Abstract
Pollen limitation may have important consequences for the reproduction and abundance of plant species. It may be especially harmful to endangered and endemic plants with small populations. In this study, we quantify the effect of pollen limitation on seed production and seedling emergence in an endangered narrow endemic crucifer, Erysimum popovii. We conducted a pollen addition experiment across the entire geographic distribution of the species, and explored the effect of pollinator assemblage, plant population size and density, and other habitat variables on pollen limitation intensity in 13 populations. We supplemented flowers in 20 plants per population with allogamous pollen. To account for potential resource reallocation, we used two types of control untreated flowers: internal control flowers from the same individual as the supplemented flowers, and external control flowers from other individuals. Our results indicate that E. popovii is pollen-limited in most of the populations studied, but only through seed production, since pollen supplementation did not enhance seedling emergence. Beefly abundance was associated with among-population differences in pollen limitation intensity. Populations in which beeflies were more abundant were less pollen-limited. In contrast, the abundance of other flower visitors, such as large bees or butterflies, was not associated with pollen limitation. Annual rainfall and bare soil cover were associated with the intensity of pollen limitation across populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant reproduction may be limited by the availability of resources (Suzuki 2000; Griffin and Barrett 2002), inadequate pollen receipt (Ashman et al. 2004; Knight et al. 2005; Aizen and Harder 2007), or a combination of both factors (Haig and Westoby 1988). Pollen limitation appears to be common across Angiosperms (Ashman et al. 2004; Knight et al. 2005). According to recent reviews, between 62 and 73 % of all insect-pollinated plants show evidence of inadequate pollen receipt (Ashman et al. 2004; García-Camacho and Totland 2009).
Pollen limitation may be caused by a decrease in pollen quantity or quality (Aizen and Harder 2007; Harder and Aizen 2010). Pollen quantity limitation occurs when pollinators are scarce or ineffective (depositing low amounts of pollen grains per visit) (Johnston 1991; Gómez et al. 2010). Pollen quality limitation occurs when pollinators deposit incompatible pollen, self-pollen, or pollen from closely related individuals, which may produce inbreeding depression and seed quality reduction (Herrera 1987; Pflugshaupt et al. 2002). Consequently, the potential effects of pollen limitation on reproductive success should ideally be measured in each of the plant’s life-cycle reproductive stages (Ehrlén and Eriksson 1995). Yet, as noted by Knight et al. (2005), most studies only examine the consequences of pollen limitation on fruit and seed set, and few studies measure potential effects on post-dispersal stages (Ehrlén and Eriksson 1995; Price et al. 2008; Gómez et al. 2010).
The consequences of pollen limitation may be dramatic for plants. Reproductive failure due to pollen limitation may lessen population growth and long-term viability, resulting in declines in population size (Eriksson and Jakobsson 1998; Knight et al. 2005). Thus, pollen limitation may strongly influence the distribution and abundance of plant species and populations (Eriksson and Jakobsson 1998). Severe and consistent pollen-limitation may even trigger local extinctions in fragmented or heterogeneous habitats harboring small populations (Aguirre and Dirzo 2008; Hill et al. 2008). Reproductive failure due to pollen limitation is likely to be stronger in small, isolated populations than in large, well-connected populations (Aizen and Feinsinger 1994). This is especially important for the persistence of endemic plants, which have restricted distribution areas, and usually show lower fertility than their widespread congeners (Lavergne et al. 2004). Exploring the importance of pollen limitation for the reproduction of narrow endemic plants living in fragmented habitats is thus essential to understand and predict the consequences of the anthropogenic-mediated pollinator crisis on the conservation of plant populations (Allen-Wardell et al. 1998; Vamosi et al. 2006; Eckert et al. 2010).
In animal-pollinated plants, insufficient pollen deposition or deposition of low-quality pollen is mostly caused by pollinator assemblage characteristics, such as pollinator abundance, diversity, and identity. Many studies have found pollen limitation to be associated with decreased pollinator abundance (Baker et al. 2000; Cunningham 2000; Cosacov et al. 2008; Gonzalez-Varo et al. 2009; Gómez et al. 2010). In addition, pollen limitation may be related to decreased pollinator diversity (Gómez et al. 2010). This relationship appears when the plant’s probability of being visited by effective pollen vectors increases with pollinator diversity (Perfectti et al. 2009). Pollen limitation is also related to the identity of pollinators, as different flower visitors differ in pollinating effectiveness and may have contrasting effects on plant fitness (Klein et al. 2003; Gómez et al. 2007; Ne’eman et al. 2010). In addition to pollinator assemblage traits, some characteristics of the plant population itself may influence pollen limitation intensity directly or indirectly. One example of direct effects is strong fragmentation, which may result in genetic impoverishment, thus setting a limit to the quality of the pollen deposited, irrespective of pollinator community (Byers 1995). Indirect effects occur when plant population characteristics modify pollinator composition and behavior. For example, plant population size, habitat fragmentation, co-occurring flower composition, and altitude influence the community and activity of flower-visiting insects, and therefore the intensity of pollen limitation (Totland 2001; Gonzalez-Varo et al. 2009; Jakobsson et al. 2009).
