Abstract
By using the density functional theory method, we investigated the heats of formation (HOFs), electronic structure, detonation properties, thermal stability and sensitivity for a set of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives with different substituents and different numbers of N-oxides. Our findings reveal that the HOFs of the derivatives decrease dramatically with the increasing number of N-oxides. The effects of the substituents on the HOMO-LUMO gaps are coupled with those of the N-oxides. The calculated detonation properties point out that −NF2, −ONO2 and an increasing number of N-oxides are very helpful for improving the detonation performance of the designed derivatives. The bond dissociation energies of the weakest bonds indicate that a majority of our designed compounds have better thermal stability. The −NH2 group is very useful to decrease the free space value. Most of the derivatives have higher h50 values compared with parent molecules. Considering the sensitivity, thermal stability and detonation performance, four compounds could be considered as potential candidates of high–energy density compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High–energy density compounds (HEDCs) have received sizable recognition in recent past due to their wide applications in national defence as well as in non-military fields such as rock breaking, drilling and bulldozing [1,2,3]. Among different kinds of HEDCs, nitrogenous heterocyclic compounds have attracted much attention owing to their amicable insensitivity, better explosive performance and environment-friendly characters over the last couple of decades [4,5,6,7,8,9,10]. Six-membered nitrogenous heterocyclic compounds have been broadly studied as HEDCs not only experimentally but also theoretically on account of their high density, large heats of formation and good thermal stability [11,12,13,14,15,16,17]. Despite all these, researchers are still making endeavours to look for excellent potential HEDC candidates in order to meet the persisting demand for HEDCs. With regard to searching for new HEDCs with admirable characteristics, one way is to use the nitrogenous heterocycles as basic skeletons, which has been proved to be an effective approach by many studies [18,19,20,21,22,23]. 1, 2, 3, 4-Tetrazine is a six-membered heterocyclic compound holding four nitrogen atoms and nitrogen content of 68.3% in its structure, making it significant for designing HEDCs. It has been examined by researchers both in fused rings and in non-fused rings as HEDC candidates [19, 24, 25]. However, heretofore, no one has fused 1, 2, 3, 4-tetrazine and pyrazine together to examine the structure and properties as HEDCs systematically. This deficiency in the present knowledge drives us to fuse 1, 2, 3, 4-tetrazine and pyrazine to get pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine and then to amend the fused system by introducing suitable substituents to modulate its energetic properties.
Recently, computer-assisted quantum mechanical calculations play a very important role in estimating the explosive nature of HEDCs [26,27,28]. Precise theoretical evaluations on designed energetic compounds make experimental researchers focus their efforts exclusively on those HEDC candidates with excellent performance. This could assist in designing optimal laboratory schemes, avoiding a large number of overpriced experiments and excluding substandard candidates from further consideration [29, 30].
The optimization of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine–based derivatives with high density and high energy is an initial step for designing HEDCs. Their explosive properties are commonly improved by modifying the structure of the parent heterocycle. Incorporating −NO2, −NH2, −NHNO2, −N3, −NF2, −ONO2 or/and N-oxide into nitrogen-rich heterocycle compounds has been proved to be a very encouraging way for enhancing overall explosive performance [31, 32]. N-Oxide is a recent new way which not only improves the oxygen balance but also helps the molecule in efficient crystal packing.
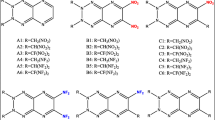
In this work, we used the density functional theory (DFT) method to calculate the heats of formation (HOFs), explosive properties and thermal stabilities of our designed energetic pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives (Fig. 1). The HOFs of the derivatives were calculated using isodesmic reactions. After that, their detonation properties were estimated using predicted HOFs and density values. Lastly, their thermal stabilities were examined based on their bond dissociation energies.
Computational methodology
The DFT-B3LYP method with 6-311 G** basis set was adopted in our calculations because it was previously employed to estimate the HOFs of energetic compounds successfully through isodesmic reactions [22, 32, 33]. Also, the B3PW91/6-31G** method was used for a comparison. In this study, we designed an isodesmic reaction where the total numbers of bonds on reactant and product sides were kept unchanged to reduce errors in calculating the HOF values. In our plotted isodesmic reactions, the basic skeletons of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine and its corresponding N-oxide derivatives were retained, while other big groups were converted to smaller ones. This method has shown to be very reliable [34,35,36].
The isodesmic reactions used to get the HOFs of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives at 298 K are shown in Scheme 1.
