997; Luijten et al. 2002).
In conservation efforts, we often have limited resources and we cannot apply the same level of conservation activities to all populations. We have to pick the most valuable and important populations. However, few studies have attempted to describe the distribution of genetic variation within a species and use this information to prioritize sites and management alternatives for capturing and maintaining maximum genetic variation within a plant species (e.g. Neel and Cummings 2003; Neel and Ellstrand 2003; Bonin et al. 2007).
The aim of this study is to estimate genetic diversity and assess its importance for plant fitness in a species belonging to the most endangered plant species in Europe, Dracocephalum austriacum L. (Lamiaceae).
Specifically, we address the following questions: (1) What is the distribution of genetic diversity within and among populations of D. austriacum and between two distant regions? (2) What is the relationship between genetic diversity and plant fitness? and (3) What are the most valuable populations for conservation in the Czech and Slovak Republic (central Europe)?
Materials and methods
Study species
Dracocephalum austriacum L. (Lamiaceae) is a perennial herb or dwarf shrub with erect or ascending stems up to 60 cm high. The species is diploid (2n = 14) (Heywood 1972). It flowers from the second half of May to the first half of June. It grows on rocky steppes and rocky sunny slopes (Hrouda 2002). The species is entomophilous (Hrouda 2000) and according to preliminary study it is self-compatible, but with a strong positive effect of outcrossing on seed production (Dostálek 2005).
In the Czech and Slovak Republic this species belongs to the category of critically endangered species (Holub and Procházka 2000). For manipulation of this species and entering its localities, we obtained permission from the Ministries of Environment of the Czech and Slovak Republic. It is also a species of high conservation interest in Europe. It is listed in Annex II of the Habitats Directive (NATURA 2000), which lists animal and plant species of societal interest. Conservation of these species requires the designation of specially protected areas (Web 1: http://www.nature.cz/publik_syst2/files08/habitats%20directive_official%20text.pdf).
The whole distribution range of this species is discontinuous and ranges eastward from the eastern Pyrenees across France, Italy, Switzerland, Austria, the Czech Republic (northern edge of the distribution range), the Slovak Republic, Hungary, Romania and to the Ukraine (Meusel et al. 1978).
Historical data regarding the distribution in the Czech and Slovak Republics are not very detailed. Heufler (1851) writes about the first discovery of D. austriacum in the area of today’s Czech Republic in 1792. Nevole (1910) and Valoušek (1928) provide a brief list of localities in the Czech Karst region and Moravia region. Nevole (1910) also mentions a locality in the České středohoří mountains on Deblík hill that has not been confirmed since 1996 (Hamerský 2000). Moucha (1990) writes about nine populations in the Czech Republic as they are known today. He also adds that stone mining destroyed some other populations, without mentioning their number. Čeřovský (1999) also writes about the nine Czech populations and notes that 3 populations in the Czech Republic were already extinct. There were thus at least 12 populations in the Czech Republic in the past. In the Slovak Republic, the data on the historical distribution of the species are even more limited. There is repeated information about populations in the Slovak Karst and Slovak Paradise on Drevenik (Moucha 1990; Čeřovský 1999). Populations in the Slovak Karst region have decreased in size in the last 50 years and some of them have already disappeared (E. Karasová, personal communication).
Study populations
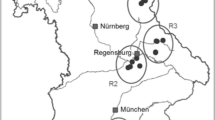
We studied all nine populations in the Czech Republic and three (out of seven) largest populations in the Slovak Republic (Table 1). Eight Czech populations are situated in the Czech Karst region (max. 20 km apart), and the last one is in Zázmoníky (M1) in southern Moravia. Slovak populations are in the Slovak Karst region (max. 30 km apart) cca 500 km from the Czech Karst region. The locality in Zázmoníky (M1) is approximately 200 and 300 km in distance from the Czech and Slovak Karst, respectively. We only took samples from three largest populations in the Slovak Republic because we obtained permission for taking samples from only those three populations. The populations are geographically grouped into three regions (Czech Karst region—C1–C8, Moravia—M1 and Slovak Karst region—S1–S3)—Table 1.
Populations C5 and C7 in the Czech Karst region were almost extinct in 1980s and they were replanted from other Czech Karst region populations (Ložek et al. 2003). For this reason we performed most of the analyses without samples from these populations.
Data on plant fitness
Plant fitness was expressed as the number of seeds per flowering stem and per flowering plant in 2005. We counted only black or dark brown large seeds with smooth surface which resisted light pressure and excluded seeds that were clearly undeveloped, with wrinkly surface, light brown or soft when pressed. We estimated the number of seeds per flowering stem in each population by sampling 20 randomly chosen flowering plants and counting number of seeds on each flowering stem. We used these numbers to estimate the mean seed production per flowering plant as well as per flowering stem. The two values give us distinct information. Number of seeds per flowering stem shows how vital individual flowering stems are. Number of seeds per flowering plant also gives information about plant size. In order to calculate ratio of developed and initialized seeds, we counted the number of calyxes per flowering stem. Each calyx can contain as many as four developed seeds and so we would expect ratio of seeds and calyxes 4:1. In addition, we counted all plants (except for seedlings, i.e., plants with one thin stem not higher than 10 cm), number of flowering plants and proportion of flowering plants in each population. In small populations (C3 and C5) all flowering individuals were sampled to estimate seed production. In population M1 seed production was not estimated. Seed production per plant is significantly related to plant size (mean stem length r = 0.23, P < 0.001, number of stems r = 0.40, P < 0.001).