The general goal of this study is to describe geographical variation of pollen limitation on seed and seedling production in an endangered Mediterranean endemic herb, Erysimum popovii, and to explore the potential factors influencing pollen limitation. Our specific objectives are: (1) to determine the geographic variation in pollen limitation throughout the entire distribution area of the species; (2) to establish at what stage, pre-dispersal (seed production) or post-dispersal (emergence rate), is pollen limitation more intense; (3) to study the association between flower visitor community and pollen limitation; and (4) to study the association between population characteristics (size, density of co-flowering species, habitat composition) and pollen limitation.
Materials and methods
Study system
Erysimum popovii Rothm. (Brassicaceae) is a narrow endemic plant from southeastern Spain, categorized as “Near Threatened” in the Red List of Andalusia Vascular Flora (Cabezudo et al. 2005). The species inhabits rocky areas and shrubland gaps in montane areas from 900 to 2,000 m. a.s.l. It is a biennial to perennial monocarpic herb, usually producing tens of flowers (mean ± SD = 37.6 ± 17.9 flowers; Table 1) on a variable number of reproductive stalks. Flowers are hermaphroditic and slightly protandrous, with four bright purple petals (Blanca et al. 1992, 2009). Flower lifespan is 1–2 days, depending on environmental and pollination conditions (authors’ personal observation). Like other Erysimum species (Gómez 2005), E. popovii is self-compatible, but requires pollen vectors for full seed set (plants from which pollinators are excluded produce seeds, but in low numbers, authors’ personal observation).
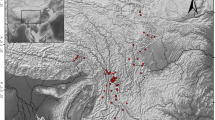
The study was conducted in 21 Erysimum popovii populations located in the Baetic Mountain range (Granada and Jaen provinces, southern Spain; Table 1), spanning the entire geographic range of the species (Fig. 1). Maximum distance between populations was 80 km. The area has a characteristic Mediterranean climate with cool wet winters and warm dry summers.
Pollen addition experiment
We conducted a pollen-supplementation experiment in the spring of 2009. In each of the 21 populations, we labeled 50 plants of average size during peak bloom. In 20 randomly designated plants, we administered pollen to 4 flowers from the central part of the flowering stalk (PA treatment). Pollen was collected in the early morning from newly opened flowers of donor individuals located at least 10 m from the receiving individual. In each receiving individual, we selected 4 additional flowers that were left untreated and served as internal controls (IC treatment). IC flowers were located immediately above or below PA flowers. By selecting PA and IC flowers from the central part of the flowering stalks, we expected to avoid potential confounding effects of flower position on reproductive outcome (Wesselingh 2007). In the 30 remaining plants, we selected 3 flowers as external controls (EC treatment). EC flowers were used to detect possible effects of pollen supplementation on resource allocation (Wesselingh 2007). Domestic and wild ungulates consumed some of our experimental plants. As a result, we could recover enough individuals to perform statistical analyses in only 13 of the 21 populations. For this reason, all subsequent pollen limitation analyses are based on 13 populations.
We measured the effect of pollen limitation during both the pre-dispersal stage of the plant’s reproductive success (seed production), and the post-dispersal stage (seedling emergence rate). Seed production was estimated as seeds per flower. At the end of the fruiting season, we collected ripe fruits produced by PA, IC, and EC flowers. For each fruit, ovules, aborted seeds, and ripe seeds were counted in the laboratory using a magnifying glass. We were able to discern ripe seeds, aborted seeds, and unfertilized ovules because, as in other crucifers, ripe seeds are light brown and larger, and aborted seeds are dark brown, with shriveled cotyledons and embryo (Gómez et al. 2010). By contrast, unfertilized ovules are creamy white and smaller. Seedling emergence rate was measured as the proportion of seeds producing seedlings. Due to the relatively low number of seeds obtained in some mother × treatment combinations, we decided to pool all seeds within each treatment and population, and randomly selected 24 seeds per treatment and population (PA, IC, and EC). In February 2010, these seeds were sown in seedbeds in a greenhouse, and emergence was monitored weekly until April.