For the isodesmic reactions, the heat of reaction ΔH298 at 298 K can be calculated from the following formula:
where ΔHf,R and ΔHf,P are the HOFs of the reactants and products at 298 K, respectively. As the experimental values of HOFs for CH3N3, CH3NHNO2, CH3NF2, pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine (P1), pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-2-N-oxide (P2) and pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-1, 3-di-N-oxide (P3) are unavailable, further calculations were performed for atomization reaction: CaHbOcNdFe → aC(g) + bH(g) + cO(g) + dN(g) + eF(g) at the G2 (Gaussian2) [37] and CBS-Q (complete basis set) levels [38] to obtain accurate HOF values. The G2 and CBS-Q methods have demonstrated to estimate the HOFs precisely [39,40,41]. As the G2 theory is much more expensive to predict the HOFs of big molecules, therefore, we performed the G2 calculations for small molecules and the CBS-Q calculations for big molecules. The experimental HOF values for reference compounds CH3NH2, CH3ONO2, CH3NO3 and CH4 already exist in literature. Now, the most essential job is to figure out ΔH298. The ΔH298 can be calculated by the following equation:
where ΔE0 is the total change in energy between the products and reactants at 0 K; ΔZPE is the difference between zero-point energies of products and reactants at 0 K; ΔHT is the thermal correction from 0 to 298 K. The Δ(PV) value is equal to ΔnRT for ideal gas reactions. Here, for our designed isodesmic reaction, Δn = 0; hence, Δ(PV) = 0.
As a majority of energetic materials exist in solid phase, their explosive properties must be estimated using the solid-phase HOFs (ΔHf,solid). According to Hess’s law [41], ΔHf,solid can be calculated from ΔHf,gas and ΔHsub by:
The ΔHsub for energetic materials can be calculated from the electrostatic interaction index (υσtot2) and molecular surface area [42] as follows:
where A is the surface area of 0.001 contour of electronic density on the isosurface, υ describes the degree of balance between positive and negative electrostatic potentials on the isosurface, and σtot2 is the measure of variability of electrostatic potential on the isosurface. The coefficients were taken from literature [43] as a = 2.670 × 10−4 kcal mol−1, b = 1.650 kcal mol−1 and c = 2.966 kcal mol−1.
The detonation pressure and detonation velocity were calculated by Kamlet–Jacobos [44] equation:
where every term in Eqs. (4) and (5) is defined as follows: D, the detonation velocity (km s−1); P, the detonation pressure (GPa); N, the number of detonation gases per gramme of explosive; M, the average molecular weight of these gases; Q, the heat of detonation (cal g−1); and ρ, the loaded density of explosives (g cm−3). For common explosives, their heat of detonation and densities could be assessed experimentally; hence, their detonation velocity and detonation pressure can be calculated from Eqs. (5) and (6), respectively. However, for some compounds whose Q and ρ cannot be calculated experimentally, it is necessary to calculate Q and ρ first before the calculations of D and P.
The following modified equation was used to calculate the theoretical density where electrostatic interaction index υσtot2 term was included [45]:
where M is the mass of the molecule (g mol−1) and V (0.001) is the volume of the 0.001 electrons bohr−3 contour of electronic density of the molecule (cm3/molecule). The numerical values of the coefficients α, β and γ are 0.9183, 0.0028 and 0.0443, respectively.
The free space per molecule in a unit cell, which can give a clue about the sensitivity of energetic materials [46, 47], was determined as the difference between the effective volume Veff and intrinsic gas-phase molecular volume Vint by:
where Veff is the volume exactly needed to entirely pack the unit cell and Vint is the volume enclosed by 0.003 electrons/bohr3 contour of molecular electronic density. Veff can be determined by the following formula:
The impact sensitivity (h50, cm) can be determined using the following expression [48]:
where V (0.002) denotes the volume enclosed by 0.002 electrons/bohr3. υσtot2 is the electrostatic interaction index determined during the same step. The coefficients α, β and γ are − 234.83, − 3.197 and 962, respectively.
The heats of detonation (Q) were figured out by HOF differences between products and energetic materials according to the principle of exothermic reactions. The detonation products are supposed to be CO2 (or C), H2O (or H2 or F2) and N2, so the liberated energy should reach its peak value. Using these values of ρ and Q, the D and P values can be assessed. The theoretical densities predicted in this study are slightly larger than the actual loaded densities; therefore, the D and P values can be regarded as their upper limits.