Genetic analysis
Genetic diversity of the populations was assessed using allozymes. Allozymes are supposed to be neutral markers (Soltis and Soltis 1989; Skibinski et al. 1993) and were repeatedly used to infer genetic diversity of populations of rare species (e.g. Petit et al. 1998; Neel and Ellstrand 2003; Neel and Cummings 2003; Godt et al. 2005). Some have questioned the appropriateness of assessing genetic diversity of a rare species using allozyme or other neutral genetic markers for conservation purposes, arguing that the correlation between molecular genetic variation and the phenotypic, adaptive, variation that is important for species survival may be weak (e.g. Hamrick et al. 1991; Storfer 1996). On the other hand, the neutral genetic markers allow describing the potential variation that may not have adaptive advantage now, but may gain it later, when habitat conditions change. We thus suggest that neutral genetic variation can provide useful information for selected populations that should get a priority in conserving the species.
Allozyme variation was analyzed for a total of 212 D. austriacum individuals sampled from 12 populations in 3 regions (Czech Karst and Moravia in the Czech Republic, and Slovak Karst in the Slovak Republic). Twenty-two individuals were sampled per population if possible (Table 1). Plants were randomly chosen within populations (seedlings, i.e., plants with one thin stem not higher than 10 cm, were excluded from selection). Plants were sampled using random distance and random angle from the middle point of the population. Only plants within 20 cm from randomly chosen point (minimum distance between two plants) were sampled. In populations larger than 50 × 50 m, several middle points were selected and sampling was done from a randomly chosen middle point in each case. In populations with less than 22 individuals, all plants (but excluding seedlings) in the locality were sampled. We decided not to sample seedlings, because seedlings often have higher genetic variation than adults, and mixing seedlings and adults in different proportions would lead to biased estimates of genetic diversity of the populations (Mandák et al. 2006; Honnay et al. 2008).
Leaf samples were kept on ice during transport to laboratory and stored in a refrigerator until extraction. Approximately 70 mg of leaf tissue was mechanically ground with Dowex-Cl (1-X8) and quartz sand and homogenized on ice in “viola” extraction buffer (0.1 M Tris–HCl pH 8.0, 70 mM 2-mercaptoethanol, 26 mM sodium metabisulfite, 11 mM ascorbic acid, 4% (w/v) polyvinylpyrrolidone). Further extraction and electrophoresis followed Kaplan et al. (2001). The following ten enzymes were analysed: leucyl aminopeptidase (LAP, E.C. 3.4.11.1), superoxide dismutase (SOD, E.C. 1.15.1.1), aspartate aminotransferase (AAT, E.C. 2.6.1.1), 6-phosphogluconate dehydrogenase (6-PGDH, E.C. 1.1.1.44), alcohol dehydrogenase (ADH, E.C. 1.1.1.1), shikimate dehydrogenase (SKDH, E.C 1.1.1.25), phosphoglucomutase (PGM, E.C. 2.7.5.1), malic enzyme (ME, E.C. 1.1.1.40), esterase (EST, E.C. 3.1.1.-), isocitrate dehydrogenase (IDH, E.C. 1.1.1.42). The systems with high variation and easy to score were selected for further analyses, e.g. LAP, SOD, 6-PGDH and AAT. The staining procedures are available in Appendix. A total of 10 loci was resolved for this species—LAP (3), SOD (3), 6-PGDH (2) and AAT (2).
Data analysis
Total number of alleles (TA), proportion of polymorphic loci (P), number of alleles per polymorphic locus (A P), observed heterozygosity (H O) and expected heterozygosity (H E) were calculated for each region (Czech Karst, Moravia and Slovak Karst) and each sampled population using Popgene 1.32 (Yeh and Boyle 1997). Inbreeding coefficient (F IS) for each population was calculated using average population expected (H E) and observed heterozygosity (H O) as
Using Popgene 1.32 we further computed population differentiation (F ST) within the Czech and Slovak Karst regions.
ARLEQUIN 3.11 (Excoffier and Schneider 2005) was used to assess the partitioning of the genetic variance within and among populations, and within groups of populations by conducting an analysis of molecular variance (AMOVA, Excoffier and Schneider 2005). Populations examined in the AMOVA procedure were grouped geographically—Czech Karst and Slovak Karst. Moravian region was excluded because there was only one population with four plants. We performed the analysis with Moravia as the third region as well but the results of AMOVA were almost the same. The significance levels of variance components were calculated by conducting 1,000 permutations of the data (variation within populations—permuting individual genotypes among populations and among groups; variation among populations within regions—permuting individual genotypes among populations but within groups; variation among regions—permuting whole populations among groups). Using ARLEQUIN 3.11 we further computed population differentiation (F ST) for each pair of populations separately.