To determine the magnitude of pollen limitation, we used a pollen limitation (PL) index calculated as 1 − (RSC/RSPA), where RSC is the mean reproductive success of the control treatment and RSPA the mean reproductive success of the pollen addition treatment. This index has a straightforward biological interpretation (Lázaro and Traveset 2006). Positive values result from higher reproductive success in PA than C, thus indicating pollen limitation, while zero or negative values indicate absence of pollen limitation. We calculated the PL index for both components of reproductive success (seed production and seedling emergence), and for the comparisons PA versus IC and PA versus EC (henceforth PLIC and PLEC). The difference between these two PL estimates provides a measure of resource allocation as a consequence of the experimental supplementation of pollen (Gómez et al. 2010).
Flower visitor assemblage
We conducted surveys of floral visitors in each of the 21 populations during the springs of 2008 and 2009. Each population was sampled on several days throughout its flowering period. Groups of plants with approximately 100 flowers were tagged and repeatedly observed over the course of the day (from 1100 to 1700 hours) and all insects seen actively visiting the flowers (collecting pollen and/or nectar) were noted. We tried to record a minimum of 200 flower–pollinator contacts per population because this number provided a good estimate of overall pollinator assemblage in E. mediohispanicum (Gómez et al. 2007). Total survey time per population ranged from 190 to 795 min. We observed a total of 5,169 flower visits (193–331 per population). Specimens (n = 486) of most morphospecies were captured for later identification in the laboratory.
We estimated flower visitation rate as the number of flower visits observed per minute of observation. We also quantified the visitation rate of each type of flower visitor, considering the following five functional groups: large bees (10 mm or more in body length), small bees (<10 mm in body length), butterflies (mostly Rhopalocera, all nectar collectors), beeflies (long-tongued Bombyliidae), and others (including ants, beetles, hoverflies, and other minor groups previously shown to have low pollinating efficiency on Erysimum; Gómez et al. 2007, 2008, 2009). Finally, we estimated the overall diversity of the flower visitor community in each population using Hurlbert’s PIE (EcoSim 7; Gotelli and Entsminger 2009). This index ranges between 0 (low diversity) and 1 (maximum diversity), and measures the probability that two randomly sampled individuals from the community belong to different species.
Plant population characteristics
We measured the following variables to characterize the E. popovii populations: population size (all reproductive individuals in each population), altitude (meters above sea level measured with a GPS), annual rainfall (in mm, obtained from the Digital Climatic Atlas of the Iberian Peninsula; Ninyerola et al. 2005), density of co-occurring flowering plants (flowers per m2), woody plant cover (percent area occupied by woody plants), bare soil cover (percent area occupied by bare soil), and rock cover (percent area occupied by rocks). Flowering plant density was measured along three transects of 10 × 2 m in which we counted the number of flowers of species blooming at the same time as E. popovii. Habitat cover was estimated along three additional transects of 25 × 2 m in which, every 0.5 m, we scored microhabitat type in the center and at the two edges of the transect width.
Data analysis
Significance of the PL index for seed production of each population was obtained with bootstrapping, running 1,000 replicates per population (Boot package; R Development Core Team 2008). Bootstrapping could not be applied to post-dispersal PL results because, by pooling all seeds within each treatment and population, internal variation required for this analysis was lost (Davison and Hinkley 1997). Thus, we used contingency tables to compare seedling emergence rates across treatments within each population.
Spatial autocorrelation of PL indices was analyzed with Moran’s I coefficient at different distance classes (Rangel et al. 2010). The number of distance classes was established by the default function of the software (SAM v.4.0; Rangel et al. 2010). The relationship of flower–visitor community and population characteristics with pollen limitation intensity was explored by means of a model selection approach. We run a set of models including all possible combinations of independent variables. Since pollen limitation was spatially autocorrelated (Appendix 1), these models were spatially-explicit, including X and Y geographic coordinates as co-variables to control for the possible effects of spatial distribution. Prior to running these models, we checked for multicollinearity by performing pairwise Pearson’s correlations amongst variables. Since no strong correlation appeared between variables (Appendices 2 and 3), we included all independent variables in the models. Due to the high number of independent variables with respect to the number of samples (13 populations), we ran separate models for flower–visitor and population variables. To further avoid potential problems derived from the small number of populations, we decided not to include more than three independent variables in each model. We thus ran all models resulting from all possible combinations of one, two, or three variables. All analyses were performed with SAM v.4.0 (Rangel et al. 2010). Because the two PL indices were consistent (see below), we only show outcomes of PLEC, which is more robust to resource reallocation (Wesselingh 2007).