The strength of a chemical bond, which could be examined based on bond dissociation energy, is elementary to understanding chemical processes [49]. The energy needed for bond homolysis at 298 K and 1 atm corresponds to the enthalpy of reaction A – B (g) → A. (g) + B. (g), which is bond dissociation enthalpy of the molecule by definition [50]. For many organic molecules, the terms “bond dissociation energy (BDE)” and “bond dissociation enthalpy” are often used interchangeably in the literature [51]. Thus, at 0 K, the homolytic bond energy can be derived from the following expression:
The BDE with zero-point correction can be calculated by the following equation:
where ΔEZPE is the difference between the ZPEs of the products and reactants.
The calculations were performed with Gaussian 09 package [52]. The optimizations were performed without any symmetry restrictions using the default convergence criteria in the program. All of the optimized structures were characterized to be true local energy minima on the potential energy surfaces without imaginary frequencies. Also, we selected two different functionals (B3LYP and B3PW91) in the calculations of some important properties such as heats of formation, densities and free space. This will let us and the readers know how differently the two functionals predict some of the most important properties of energetic materials.
Results and discussion
Heats of formation
HOF is often considered an indicatory factor of the “energy content” of energetic materials. That is why it is crucial to predict HOFs accurately. Here, we studied the effect of N-oxide and different substituents on the HOFs of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine. The molecular numbering of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives in Fig. 1 is shown as A1–A6, B1–B6, C1–C5, D1–D6, E1–E6, F1–F5, G1–G6, H1–H6 and I1–I5. Total energies, thermal corrections and ZPEs for the reference compounds in the isodesmic reactions are listed in Table S1 of Supporting Information. The experimental data of ΔHf,gas for CH4, CH3NH2, CH3NO2 and CH3ONO2 were taken from literature. Since CH3N3, CH3NHNO2, CH3NF2, pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine, pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-2-N-oxide and pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-1, 3-di-N-oxide have no experimental HOF values, their HOF values were calculated via atomization reactions by using the G2 and CBS-Q methods. In order to validate the credibility of the G2 theory and CBS-Q method, we calculated the HOFs for all reference compounds by the above two methods. However, the sizes of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine, pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-2-N-oxide and pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-1, 3-di-N-oxide are quite large. Therefore, we could not calculate their HOFs at the G2 level. It is seen in Table 1 that their HOF values by the G2 and CBS-Q methods can be compared with the experimental values. The mean absolute deviation of the HOFs at the CBS-Q level from the experimental values is 3.82663, while that at the G2 level is 5.9284. The linear relationship between the CBS-Q and G2 calculated HOFs is pretty good: HOFG2 = 0.9729HOFCBS-Q + 3.8547 with R2 = 0.9991. This indicates that the two methods produce similar results, but the former has comparatively lower mean absolute deviation than the latter. The HOF values of CH4, CH3NH2 and CH3NO2 in parentheses calculated at the G2 level were taken from refs. [39, 53], which are very much closer to our values, indicating that our calculations are reliable.
Table S2 of Supporting Information lists thermal corrections, zero-point energies, total energies, heat of sublimation and solid- and gas-phase HOFs of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives. We calculated their HOFs at two different methods and basis sets: B3LYP/6-311G** and B3PW91/6-31G**. The results show that the HOFs calculated at two different methods and basis sets are very similar. They exhibit a very nice linear relationship: HOFB3PW91/6 − 31G ∗ ∗ = 0.9984B3LYP/6 − 311G ∗ ∗ – 3.486 with R2 = 0.9992 and root mean square error = 5.47, which shows that the two methods with different basis sets produce similar results for our compounds.
Prior investigations [34, 35] revealed that the calculated HOF values of energetic organic compounds were in good accordance with experimental results when suitable reference compounds were designed in isodesmic reactions. An adequate approach of decreasing the errors in calculating the HOF values is to preserve the cage or conjugated skeleton invariable in the designed isodesmic reactions. This technique has shown to be very reliable [54]. For A and B series, when the substituent −N3, −NHNO2 or −NO2 is included, the HOF of its substituted pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine increases as compared with the unsubstituted one. However, the case is the contrary for −NH2, −ONO2 or −NF2. For C series, incorporating −NF2, −NHNO2 or −N3 together with −NO2 raises the HOF of parent (P1), while for −ONO2 and −NH2, the situation is the opposite. Among the same series, the substitution of −N3 exceedingly elevates its HOF value. This indicates that −N3 is the most important substituent for improving the HOF value of P1, in accordance with previous work [32, 55]. Our study points out that −NHNO2, −N3 and −NO2 have a vital role in increasing the HOFs of the derivatives.