We also counted the number of unique alleles (alleles detected only in one population/region). A Mantel test (Mantel 1967) was used to test whether the matrix of genetic distances was correlated with the matrix of geographical distances (ARLEQUIN 3.11; Excoffier and Schneider 2005, 10,000 permutations). We also calculated the correlation between genetic and geographical distance only for Czech and Slovak Karst populations separately.
Correlations between genetic diversity measures and various fitness measures were tested with linear regression in S-Plus (2000). Individual fitness was expressed as number of seeds per flowering stem, number of seeds per flowering plant and the proportion of developed seeds. The status of a population was expressed as the logarithm of population size (total number of plants) and the proportion of flowering plants in a population. We tested also net effect of population size (logarithm) after removing the effect of genetic diversity measures (H E, H IS and F IS) on fitness measures (number of seeds per flowering stem and per flowering plant) and vice versa using multiple regression.
In spite of the high number of tests, we did not apply the overly conservative Bonferroni correction, but report all results indicating exact P-values, as suggested by Moran (2003). Moreover, to assess whether the number of significant tests could be due to chance alone, we calculated the probability of obtaining a given number of significant tests at the 5% significance level based on binomial distribution.
The localities C5 and C7 from the Czech Karst were excluded from AMOVA analyses, Mantel test, overall F ST calculations and F ST calculations for the Czech Karst region and from correlations of genetic diversity measures and fitness because they were newly replanted in 1980s.
We ranked the populations by their TA, P, AP, H E, H O, F ST and F IS values and by number of unique alleles. The highest values of all parameters except for F IS got the lowest rank (they got the highest rank for F IS). Populations with the lowest mean rank were considered to have a higher priority for conservation of genetic diversity within the species within each region (Neel and Ellstrand 2003).
Results
At the species level, 6 of the 10 loci examined were polymorphic. A total of 24 alleles were detected within all loci combined, yielding an average of 2.40 (SD = 2.12) alleles per locus and 3.33 (SD = 2.13) alleles per polymorphic locus. Expected heterozygosity (H E) for the whole dataset was 0.15 (SD = 0.19) and observed heterozygosity (H O) was 0.08 (SD = 0.11).
The proportion of loci that were polymorphic within the 12 sampled populations ranged from 0 (C5) to 0.5 (C4 and C7). The number of alleles per population ranged between 10 (C5) and 19 (C8). The number of alleles per polymorphic locus within populations ranged from 2.20 (C4) to 3.50 (M1). H E within populations ranged from 0 (C5) to 0.18 (S1). H O within populations varied from 0 (C5) to 0.14 (S3)—Table 1.
One unique allele was found in population S2 and one in S3. Three unique alleles were found in the Czech Karst region and three in the Slovak Karst region (Table 1). The inbreeding coefficient (F IS) ranged from 0.079 (M1) to 0.638 (C3). Observed heterozygosity was lower than expected heterozygosity in all populations (Table 1).
The lowest mean rank was observed in Moravian population (M1) and all three Slovak populations. In the Czech Karst the most valuable populations were C2, C3 and C8 (Table 1).
The analysis of molecular variance (AMOVA) showed highest variance within populations (80.14%), while 10.45% of variance was divided between the two regions. Variance amongst the populations within regions was 9.42%. Similar results were also found for single loci (Table 2). Differentiation among regions was significant only in two out of six polymorphic loci. However, variation amongst populations was significant almost in all loci (Table 2). The recorded value 0.177 of F ST (variation among populations among regions) implies a low gene flow between populations. F ST for the Czech and Slovak Karst regions were 0.143 and 0.088, respectively. It implies that populations in the Czech Karst region are more genetically separated.
FST values for all pairs of populations ranged from −0.022 to 0.637. All (with the exception of 12 low values) of the 66 paired comparisons were significant (Table 3). Genetic distance amongst populations was significantly correlated with geographical distance (r = 0.44, P = 0.002). Mantel tests for populations from the Czech and Slovak Karst regions separately were not significant (P = 0.58 and P = 0.66, respectively).
There was significant positive correlation between H E and H O (r = 0.83, P = 0.003), TA and P (r = 0.66, P = 0.036) and TA and A P (r = 0.73, P = 0.024) and significant negative correlation between F IS and H O (r = −0.70, P = 0.025). Correlations between all other genetic diversity measures were not significant.
We found significant positive correlation between seed production per flowering stem and genetic diversity measures—expected heterozygosity (r = 0.70, P = 0.036, Fig. 1) and observed heterozygosity (r = 0.80, P = 0.009). Seed production per flowering plant was significantly correlated with observed heterozygosity (r = 0.78, P = 0.012) and negatively correlated with inbreeding coefficient (r = −0.71, P = 0.033, Fig. 2). We also found correlation between the total number of alleles and seed production per flowering plant (r = 0.71, P = 0.034). The proportion of developed seeds was significantly related to the total number of alleles (r = 0.75, P = 0.020). The probability of obtaining these numbers of significant tests by chance is 0.020 for seed production per flowering stem and 0.001 for seed production per flowering plant.