To select the best model(s), we used an information-theoretic approach (Burnham and Anderson 2002; Richard 2005; Stephens et al. 2007). We first selected those models providing an appropriate goodness of fit (P < 0.05; Grace 2006). For this subset of candidate models, we calculated the Akaike information criterion (AIC) and the Akaike weights (AICwi). All models having AICwi > 0.2 were considered an appropriate representation of the raw data (Burnham and Anderson 2002). By means of a multimodel inference process, we determined the importance of each independent variable across all models weighted by their AIC values (Richard 2005).
Results
Pollen limitation
Despite large differences among populations, flowers from PA treatments produced more seeds than the two control treatments in all populations but one (Fig. 2). Excluding population Ep03 (in which IC flowers produced more seeds than PA flowers), PA flowers produced a mean of 3.76 more seeds (SD = 2.03; range 0.74–7.37; n = 12 populations) than IC flowers. When considering entire plants, this represents a mean increase of 155 ± 115 seeds per individual (range 26–416). Of the 13 populations analyzed, PLIC was significant in 8, and PLEC in 10 (Table 2), although PL values were mostly moderate (PLIC = 0.18–0.45; PLEC = 0.16–0.49). When pooling all populations, pollen limitation for seed production was significantly different from zero for both PL indices (Table 2).
Seed production (seeds per flower) in PA (pollen addition), IC (internal control), and EC (external control) flowers from populations for which we could conduct bootstrapping analysis of pollen limitation (see Table 2). Bars means (+SE)
On the other hand, we found no significant pollen limitation for seedling emergence rate (PLIC = 0.012, P = 0.46; PLEC = −0.039, P = 0.31). In fact, only two populations (Ep01 and Ep15) were pollen limited for seedling emergence and only when comparing PA and EC treatments (Table 2).
We did not find a consistently better performance of EC treatment compared to IC treatment (binomial test of EC performance >IC performance, P = 0.5), with no differences between EC and IC in seed production when data from all populations were lumped together (Fig. 2). In addition, in most populations (9 out of 13), PLIC and PLEC values were similar (Table 2). These results provide no evidence of reallocation between internal control and pollen-supplemented flowers.
Flower visitors
The flower visitor community of Erysimum popovii was very diverse, including large bees, small bees, beeflies, butterflies, hoverflies, beetles, and ants (Fig. 1). Taking all populations together, we observed 166 insect species visiting the flowers of E. popovii, ranging from 11 to 32 species per population (Appendix 4). Flower visitor diversity ranged between 0.62 and 0.94 (Hurlbert’s PIE index). Mean visitation rate was 0.348 visits per flower per hour (range 0.084–1.050) (Appendix 4). Large bees and beeflies were the most abundant flower visitors (Fig. 1).
Factors associated with pollen limitation
As we found no evidence of pollen limitation on germination rates, analyses exploring the factors associated to pollen limitation were only conducted for seed production. The visitation rate of beeflies was the main variable influencing pollen limitation, with an importance value of 0.966, which is much higher than the importance values of the others variables (Table 3). Consequently, the best model included only beefly visitation rate (Model A; Tables 3, 4). According to this model, beefly visitation rate was negatively correlated with the intensity of pollen limitation (Table 3), so that populations with higher beefly abundance showed less pollen limitation. The abundance of the other flower visitor groups (large bees, small bees, butterflies, and others), as well as the visitation rate and diversity of the whole flower visitor assemblage revealed no relationship to pollen limitation (Table 3). These variables showed very low importance values (Table 3), and did not appear in the selected models (Table 4).
When exploring the effect of population characteristics, we found that annual rainfall and bare soil cover were the two most important variables (0.50 and 0.44 importance values, respectively; Table 3). The selection model process yielded two equally good models (Models B and C; Table 4). Annual rainfall was positively and significantly related to pollen limitation intensity (Table 3). That is, pollen limitation was stronger in populations receiving more precipitation. Model C only included bare soil cover, which was marginally and negatively correlated with PLEC (Table 3). In other words, pollen limitation tended to be lower in populations with extensive bare soil cover. The remaining population characteristics did not correlate with pollen limitation intensity (Table 3).