When one N-oxide (D1-F5) and two N-oxides (G1-I5) were gradually introduced into P1, its HOF decreases dramatically with the increasing number of N-oxide. However, the effect of the substituent on the variation trend of HOF for the molecules with N-oxide is almost the same as that for the ones without N-oxide. The substitution of −N3, −NHNO2 or −NO2 increases the HOF of parent P2 (or P3), while incorporating −NH2, −ONO2 or −NF2 produces opposite effects. For F and I series, the substitution of −NF2 together with −NO2 surprisingly increases the HOF values compared with parents P2 and P3.
Figure 2 shows the comparison of the HOF values of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives without and with N-oxide. It is found that the HOFs of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives without N-oxide are larger than those of the ones with one and two N-oxides. This indicates that increasing the number of N-oxide decreases the HOF values of the derivatives. Figure 3 compares the solid- and gas-phase HOFs for different derivatives of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine. As the substituent changes, the gas-phase HOFs present a similar variation trend to the solid-phase HOFs for the derivatives, which means that the heat of sublimation does not change the HOF variation trend.
Electronic structure
Table 1 enlists the lowest unoccupied molecular orbitals (LUMO) and highest occupied molecular orbitals (HOMO) energies and the energy gaps (ΔE = ELUMO − EHOMO) for pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives. It is clear that ELUMO and EHOMO at B3LYP/6-311G** are larger than those at B3PW91/6-31G**, while for ΔELUMO-HOMO, the case is just the opposite for most of the derivatives. There is a good linear relationship between B3PW91/6-31G** and B3LYP/6-31G** calculated energy gaps: ΔEB3PW91/6 − 31G ∗ ∗ = 1.102ΔEB3LYP/6 − 311G ∗ ∗ − 0.0025 with R2 = 0.9889 and root mean square error = 0.001335.
Some of the substituents enlarge the HOMO-LUMO gaps of the unsubstituted parent molecules, while others decrease these. For A, B and D series, the HOMO-LUMO gaps are increased when the substituent −NH2, −ONO2 or −N3 is introduced, reflecting a shift towards higher frequencies in electronic absorption spectra, while the effect of −NO2, −NF2 or −NHNO2 is quite the opposite, indicating a shift towards lower frequencies in their electronic absorption spectra. Only the substitution of −ONO2 in the E and F series causes an increase in the HOMO-LUMO gap. The HOMO-LUMO gaps of all the derivatives in the C, G, H and I series decrease. This shows that not only the substituents but also the N-oxides have an important effect on the HOMO and LUMO energies. Among all the derivatives, D1 has the highest value of HOMO-LUMO gap, while C1 has the smallest one. The derivatives with one N-oxide have the highest HOMO-LUMO gaps, followed by two N-oxides, and then without any N-oxide when they have the same substituents. Hence, it may be concluded that the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives with one N-oxide have higher stability than those with two N-oxides and without N-oxide.
Energetic properties
Detonation pressure (P), detonation velocity (D), heat of detonation (Q) and density (ρ) are considered influential performance factors for energetic materials. Table S3 of Supporting Information presents calculated P, Q, D, ρ and oxygen balance of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives along with experimental detonation properties of two most common explosives RDX and HMX. The densities were calculated at the B3PW91/6-31G** and B3LYP/6-311G** levels. It is clear that for all the derivatives, the densities calculated by the B3PW91/6-31G** are larger than those by the B3LYP/6-311G**. The linear relationship between the densities at the B3PW91/6-31G** and B3LYP/6-311G** levels is as follows: ρB3PW91/6 − 31G ∗ ∗ = 1.0748ρB3LYP/6 − 311G ∗ ∗ − 0.0972 with R2 = 0.9803 and root mean square error = 0.0107. The densities calculated at the B3PW91/6-31G** level were used to calculate the detonation properties of the title derivatives by the Kamlet–Jacobos equations [56,57,58].
Figures 4 and 5 display the ρ, Q, D and P of different derivatives of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine. Pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives with different substituents and different numbers of N-oxides have different ρ values, for instance, the largest and smallest ρ values are 2.09 and 1.73 g cm−3, respectively. The ρ values of substituted pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives increase as compared with unsubstituted parent molecule. The substitution of −NF2, −ONO2 or −NO2 causes a sufficiently large increase in the values of ρ compared with the parent one. Especially, the disubstituted derivatives with −NF2 and two N-oxides have the highest ρ value among all the derivatives. This may be because the substitution of −NF2 could contribute extensively to the mass but little to the volume.