There was no significant correlation between any measure of genetic diversity and population size (see non-significant relationship between population size and expected heterozygosity in Fig. 4), number of flowering plants or proportion of flowering plants in population, except for a significant positive correlation between proportion of polymorphic loci and population size (r = 0.78, P = 0.008).
There was a significant positive correlation between population size and proportion of developed seeds (r = 0.67, P = 0.049) and seed production per flowering plant (r = 0.73, P = 0.026, Fig. 3), but not between population size and seed production per flowering stem (P = 0.368).
Multiple regressions indicated significant positive effect of population size on seed production per flowering plant even after removing the effect of H E (r = 0.12, P = 0.048) and effect of H O after removing the effect of population size (r = 0.44, P = 0.012). All other tests done using multiple regression were not significant (Fig. 4).
Discussion
The study indicates high genetic diversity within the populations of D. austriacum, suggesting that the populations still host sufficient genetic diversity within regions. In contrast to our expectations, the genetic differentiation between the two studied regions was quite low. Similar low differentiation was observed for species with good pollen or seed dispersal (e.g. Brzosko et al. 2002). However, this is not the case of D. austriacum (see below).
Despite the low differentiation between the two regions, we found quite high genetic differentiation among the studied populations (F ST = 0.177). This value corresponds to the F ST values for animal pollinated species in a review of Hamrick and Godt (1989), which provides F ST values for a range of allozyme studies. The value also agrees with the results of a summary of studies using RAPD markers indicating mean F ST values of 0.25 for long-lived perennials (N = 14, Nybom and Bartish 2000). Similar results were also shown in other studies (e.g. Ayres and Ryan 1999; Martínez-Palacios et al. 1999).
The high genetic isolation of the studied populations is in agreement with the fact that D. austriacum is pollinated mostly by bumblebees and bees (Castro unpublish), and it is quite unlikely that they are able to transfer pollen from one locality to another (Darvill et al. 2004). Seed dispersal between populations is also unlikely (Widén and Widén 1990), because the seeds are large without any specific adaptations to disperse. Similar high genetic isolation was also found in other animal pollinated plants (e.g. Colas et al. 1997; Martínez-Palacios et al. 1999). In contrast, Ben Fadhel and Boussaid (2004) showed in the populations of Mentha pulegium, from the same family as D. austriacum, quite high intra-population genetic variability but lower inter-population differentiation. They hypothesize that it could be due to the relatively recent origin of the studied populations.
Population differentiation is higher in the Czech Karst region (F ST = 0.143) than in the Slovak Karst region (F ST = 0.088). This difference could be due to the fact that there only three populations were sampled in the Slovak Karst region; whereas eight populations were sampled in the Czech Karst region. When only the three largest Czech populations were taken for analysis their F ST was 0.068, which is comparable with the Slovak Karst region. An alternative explanation for the observed pattern could be that in the Czech Karst region there are more very small populations than in the Slovak Karst region. In the small populations genetic drift plays an important role and leads to random genetic differentiation among populations (Lowe et al. 2004).
Values of expected heterozygosity at the population level for D. austriacum are quite high when compared to the values provided by Hamrick and Godt (1989) for animal-pollinated species with mixed mating. We found lower mean genetic diversity in the Czech Karst region populations than in the Slovak Karst region populations. This could be due to the position of Czech populations in the northern periphery of the distribution range of the species. Lower genetic diversity at the edge of the distribution range was also reported by Lamni et al. (1999) and Faugeron et al. (2004).
We found significant correlation between genetic and geographical distances when the data from the two regions were combined, but not within regions. This was probably because of long distance between Czech and Slovak Karst populations. The absence of correlation between genetic and geographical distances within regions suggests the important role of genetic drift in D. austriacum populations. The effect of genetic drift on the distribution of genetic diversity in small populations was also suggested as an explanation for the absence of spatial patterns for Taxus baccata in Switzerland by Hilfiker et al. (2004), and for small unconnected populations of Ranunculus reptans by Fischer et al. (2000).
Seed production was significantly higher in genetically more diverse and less inbred populations. Similar results were also found e.g. by Husband and Schemske (1996), Fischer and Matthies (1998), Schmidt and Jensen (2000) and Hensen and Oberprieler (2005). In agreement with these studies, we also found significant correlation between seed production per flowering plant and population size. Such correlation is often reported for species whose populations decreased due to habitat fragmentation or habitat destruction (e.g. Luijten et al. 2000). This is probably also the case of D. austriacum. Some populations decreased in last 50 years (Karasová in verb.) and some of them were destructed with stone mining (Moucha 1990). Lower seed set in small and less variable populations could be caused by inbreeding. It can play an important role in populations of species with mixed mating system (Kephart et al. 1999) such as D. austriacum. Inbreeding could result from increased outcrossing between close relatives in small populations and/or increased outcrossing within genetic neighborhoods (e.g. spatially separated patches within a large population). Other reason for the relationship between population size and seed production could be increased frequency of geitonogamy in small populations (Lloyd and Schoen 1992). This explanation is likely because the plants of D. austriacum are quite large with many flowers and it is much more probable that the pollinators fly between flowers of the same plant. Another explanation, supported by unpublished experiments on D. austriacum (Castro unpublish) is simply a lack of pollinator service leading to increased spontaneous selfing.