Discussion
Our study has shown that E. popovii reproduction was pollen limited throughout most of its distribution range. This finding was somewhat unexpected because E. popovii is self-compatible and its flowers are visited by over 166 insect species belonging to very disparate functional and taxonomic groups, from large bees and butterflies to hoverflies, beeflies, beetles, and ants. In addition, intrapopulation diversity of flower visitors was very high, with Hurlbert’s PIE values around 0.84. These results indicate that the pollination system of E. popovii is very generalist, as found in other Erysimum species, such as E. mediohispanicum, E. nevadense, and E. baeticum (Gómez et al. 2007; Ortigosa and Gómez 2010). Self-compatible and/or pollinator generalist plants are less prone to pollen limitation than self-incompatible and/or specialist species (Ashman et al. 2004; Knight et al. 2005). Nevertheless, pollen limitation has been found in another Erysimum species with a similar pollination system (Gómez et al. 2010). In E. popovii, pollen limitation affected seed production rather than emergence rate. This outcome points to a quantitative rather than a qualitative pollen limitation (Aizen and Harder 2007). However, because inbreeding depression in self-compatible species is more pronounced in late stages of the life cycle (Husband and Schemske 1996), further studies would be needed to confirm this conclusion in E. popovii.
Resource reallocation from untreated to pollen-supplemented flowers is always a concern in PL experiments. To avoid confounding results related to resource reallocation, some studies have submitted whole individuals to control or experimental treatments (Baker et al. 2000). Although we could not follow this approach, because E. popovii individuals often produce several hundred flowers, we used two complementary controls, one internal and the other external, to detect potential reallocation (Wesselingh 2007; Gómez et al. 2010). We found that the reproductive output of the two control treatments was similar and consistent, suggesting that resource reallocation, if existing, was weak. In fact, under severe resource reallocation, we would have consistently expected better performance by external rather than internal control flowers. However, this was not so, as only half the populations showed this pattern. In addition, we found a high degree of congruence between our two measures of pollen limitation (PLIC and PLEC), which showed the same trend in 12 of the 13 populations that could be statistically analyzed (Table 2). Finally, we found no differences between the two controls for seedling emergence rate.
Variation among populations in pollinator assemblage may produce inter-population differences in pollen movement patterns and pollen transfer effectiveness, because different types of flower visitors have different flower-handling behaviors and show important differences in the amount and quality of pollen deposited per visit (Gómez and Zamora 1999; Fenster and Dudash 2001; Cosacov et al. 2008). Consequently, several studies have found that plant populations differ in the intensity of pollen limitation as a consequence of differences in pollinator abundance and diversity (Baker et al. 2000; Gonzalez-Varo et al. 2009; Gómez et al. 2010). In E. mediohispanicum, a co-ocurring and ecologically similar species to E. popovii, pollinator diversity and abundance significantly affect pollen limitation (Gómez et al. 2010). Under this perspective, it is remarkable that none of the two variables were correlated with pollen limitation intensity in E. popovii. E. mediohispanicum was found to be pollen limited in 50 % of the populations studied (Gómez et al. 2010), whereas E. popovii is pollen limited throughout most of its geographic range. We believe that this disparity between the two plant species is a consequence of differences pollinator visitation rate between both species. Pollinator visitation rates in E. popovii (mean ± SD: 0.348 visits flower−1 h−1; Appendix 4) are three times lower than those reported for E. mediohispanicum (mean ± SD: 0.960 visits flower−1 h−1; Gómez et al. 2007). More important, some populations received an even lower amount of visits. Since E. popovii flowers last only 1 or 2 days, we believe that this difference in pollinator abundance at flowers may cause the strong pollen limitation observed in this plant species. These differences could be due to traits intrinsic to each species (e.g., E. popovii individuals produce fewer flowers than E. mediohispanicum; Blanca et al. 2009), or to extrinsic factors such as differences in co-blooming plant composition. Lay et al. (2011) have recently found Erysimum capitatum not to be pollen limited, despite having a pollinator visitation frequency similar to E. mediohispanicum. They argue that this could be due to E. capitatum pollinators being more effective than those of E. mediohispanicum.