As the number of N-oxide increases, the densities of its substituted derivatives increase. The substituted pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives with two N-oxides have higher densities than corresponding ones with one N-oxide, while the derivatives with one N-oxide have higher densities than corresponding ones without N-oxide. This could be probably because the introduction of N-oxide not only enhances oxygen balance but also helps the molecules in better crystal packing. Among different substituents, the substitution of −N3 produces poor effects on the densities of its substituted derivatives.
The calculated heats of detonation in Table S3 of Supporting Information indicate that the substitution of −NO2, −ONO2, −N3, −NHNO2 or −NF2 increases the heat of detonation as compared with unsubstituted parent molecule, while the case is the contrary for the NH2-substituted derivatives. For a majority of the derivatives, increasing the number of N-oxide is favourable for increasing the heat of detonation. The disubstituted derivative with −NO2 and without N-oxide (B2) has the largest heat of detonation, while the disubstituted derivative with −NH2 and without N-oxide (B1) has the smallest value among all the derivatives.
Oxygen balance is another valuable factor for choosing promising HEDCs, which shows the extent to which an energetic material can be oxidized. As a whole, the larger the oxygen balance value is, the higher are the P and D values, and the better is the explosive performance of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives. The −ONO2, −NO2 and an increasing number of N-oxides are very helpful for improving oxygen balance. However, too much oxygen is not helpful in promoting the detonation performance of HEDCs. The main reason is that extra oxygen will produce O2 that brings away plenty of energy during the explosion of HEDCs. Therefore, keeping oxygen balance value near 0 is a good idea.
Different substitution and introduction of N-oxide to pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives result in different values of densities, which in turn affects the D and P values. For all the substituted pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives, their D and P values increase when compared with the unsubstituted one. The same case is valid for pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-2-N-oxide and pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-1, 3-di-N-oxide derivatives. It is noted in Table S3 that the density values of B-(R1 = R2 = ONO2), B-(R1 = R2 = NF2), E-(R1 = R2 = ONO2), E-(R1 = R2 = NF2), F-(R1 = NO2 and R2 = NF2), H-(R1 = R2 = ONO2), H-(R1 = R2 = NF2), H-(R1 = R2 = NHNO2), I-(R1 = NO2 and R2 = ONO2) and I-(R1 = NO2 and R2 = NF2) are fairly large and are either close to or above 1.9 g cm−3. Likewise, their detonation pressures and detonation velocities values are really high and are either close to or above 40.0 GPa and 9.0 km s−1, respectively. The introduction of the increasing number of N-oxides to pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine system gradually increases their D and P values. It is also found that H4, the derivative with two −NF2 groups and two N-oxides, has the highest values of D and P among all the derivatives. This indicates that the N-oxide, −NO2, −ONO2 and −NF2 groups are useful structural fragments for enhancing the detonation properties of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives.
Thermal stability
Bond dissociation energy (BDE) gives valuable knowledge for judging the stability of energetic compounds. To assess the stability of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives, we examined the BDE of the weakest bond among all the bonds and compared them with those of well-known explosives HMX and RDX. Table 2 lists the bond orders and BDE of the weakest bond for the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives.
It is noted that for all the derivatives in A–C series, the values of bond order for the bonds outside the rings are smaller than those inside the rings. This shows that the ring cleavage may not happen during thermal decomposition. We also noted that for most of the derivatives in D–F series, after introducing one N-oxide, the N2–N3 bond of the tetrazine becomes the bond with the lowest bond order, indicating that the ring cleavage may be a trigger reaction in thermal decomposition. For G–I series, after introducing two N-oxides, the C–N1 bond of tetrazine close to N-oxide gets the lowest bond order for most of the derivatives, showing that the ring cleavage may be a trigger reaction in thermal decomposition.
According to the principle of smallest bond order (PSBO) [34], the smallest bond in a compound could be a possible trigger bond in the thermal decomposition of energetic compounds. We determined the smallest Mayer bond order among all the bonds for every derivative as listed in Table 2. We broke these weak bonds to calculate their BDEs at the B3LYP/6-311G** level. For a majority of the title compounds, the BDE values of the derivatives without N-oxide are highest, followed by the derivatives with two N-oxides, and then the derivatives with one N-oxide. The substituted derivative with one −NH2 and without N-oxide (A1) has the largest BDE value of 433.79 kJ/mol, while the substituted derivative with −NO2 and −N3 (I4) has the smallest BDE value of 8.91 kJ/mol among all the derivatives. In comparison with HMX and RDX, most of our designed compounds are more stable.