In contrast with previous studies (see Ellstrand and Elam 1993; Frankham 1996; Leimu et al. 2006 for reviews), we did not find significant correlation between the genetic diversity measures (TA, A P, H E or H O with the exception of P) and population size (number of plants, number of flowering plants). Similar absence of correlation between genetic variation and population size was found by Oostermeijer et al. (1994), Schmidt and Jensen (2000) and Leimu and Mutikainen (2005). Leimu and Mutikainen (2005) assume that the populations are of recent common ancestry and may also have relatively high levels of current gene flow. However, neither recent common ancestry nor high level of current gene flow is definitely the case for our species. In review of Leimu et al. (2006) they also found positive relationship between genetic diversity and population size but they showed that this relationship was weaker for self-compatible species and for allozyme studies. The absence of this relationship in D. austriacum could be due to the low number of examined populations, or the fact that some populations are very small and there are not multiple large populations in Central Europe. Alternatively, the lack of the pattern could be due to historical factors (such as population size) being more important than current population size in determining patterns of genetic diversity of the populations (see Ellstrand and Elam 1993; Ouborg and van Treuren 1995; Schmidt and Jensen 2000). Another explanation could be the lower resolution of the marker type used in comparison to DNA markers such as AFLP’s.
Conservation implications
In this study, we explored genetic diversity of populations of D. austriacum in two regions at the periphery of the total distribution range. While it would be useful to have a comparison of genetic diversity from a higher number of countries, the decision on conservation priorities are still done mainly within the single countries. The knowledge on genetic diversity of Czech and Slovak populations will thus provide useful background information for conservation of this species within this region.
All studied Slovak populations had high genetic diversity; indicating that all three examined Slovak populations should be of high conservation priority. All three Slovak populations should be maintained to ensure that all alleles identified in our analyses could be preserved. Each one of them has some unique genetic information within the Slovak Karst region. In the Czech Republic, three populations (C2, C4 and C8) seem to be the most valuable populations for conserving genetic diversity within D. austriacum. However, if we would like to maintain all possible genetic diversity within the Czech Republic we should also protect the C6 population as well. The C6 population contains alleles, which are not present in other populations in the Czech Republic (but are included in the Slovak populations). Results of parallel demographic study indicate that the populations are not currently declining (Dostálek 2005). It should be sufficient to keep the localities in their current state. The restoration of degraded sites to increase the size of populations could be useful but should be a lower priority than protecting existing populations where the largest amount of genetic diversity is concentrated. According to our analysis, most of the small populations do not substantially contribute to the genetic variability of the species in each studied region (with the exception of the isolated small population M1).
References
Anderson S, Waldmann P (2002) Inbreeding depression in a rare plant, Scabiosa canescens (Dipsacaceae). Hereditas 136:207–211. doi:10.1034/j.1601-5223.2002.1360305.x
Ayres DR, Ryan FJ (1999) Genetic diversity and structure of the narrow endemic Wyethia reticulata and its congener W. bolanderi (Asteraceae) using RAPD and allozyme techniques. Am J Bot 86:344–353. doi:10.2307/2656756
Ben Fadhel N, Boussaid M (2004) Genetic diversity in wild Tunisian populations of Mentha pulegium L. (Lamiaceae). Genet Resour Crop Evol 51:309–321. doi:10.1023/B:GRES.0000024016.13743.8b
Bonin A, Nicole F, Pompanon F, Miaud C, Taberlet P (2007) Population adaptive index: a new method to help measure intraspecific genetic diversity and prioritize populations for conservation. Conserv Biol 21:697–708. doi:10.1111/j.1523-1739.2007.00685.x
Brzosko E, Wróblewska A, Ratkiewicz M (2002) Spatial genetic structure and clonal diversity of island populations of lady’s slipper (Cypripedium calceolus) from the Biebrza National Park (northeast Poland). Mol Ecol 11:2499–2509. doi:10.1046/j.1365-294X.2002.01630.x
Čeřovský J (1999) Dracocephalum austriacum L. In: Čeřovský J, Feráková V, Holub J, Maglocký Š, Procházka F (eds) Red book of endangered and rare plant and animal species in the Czech and Slovak Republic, vol 5. Príroda, Bratislava, p 138 (in Czech)