In E. popovii, beefly abundance was associated with low intensity of pollen limitation. Beeflies are likely to be very efficient pollinators of E. popovii because, compared to most other flower visitors, they move frequently between plants and visit few flowers per individual. This kind of foraging behavior has also been observed on E. mediohispanicum (Gómez et al. 2011). This foraging pattern potentially maximizes deposition of allogamous pollen per visit, which may reduce abortion rate and decrease pollen limitation during pre-dispersal stages (Vaughton and Ramsey 2010). Beeflies have been shown to be highly efficient pollinators in other plant species (Motten et al. 1981; Johnson and Dafni 1998; Kastinger and Weber 2001; Anderson et al. 2005; Koopman and Ayers 2005), including the con-specific E. mediohispanicum (Gómez et al. 2009, 2011). These results show that the abundance of highly efficient pollinator groups, rather than the abundance of the overall flower visitor assemblage, may be the most important determinant of pollen limitation in some pollination systems (Lay et al. 2011).
Our experiments also demonstrate that some environmental variables, such as annual rainfall and bare soil cover, are associated with pollen limitation in E. popovii. Annual rainfall was positively related to pollen limitation. This is probably due to the fact that plants growing in areas with low rainfall are unable to respond to supplementary pollination by increasing their seed number. In other words, in populations with low rainfall, seed production is likely to be limited by water availability or by a combination of water availability and pollen (Haig and Westoby 1988). Bare soil cover had a marginally significant negative effect on pollen limitation. A potential explanation could be related to the fact that most bees nest underground in areas deprived of vegetation. Consequently, bare soil availability may enhance the abundance and diversity of bees and other ground-nesting pollinators (Potts et al. 2005). In addition, by promoting bee abundance, bare soil may also enhance beefly abundance, as most beeflies are parasitic on ground-nesting bees and wasps (Boesi et al. 2009).
In sum, our study shows that E. popovii is pollen limited throughout most of its geographic range, and, although visited by a diverse community of pollinating insects, the intensity of pollen limitation in this species is mostly attributable to a specific pollinator group, namely beeflies.
References
Aguirre A, Dirzo R (2008) Effects of habitat fragmentation on pollinator abundance and fruit set on an abundant understory palm in a Mexican tropical forest. Biol Conserv 141:375–384
Aizen MA, Feinsinger P (1994) Forest fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina. Ecology 75:330–351
Aizen MA, Harder LD (2007) Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88:271–281
Allen-Wardell G, Bernhardt P, Bitner R, Burquez A, Buchmann S, Cane J, Allen Cox P, Dalton V, Feinsinger P, Ingram M, Inouye D, Jones CE, Kennedy K, Kevan P, Koopowitz H, Medellin R, Medillin-Morales S, Nabhan GP (1998) The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv Biol 12:8–17
Anderson B, Johnson SD, Carbutt C (2005) Exploitation of a specialized mutualism by a deceptive orchid. Am J Bot 92:1342–1349
Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DR, Dudash MR, Johnston MO, Mazer SJ, Mitchell RJ, Morgan MT, Wilson WG (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421
Baker AM, Barrett SCH, Thompson JD (2000) Variation of pollen limitation in the early flowering Mediterranean geophyte Narcissus assoanus (Amaryllidaceae). Oecologia 124:529–535
Blanca G, Morales C, Ruíz-Rejón M (1992) El género Erysimum L. (Cruciferae) en Andalucía (España). Ann Jardín Bot Madrid 49:201–214
Blanca G, Cabezudo B, Cueto M, Fernández C, Morales C (2009) Flora Vascular de Andalucía Oriental. Consejería de Medio Ambiente, Junta de Andalucía
Boesi R, Polidori C, Andrietti F (2009) Searching for the right target: oviposition and feeding behavior in Bombylius bee flies (Diptera: Bombyliidae). Zool Stud 48:141–150
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach. Springer, New York
Byers DL (1995) Pollen quantity and quality as explanations for low seed set in small populations exemplified by Eupatorium (Asteraceae). Am J Bot 82:1000–1006