Impact sensitivity
Free space in a unit cell per molecule, termed as ΔV, could be used to assess the sensitivity of energetic materials. Commonly, the larger the value of ΔV is, the less insensitive the compound is. Table 3 enlists the ΔV values of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives together with the experimental results of HMX and RDX. The results indicate that the calculated ΔV values at two different levels are really close. There exists a fine linear relationship: ΔVB3PW91/6 − 31G ∗ ∗ = 0.9915ΔVB3LYP/6 − 311G ∗ ∗ − 2.1406 with R2 = 0.992 and root mean square error = 0.7745. It can be noted that all the derivatives have higher ΔV than parent ones except for the NH2-substituted derivatives. All the mono- and disubstitution of the −NH2 group cause a decrease in ΔV of the derivatives as compared with parent ones. It means that −NH2 is very helpful for decreasing the free space per molecule and hence the sensitivity. Generally, the derivatives without N-oxide have lower ΔV values than corresponding derivatives with one and two N-oxides.
Impact sensitivity, denoted as h50, is commonly described as a measure of the height in centimetres from which a given mass falling onto a sample of energetic material causes 50% chances of initiating a chemical reaction. The h50 is often used to estimate the sensitivity of energetic materials. The higher the h50 value is, the less sensitive the compound is. The calculated h50 values of the title compounds at B3LYP/6-311G** are also listed in Table 3 along with experimental results of HMX and RDX. Most of the pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine derivatives have larger h50 values than HMX and RDX. As evident from Table 3, the substitution of all the substituents decreases the h50 values when compared with parent molecules. A2 has the highest h50 value of 134.56 cm among all the derivatives. When the second −NO2 is introduced into A2 with one −NO2 to produce B2, the h50 value of B2 comes down to 108.82 cm. This indicates that the less the −NO2 groups are, the more insensitive the compound is, which is according to a previous result [27]. B3 with two −ONO2 groups has the lowest h50 value of 37.65 cm among all the derivatives. Generally, the derivatives of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine with one N-oxide have higher h50 values than corresponding derivatives without and with two N-oxides, in agreement with our ΔELUMO-HOMO results. This suggests that introducing one N-oxide is favourable for increasing the h50 values of energetic materials and hence decreasing their sensitivity.
Also, the heat of detonation can provide valuable information to understand the sensitivity of energetic materials. An energetic compound with larger Q does not guarantee it to have higher detonation velocity and detonation pressure, but such a high Q value could increase the chances of high sensitivity [59, 60]. Among every series from A to I, the heat of detonation values of our designed compounds with mono- or disubstituted −NH2 are the smallest. Exactly the same trend is followed by the free space values. The results indicate that the substitution of the −NH2 group decreases the Q and ΔV values and hence decreases the sensitivity. The smallest Q value among all the derivatives is 896.2 cal g−1 when the substituents are two −NH2 groups without N-oxide (B1) and the ΔV for corresponding derivative is 22.66 Å3, which is the smallest value too among all derivatives. Our results show that there is a link between heat of detonation and sensitivity of energetic compounds.
Conclusions
In the present work, we studied the HOFs, electronic structure, detonation properties, thermal stability and impact sensitivity for a series of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine with different numbers of N-oxide using the density functional theory. The results indicate that the HOFs calculated at the B3LYP with 6-311G** and B3PW91 with 6-31G** levels are very close. The substitution of −N3 and −NO2 causes a very large increase in the HOFs of the derivatives, whereas increasing the number of N-oxide gradually decreases the HOFs.
For A–C series, the substitution of −NH2, −ONO2 or −N3 causes an increase in the HOMO-LUMO gap of the derivative. In the case of D series, the incorporation of all the substituent groups except for −NO2 and −NF2 increases the HOMO-LUMO gap. For E and F series, only the substitution of −ONO2 enlarges the HOMO-LUMO gap, while for G–I series, the substitution of all the substituents decreases that. This indicates that the HOMO-LUMO gap can be modulated by integrating the substituents with different numbers of N-oxide.