Colas B, Olivieri I, Riba M (1997) Centaurea corymbosa, a cliff-dwelling species tottering on the brink of extinction: a demographic and genetic study. Proc Natl Acad Sci USA 94:3471–3476. doi:10.1073/pnas.94.7.3471
Darvill B, Knight M, Goulson D (2004) Use of genetic markers to quantify bumblebee foraging range and nest density. Oikos 107:471–478. doi:10.1111/j.0030-1299.2004.13510.x
DeMauro M (1993) Relationship of breeding system to rarity in the lakeside daisy (Hymenoxys acaulis var. glabra). Conserv Biol 7:542–550. doi:10.1046/j.1523-1739.1993.07030542.x
Dostálek T (2005) Identification of critical life history stages in the life cycle of endangered species, Dracocephalum austriacum L. Ms. Master thesis, depon. Department of Botany, Faculty of Science, Charles University, Prague
Ellstrand N, Elam D (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–242. doi:10.1146/annurev.es.24.110193.001245
Excoffier LGL, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Faugeron S, Martínez EA, Correa JA, Cardenas L, Destombe C, Valero M (2004) Reduced genetic diversity and increased population differentiation in peripheral and overharvested populations of Gigartina skottsbergii (Rhodophyta, Gigartinales) in southern Chile. J Phycol 40:454–462
Fischer M, Matthies D (1998) RAPD variation in relation to population size and plant fitness in the rare Gentianella germanica (Gentianaceae). Am J Bot 85:811–819. doi:10.2307/2446416
Fischer M, Husi R, Prati D, Peintinger M, van Kleunen M, Schmidt B (2000) RAPD variation among and within small and large populations of the rare clonal plant Ranunculus reptans (Ranunculaceae). Am J Bot 87:1128–1137. doi:10.2307/2656649
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508. doi:10.1046/j.1523-1739.1996.10061500.x
Frankham R, Ralls K (1998) Inbreeding leads to extinction. Nature 392:441–442. doi:10.1038/33022
Gaudeul M, Taberlet P, Till-Bottraud I (2000) Genetic diversity in an endangered alpine plant, Eryngium alpinum L. (Apiaceae), inferred from amplified fragment length polymorphism markers. Mol Ecol 9:1625–1638. doi:10.1046/j.1365-294x.2000.01063.x
Glémin S, Gaude T, Guillemin M, Lourmas M, Olivieri I, Mignot A (2005) Balancing selection in the wild: testing population genetics theory of self-incompatibility in the rare species Brassica insularis. Genetics 171:279–289. doi:10.1534/genetics.104.035915
Godt MJW, Caplow F, Hamrick JL (2005) Allozyme diversity in the federally threatened golden paintbrush, Castilleja levisecta (Scrophulariaceae). Conserv Genet 6:87–99
Hamerský R (2000) Dracocephalum austriacum L. In: NATURA 2000—maping and botanical survey in localities of endangered species of vascular plants (České středohoří Mts.). Ms., depon. in AOPK ČR, Prague (in Czech)
Hamrick JL, Godt MJW (1989) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics, breeding and genetic resources. Sinauer, Sunderland, MA, pp 43–63
Hamrick JL, Murawski DA, Loveless MD (1991) Correlations between species traits and allozyme diversity: implications for conservation biology. In: Falk DA, Holsinger KE (eds) Genetics and conservation of rare plants. Oxford University Press, New York, pp 75–86
Hensen I, Oberprieler C (2005) Effects of population size on genetic diversity and seed production in the rare Dictamnus albus (Rutaceae) in central Germany. Conserv Genet 6:63–73. doi:10.1007/s10592-004-7745-6
Heufler L (1851) Trattnick’s briefwechsel. Oesterreichisches Botanisches Wochenblatt 1:157–167. doi:10.1007/BF02052745
Heywood VH (1972) Dracocephalum austriacum L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DM, Waltres SM, Webb SA (eds) Flora Europea, vol 3. Cambridge University Press, London, UK, p 161
Hilfiker K, Gugerli F, Schütz J, Rotach P, Holderegger R (2004) Low RAPD variation and female-biased sex ratio indicate genetic drift in small populations of the dioecious conifer Taxus baccata in Switzerland. Conserv Genet 5:357–365. doi:10.1023/B:COGE.0000031144.95293.1b
Holub J, Procházka F (2000) Red list of vascular plants of the Czech Republic. Preslia 72:187–230
Honnay O, Bossuyt B, Jacquemyn H, Shimono A, Uchiyama K (2008) Can a seed bank maintain the genetic variation in the above ground plant population? Oikos 117:1–5. doi:10.1111/j.2007.0030-1299.16188.x
Hrouda L (2000) Dracocephalum austriacum L. In: Slavík B (ed) Květena České republiky. [Flora of the Czech Republic.], vol 6. Academia, Prague, pp 638–639 (In Czech)
Hrouda L (2002) Dracocephalum austriacum L. In: Kubát K, Hrouda L, Chrtek J Jr, Kaplan Z, Kirschner J, Štěpánek J (eds) Klíč ke květeně České republiky. [Key to the Flora of the Czech Republic.]. Academia, Prague, p 592 (in Czech)