Cabezudo B, Talavera S, Blanca G, Salazar C, Cueto M, Valdés B, Hernández JE, Herrera CM, Rodríguez C, Navas D (2005) Lista Roja de la flora vascular de Andalucía. Consejería de Medio Ambiente, Junta de Andalucía
Cosacov A, Nattero J, Cocucci AA (2008) Variation of pollinator assemblages and pollen limitation in a locally specialized system: the oil-producing Nierembergia linariifolia (Solanaceae). Ann Bot 102:723–734
Cunningham SA (2000) Depressed pollination in habitat fragments causes low fruit set. Proc R Soc Lond B 267:1149–1152
Davison AC, Hinkley DV (1997) Bootstrap methods and their application. Cambridge University Press, Cambridge
Eckert CG, Kalisz S, Geber MA, Sargent R, Elle E, Cheptou P-O, Goodwillie C, Johnston MO, Kelly JK, Moeller DA, Porcher E, Ree RH, Vallejo-Marin M, Winn A (2010) Plant mating systems in a changing world. Trends Ecol Evol 25:35–43
Ehrlén J, Eriksson O (1995) Pollen limitation and population growth in a herbaceous perennial legume. Ecology 76:652–656
Eriksson O, Jakobsson A (1998) Abundance, distribution and life histories of grassland plants: a comparative study of 81 species. J Ecol 86:922–933
Fenster CB, Dudash MR (2001) Spatiotemporal variation in the role of hummingbirds as pollinators of Silene virginica. Ecology 82:844–851
García-Camacho R, Totland O (2009) Pollen limitation in the alpine: a meta-analysis. Arct Antarct Alp Res 41:103–111
Gómez JM (2005) Non-additive effects of pollinators and herbivores on Erysimum mediohispanicum (Cruciferae) fitness. Oecologia 143:412–418
Gómez JM, Zamora R (1999) Generalization vs. specialization in the pollination system of Hormathophylla spinosa (Cruciferae). Ecology 80:796–805
Gómez JM, Bosch J, Perfectti F, Fernandez JD, Abdelaziz M (2007) Pollinator diversity affects plant reproduction and demography: the trade-offs of generalization. Oecologia 153:597–605
Gómez JM, Bosch J, Perfectti F, Fernández JD, Abdelaziz M, Camacho JPM (2008) Spatial variation in selection on corolla shape in a generalist plant is promoted by the preference patterns of its local pollinators. Proc R Soc Lond B 275:2241–2249
Gómez JM, Abdelaziz M, Camacho JPM, Muñoz-Pajares J, Perfectti F (2009) Local adaptation and maladaptation to pollinators in a generalist geographic mosaic. Ecol Lett 12:672–682
Gómez JM, Abdelaziz M, Lorite J, Muñoz-Pajares AJ, Perfectti F (2010) Changes in pollinator fauna cause spatial variation in pollen limitation. J Ecol 98:1243–1252
Gómez JM, Perfectti F, Jordano P (2011) The functional consequences of mutualistic network architecture. PLoS One 6(1):e16143
Gonzalez-Varo JP, Arroyo J, Aparicio A (2009) Effects of fragmentation on pollinator assemblage, pollen limitation and seed production of Mediterranean myrtle (Myrtus communis). Biol Conserv 142:1058–1065
Gotelli NJ, Entsminger GL (2009) EcoSim: null models software for ecology. Version 7. Acquired Intelligence Inc. and Kesey-Bear, Jericho, VT 24 05465. http://garyentsminger.com/ecosim.htm
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Griffin SR, Barrett SCH (2002) Factors affecting low seed: ovule ratios in a spring woodland herb, Trillium grandiflorum (Melanthiaceae). Int J Plant Sci 163:581–590
Haig D, Westoby M (1988) On limits to seed production. Am Nat 131:757–759
Harder LD, Aizen MA (2010) Floral adaptation and diversification under pollen limitation. Philos Trans R Soc Lond B 365:529–543
Herrera C (1987) Components of pollinator quality: comparative analysis of a diverse insect assemblage. Oikos 50:79–90
Hill LM, Brody AK, Tedesco CL (2008) Mating strategies and pollen limitation in a globally threatened perennial Polemonium vanbruntiae. Acta Oecol 33:314–323
Husband BC, Schemske DW (1996) Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 46:216–225
Jakobsson A, Lázaro A, Totland Ø (2009) Relationships between the floral neighborhood and individual pollen limitation in two self-incompatible herbs. Oecologia 160:707–719
Johnson SD, Dafni A (1998) Response of bee-flies to the shape and pattern of model flowers: implications for floral evolution in a Mediterranean herb. Funct Ecol 12:289–297
Johnston M (1991) Pollen limitation of female reproduction in Lobelia cardinalis and L. siphiliaca. Ecology 72:1500–1503
Kastinger C, Weber A (2001) Bee-flies (Bombylius spp., Bombyliidae, Diptera) and the pollination of flowers. Flora 196:3–25