The calculated detonation properties show that introducing −NF2 and −ONO2 and increasing the number of N-oxides are a very useful way to improve the explosive performance of our designed compounds. An analysis of the bond orders reveals that the ring cleavage may not happen for A−C series, while the introduction of one N-oxide will weaken the N−N bond of the tetrazine cycle, indicating that possible ring cleavage may happen in thermal decomposition. Also, introducing two N-oxides will weaken the C−N bond of the tetrazine, showing there may be a possible ring cleavage. The substitution of the −NH2 group decreases the free space. Most of the derivatives have higher h50 values compared with parent molecules. Taking the sensitivity, thermal stability and detonation performance into account, B4, H4, H6 and I3 could be regarded as potential candidates of HEDCs for experimental synthesis.
References
Millar RW, Philbin SP, Claridge RP, Hamid J (2004) Studies of novel heterocyclic insensitive high explosive compounds: pyridines, pyrimidines, pyrazines and their bicyclic analogues. Propellants, Explosives, Pyrotechnics: An International Journal Dealing with Scientific and Technological Aspects of Energetic Materials 29(2):81–92
Badgujar D, Talawar M, Asthana S, Mahulikar P (2008) Advances in science and technology of modern energetic materials: an overview. J Hazard Mater 151(2–3):289–305
Pichtel J (2012) Distribution and fate of military explosives and propellants in soil: a review. Applied and Environmental Soil Science:2012
Churakov AM, Smirnov OY, Ioffe SL, Strelenko YA, Tartakovsky VA (2002) Benzo-1, 2, 3, 4-tetrazine 1, 3-dioxides: synthesis and NMR study. Eur J Org Chem 2002(14):2342–2349
He P, Zhang J-G, Wang K, Yin X, Jin X, Zhang T-L (2015) Extensive theoretical studies on two new members of the FOX-7 family: 5-(dinitromethylene)-1, 4-dinitramino-tetrazole and 1, 1′-dinitro-4, 4′-diamino-5, 5′-bitetrazole as energetic compounds. Phys Chem Chem Phys 17(8):5840–5848
Klapötke TM, Mayer P, Schulz A, Weigand JJ (2005) 1, 5-Diamino-4-methyltetrazolium dinitramide. J Am Chem Soc 127(7):2032–2033
W-p L, Lian P, Liu Y-z YT, Zhu W-l, Z-x G, Lv J (2014) Design and theoretical study of 15 novel high energy density compounds. J Mol Model 20(11):2479
Talawar M, Sivabalan R, Senthilkumar N, Prabhu G, Asthana S (2004) Synthesis, characterization and thermal studies on furazan- and tetrazine-based high energy materials. J Hazard Mater 113(1–3):11–25
Tan B, Huang M, Huang H, Long X, Li J, Nie F, Huang J (2013) Theoretical investigation of several 1, 2, 3, 4-tetrazine-based high-energy compounds. Propellants, Explosives, Pyrotechnics 38(3):372–378
Vij A, Wilson WW, Vij V, Tham FS, Sheehy JA, Christe KO (2001) Polynitrogen chemistry. Synthesis, characterization, and crystal structure of surprisingly stable fluoroantimonate salts of N5+. J Am Chem Soc 123(26):6308–6313
Liu H, Du H, Wang G, Liu Y, Gong X (2012) Molecular design of new nitramine explosives: 1, 3, 5, 7-tetranitro-8-(nitromethyl)-4-imidazolino [4, 5-b] 4-imidazolino-[4, 5-e] pyridine and its N-oxide. J Mol Model 18(4):1325–1331
Lai W-P, Lian P, Yu T, Chang H-B, Xue Y-Q (2011) Design and density functional theoretical study of three novel pyrazine-based high-energy density compounds. Computational and Theoretical Chemistry 963(1):221–226
He P, Zhang J-G, Wang K, Yin X, Zhang T-L (2015) Combination multinitrogen with good oxygen balance: molecule and synthesis design of polynitro-substituted tetrazolotriazine-based energetic compounds. The Journal of Organic Chemistry 80(11):5643–5651