Husband B, Schemske D (1996) Evolution of the magnitude and timing of inbreeding depression in plants. Evol Int J Org Evol 50:54–70. doi:10.2307/2410780
Ishihama F, Ueno S, Tsumura Y, Washitani I (2005) Gene flow and inbreeding depression inferred from fine-scale genetic structure in an endangered heterostylous perennial, Primula sieboldii. Mol Ecol 14:983–990. doi:10.1111/j.1365-294X.2005.02483.x
Kaplan Z, Plačková I, Štěpánek J (2001) Potamogeton × fluitans (P. fluitans × P. lucens) in the Czech Republic II. Isozyme analysis. Preslia 74:187–195
Kephart S, Brown E, Hall J (1999) Inbreeding depression and partial selfing: evolutionary implications of mixed-mating in a coastal endemic, Silene douglasii var. oraria (Caryophyllaceae). Heredity 82:543–554. doi:10.1038/sj.hdy.6885250
Lamni A, Siikamaki P, Mustajarvi K (1999) Genetic diversity, population size, and fitness in central and peripheral populations of a rare plant Lychnis viscaria. Conserv Biol 13:1069–1078. doi:10.1046/j.1523-1739.1999.98278.x
Leimu R, Mutikainen P (2005) Population history, mating system, and fitness variation in a perennial herb with a fragmented distribution. Conserv Biol 19:349–356. doi:10.1111/j.1523-1739.2005.00480.x
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? J Ecol 94:942–952. doi:10.1111/j.1365-2745.2006.01150.x
Lloyd DG, Schoen DJ (1992) Self-and cross-fertilization in plants. I. Functional dimensions. Int J Plant Sci 153:358–369
López-Pujol J, Bosch M, Simon J, Blanché C (2003) Population genetics and conservation priorities for the critically endangered island endemic Delphinium pentagynum subsp. formenteranum (Ranunculaceae). Biodivers Conserv 12:1937–1951. doi:10.1023/A:1024103714274
Lowe A, Harris S, Ashton P (2004) Ecological genetics: design, analysis, and application. Blackwell publishing, Oxford
Ložek V et al (2003) Localities of Dracocephalum austriacum in Czech Karst in 1983–2003. Ms. depon. in CHKO Czech Karst, Karlštejn, Czech Republic (in Czech)
Luijten SH, Dierick A, Oostermeijer JGB, Raijmann LEL, den Nijs JCM (2000) Population size, genetic variation, and reproductive success in a rapidly declining, self-incompatible perennial (Arnica montana) in the Netherlands. Conserv Biol 14:1776–1787. doi:10.1046/j.1523-1739.2000.99345.x
Luijten SH, Marckéry J, Oostermeijer JGB, den Nijs JCM (2002) Demographic consequences of inbreeding and outbreeding in Arnica montana: a field experiment. J Ecol 90:593–603. doi:10.1046/j.1365-2745.2002.00703.x
Mandák B, Bímová K, Mahelka V, Plačková I (2006) How much genetic variation is stored in the seed bank? A study of Atriplex tatarica (Chenopodiaceae). Mol Ecol 15:2653–2663. doi:10.1111/j.1365-294X.2006.02953.x
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–212
Martínez-Palacios A, Eguiarte LE, Furnier GR (1999) Genetic diversity of the endangered endemic Agave victoriae-reginae (Agavaceae) in the Chihuahuan desert. Am J Bot 86:1093–1098. doi:10.2307/2656971
Meusel H, Jäger E, Rauschert S, Weinert E (1978) Vergleichende Chorologie der zentraleuropäischen Flora—Karten, Band 2. Veb Gustav Fischer Verlag, Jena
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405. doi:10.1034/j.1600-0706.2003.12010.x
Moucha P (1990) Včelník rakouský. Nika Prague 11:34. Dracocephalum austriacum (in Czech)
Neel MC, Cummings MP (2003) Effectiveness of conservation targets in capturing genetic diversity. Conserv Biol 17:219–229. doi:10.1046/j.1523-1739.2003.01352.x
Neel MC, Ellstrand N (2003) Conservation of genetic diversity in the endangered plant Eriogonum ovalifolium var. vineum (Polygonaceae). Conserv Genet 4:337–352. doi:10.1023/A:1024017029933
Nevole J (1910) Studien über die Verbreitung von sechs südeuropäischen Pflanzenarten. Mitteilungen des naturwissenschaftlichen Vereines für Steiermark 46:3–25
Newman D, Pilson D (1997) Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchella. Evol Int J Org Evol 51:345–362. doi:10.2307/2411107
Nybom H, Bartish IV (2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect Plant Ecol Evol Syst 3:93–114. doi:10.1078/1433-8319-00006
Oostermeijer JGB, Eijck MW, Nijs JCM (1994) Offspring fitness in relation to population size and genetic variation in the rare perennial plant species Gentiana pneumonanthe (Gentianaceae). Oecologia 97:289–296
Oostermeijer JGB, Luijten SH, den Nijs JCM (2003) Integrating demographic and genetic approaches in plant conservation. Biol Conserv 113:389–398. doi:10.1016/S0006-3207(03)00127-7
Ouborg NJ, Van Treuren R (1995) Variation in fitness-related characters among small and large populations of Salvia pratensis. J Ecol 83:369–380
Petit RJ, El Mousadik A, Pons O (1998) Identifying populations for conservation on the basis of genetic markers. Conserv Biol 12:844–855
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17:230–237. doi:10.1046/j.1523-1739.2003.01236.x