Klein AM, Steffan-Dewenter I, Tscharntke T (2003) Fruit set of highland coffee increases with the diversity of pollinating bees. Proc R Soc Lond B 270:955–961
Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston M, Mitchell RJ, Ashman TL (2005) Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497
Koopman MM, Ayer TJ (2005) Nectar spur evolution in the Mexican lobelias (Campanulaceae: Lobelioideae). Am J Bot 92:558–562
Lavergne S, Thompson JD, Garnier E, Debussche M (2004) The biology and ecology of endemic and widespread plants: a comparative study of trait variation in 20 congeneric pairs. Oikos 107:505–518
Lay CR, Linhart YB, Diggle PK (2011) The good, the bad and the flexible: plant interactions with pollinators and herbivores over space and time are moderated by plant compensatory responses. Ann Bot. doi:10.1093/aob/mcr152
Lázaro A, Traveset A (2006) Reproductive success of the endangered shrub Buxus balearica Lam. (Buxaceae): pollen limitation, and inbreeding and outbreeding depression. Plant Syst Evol 261:117–128
Motten AF, Campbell DR, Alexander DE (1981) Pollination effectiveness of specialist and generalist visitors to a North Carolina population of Claytonia virginica. Ecology 62:1278–1287
Ne’eman G, Jürgens A, Newstrom-Lloyd L, Potts SG, Dafni A (2010) A framework for comparing pollinator performance: effectiveness and efficiency. Biol Rev 85:435–451
Ninyerola M, Pons X, Roure JM (2005) Atlas Climático Digital de la Península Ibérica. Metodología y aplicaciones de bioclimtología y geobotánica. Autonomous University of Barcelona, Bellaterra
Ortigosa AL, Gómez JM (2010) Differences in the diversity and composition of the pollinator assemblage of two co-flowering congeneric alpine wallflowers, Erysimum nevadense and E. baeticum. Flora 205:266–275
Perfectti F, Gómez JM, Bosch J (2009) The functional consequences of diversity in plant-pollinator interactions. Oikos 118:1430–1440
Pflugshaupt K, Kollmann J, Fischer M, Roy B (2002) Pollen quantity and quality affect fruit abortion in small populations of a rare fleshy-fruited shrub. Basic Appl Ecol 3:319–327
Potts SG, Vulliamy B, Robert S, O’Toole C, Dafni A, Neeman G, Willmer P (2005) Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol Entomol 30:78–85
Price MV, Campbell DR, Waser NM, Brody AK (2008) Bridging the generation gap in plants: pollination, parental fecundity, and offspring demography. Ecology 89:1596–1604
R Development Core Team (2008) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna. http://www.R-project.org
Rangel TF, Diniz-Filho JAF, Bini LM (2010) SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33:46–50
Richard SA (2005) Testing ecological theory using the information-theoretic approach: example and cautionary results. Ecology 86:2805–2814
Stephens PA, Buskirk SW, Martínez del Rio C (2007) Inference in ecology and evolution. Trends Ecol Evol 22:192–197
Suzuki N (2000) Pollinator limitation and resource limitation of seed production in the Scotch broom, Cytisus scoparius (Leguminosae). Plant Species Biol 15:187–193
Totland Ø (2001) Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82:2233–2244
Vamosi JC, Knight TM, Steets JA, Mazer SJ, Burd M, Ashman T-L (2006) Pollination decays in biodiversity hotspots. Proc Natl Acad Sci USA 103:956–961
Vaughton G, Ramsey M (2010) Floral emasculation reveals pollen quality limitation of seed output in Bulbine bulbosa (Asphodelaceae). Am J Bot 97:174–278
Wesselingh RA (2007) Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytol 174:26–37
Acknowledgments
Modesto Berbel, Jesús Muñoz, Juan Lorite, Ángela Cano, Helena Barril, Francisco Perfectti and Mª Belén Herrador helped us during various stages of the research. We also thank the handling editor and two anonymous reviewers for their comments, which helped improve the manuscript. This study was partially funded by MARM (078/2007), Junta de Andalucía (P07-RNM-02869), MICINN (FPU-2006) and Consolider-Ingenio (CSD2008-00040) grants. All experiments complied with the current Spanish laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jeff Karron.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernández, J.D., Bosch, J., Nieto-Ariza, B. et al. Pollen limitation in a narrow endemic plant: geographical variation and driving factors. Oecologia 170, 421–431 (2012). https://doi.org/10.1007/s00442-012-2312-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2312-1