S- Plus (2000) Professional edition for windows, release 2. MathSoft, Inc, Massachusetts
Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I (1998) Inbreeding and extinction in a butterfly metapopulation. Nature 392:491–494. doi:10.1038/33136
Schmidt K, Jensen K (2000) Genetic structure and AFLP variation of remnant populations in the rare plant Pedicularis palustris (Scrophulariaceae) and its relation to population size and reproductive components 1. Am J Bot 87:678–689. doi:10.2307/2656854
Skibinski DOF, Woodwark M, Ward RD (1993) A quantitative test of the neutral theory using pooled allozyme data. Genetics 135:233–248
Soltis DE, Soltis OS (1989) Isozymes in plant biology. Dioscorides Press, Portland
Storfer A (1996) Quantitative genetics: a promising approach for the assessment of genetic variation in endangered species. Trends Ecol Evol 11:343–348
Vallejos CE (1983) Enzyme activity staining. In: Tanksley SD, Orton TJ (eds) Isozyme in plant genetics and breeding, part A. Elsevier, Amsterdam, pp 469–516
Valoušek B (1928) Včelník rakouský (Dracocephalum austriacum L.) nová rostlina na Moravě. Priroda 21:258–260 (in Czech)
Wendel JF, Weeden NF (1989) Visualisation and interpretation of plant isozymes. In: Soltis DE, Soltis PS (eds) Isozymes in plant biology. Dioscoroides Press, Portland, pp 5–45
Widén B, Widén M (1990) Pollen limitation and distance-dependent fecundity in females of the clonal gynodioecious herb Glechoma hederacea (Lamiaceae). Oecologia 83:191–196. doi:10.1007/BF00317751
Yeh FC, Boyle TJB (1997) Population genetic analysis of co-dominant and dominant markers and quantitative traits. Belg J Bot 129:157
Acknowledgments
We would like to thank to E. Karasová from the Slovak Karst National park for information about the species and help in the field, K. Kottová for help with allozyme analyses and two anonymous reviewers for useful comments on the previous version of the paper. Workers of the Czech Karst Protected Area Landscape Administration also provided us all information about localities in the Czech Karst region. This study was supported by GAUK 198/2005, MŠMT 2B06178, GAČR 206/08/H049, MŠMT 0021620828 and AV0Z6005908.
Author information
Authors and Affiliations
Corresponding author
Appendix: The gel staining procedures
Appendix: The gel staining procedures
The staining procedures followed Vallejos (1983) to visualize ADH, 6-PGDH, PGM, EST, IDH and Wendel and Weeden (1989) for SOD and SKDH with the following modifications: ADH (20 ml ethanol), 6-PGDH (0.1 M Tris–HCl pH 8.4, 30 mg 6-phosphogluconic acid), PGM (24 mg MgCl2), EST (Na-phosphate buffer pH 6.45, 30 mg β-naphthylphosphate, 30 mg α-naphtylacetate), IDH (50 ml 0.1 M Tris–HCl pH 8.0, 50 mg isocitric acid, 80 mg MgCl2), SKDH (30 ml 0.1 M Tris–HCl pH 8.4, 30 mg shikimic acid, 5 mg NADP, 6 mg MTT). Enzyme systems AAT and LAP were stained using the following methods. Two staining solutions were prepared for AAT: A (260 mg aspartic acid and 45 mg α-ketoglutaric acid dissolved in warm 20 ml 0.1 M Tris–HCl pH 8.4) and B (20 ml 0.1 M Tris–HCl pH 8.4, 50 mg Fast Blue BB Salt, 50 mg Fast Violet B). The solution A was prepared at least 15 min before the application. The gel was rinsed in water and then in buffer Tris–HCl pH 7. Solutions A and B were mixed and poured on the gel. The gel was incubated in the dark at 32°C until bands appeared. Then it was rinsed and fixed (1:1:3:5, glycerine, acetic acid, H2O, methanol). The gel stained for LAP was rinsed in buffer 0.2 M Tris-maleate pH 6 and incubated 10 min with 45 mg l-leucyl-β-naphthylamide-HCl (in 50% acetone) and 60 mg MgCl2 (both dissolved in 30 ml buffer). Afterwards solution of 25 mg Fast Black K Salt in 30 ml buffer was added and gel was incubated in the dark at 32°C until bands appeared. For SOD ingredients, 50 ml of 0.05 M Tris–HCl (pH 8.2), 5 mg of EDTA, 5 mg of NBT and 2 mg of riboflavin were combined and poured over the gel. This was incubated for 20 min in the dark at 32°C then removed and illuminated under a lamp until bands appeared on the blue background. A standard staining solution for ME was prepared by dissolving 150 mg malic acid in 25 ml 0.05 M Tris–HCl (pH 8.0) and adjusted to pH 7.5 with 1N NaOH; to this was added a solution of 10 mg of MTT, 5 mg of NADP and 2 mg of PMS in 25 ml of 0.05 M Tris–HCl (pH 8.0), and the resulting staining solution was poured over the gel. Afterwards, all gels were thoroughly rinsed in distilled water, dried between two cellophane sheets and stored.
Rights and permissions
About this article
Cite this article
Dostálek, T., Münzbergová, Z. & Plačková, I. Genetic diversity and its effect on fitness in an endangered plant species, Dracocephalum austriacum L.. Conserv Genet 11, 773–783 (2010). https://doi.org/10.1007/s10592-009-9879-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-009-9879-z