Huynh MHV, Hiskey MA, Hartline EL, Montoya DP, Gilardi R (2004) Polyazido high-nitrogen compounds: hydrazo- and azo-1, 3, 5-triazine. Angew Chem Int Ed 43(37):4924–4928
Pagoria PF, Lee GS, Mitchell AR, Schmidt R (2001) Synthesis of amino- and nitro-substituted heterocycles as insensitive energetic materials. Lawrence Livermore National Lab, CA (US)
Licht H, Ritter H (1988) 2, 4, 6-Trinitropyridine and related compounds, synthesis and characterization. Propellants, explosives, pyrotechnics 13(1):25–29
Chavez DE, Hiskey MA (1998) Synthesis of the bi-heterocyclic parent ring system 1, 2, 4-triazolo [4, 3-b][1, 2, 4, 5] tetrazine and some 3, 6-disubstituted derivatives. J Heterocyclic Chem 35(6):1329–1332
Deswal S, Ghule VD, Kumar TR, Radhakrishnan S (2015) Quantum-chemical design of tetrazolo [1, 5-b][1, 2, 4, 5] tetrazine based nitrogen-rich energetic materials. Computational and Theoretical Chemistry 1054:55–62
Kiselev V, Gritsan N, Zarko V, Kalmykov P, Shandakov V (2007) Multilevel quantum chemical calculation of the enthalpy of formation of [1, 2, 5] oxadiazolo [3, 4-e][1, 2, 3, 4]-tetrazine-4, 6-di-N-dioxide. Combustion, Explosion, and Shock Waves 43 (5):562–566
Rezchikova K, Churakov A, Shlyapochnikov V, Tartakovsky V (1999) A quantum-chemical study of 1, 2, 3, 4, 5, 6, 7, 8-octaazanaphthalene and its N-oxides. Russ Chem Bull 48(5):870–872
Upadhyay MK, Sengupta SK, Singh HJ (2015) Nitro and dinitroamino N-oxides of octaazaanthracene as high energy materials. J Mol Model 21(1):18
Wei T, Zhu W, Zhang J, Xiao H (2010) DFT study on energetic tetrazolo-[1, 5-b]-1, 2, 4, 5-tetrazine and 1, 2, 4-triazolo-[4, 3-b]-1, 2, 4, 5-tetrazine derivatives. J Hazard Mater 179(1–3):581–590
Yang J (2015) Theoretical studies on the structures, densities, detonation properties and thermal stability of tris (triazolo) benzene and its derivatives. Polycycl Aromat Compd 35(5):387–400
Liu Z, Wu Q, Zhu W, Xiao H (2013) Theoretical study of energetic trinitromethyl-substituted tetrazole and tetrazine derivatives. J Phys Org Chem 26(11):939–947
Wu Q, Pan Y, Xia X, Shao Y, Zhu W, Xiao H (2013) Theoretic design of 1, 2, 3, 4-tetrazine-1, 3-dioxide-based high-energy density compounds with oxygen balance close to zero. Struct Chem 24(5):1579–1590
Ghule VD, Srinivas D, Sarangapani R, Jadhav PM, Tewari SP (2012) Molecular design of aminopolynitroazole-based high-energy materials. J Mol Model 18(7):3013–3020
Wu Q, Zhu W, Xiao H (2014) A new design strategy for high-energy low-sensitivity explosives: combining oxygen balance equal to zero, a combination of nitro and amino groups, and N-oxide in one molecule of 1-amino-5-nitrotetrazole-3 N-oxide. J Mater Chem A 2(32):13006–13015
Wu Q, Zhu W, Xiao H (2014) Computer-aided design of two novel and super-high energy cage explosives: dodecanitrohexaprismane and hexanitrohexaazaprismane. RSC Adv 4(8):3789–3797
Miller MS, Rice BM, Kotlar AJ, Cramer RJ (2000) A new approach to propellant formulation: minimizing life-cycle costs through science-based design. Clean Products and Processes 2(1):37–46
Muthurajan H, Sivabalan R, Talawar M, Anniyappan M, Venugopalan S (2006) Prediction of heat of formation and related parameters of high energy materials. J Hazard Mater 133(1–3):30–45
Rozhkov VY, Batog L, Struchkova M (2011) Synthesis of 3-nitramino-4-(1H-1, 2, 3-triazol-1-yl)-1, 2, 5-oxadiazoles and their salts. Russ Chem Bull 60(8):1712–1718
Wei T, Zhu W, Zhang X, Li Y-F, Xiao H (2009) Molecular design of 1, 2, 4, 5-tetrazine-based high-energy density materials. J Phys Chem A 113(33):9404–9412
Fan XW, Ju XH (2008) Theoretical studies on four-membered ring compounds with NF2, ONO2, N3, and NO2 groups. J Comput Chem 29(4):505–513
Chen Z, Xiao J, Xiao H, Chiu Y (1999) Studies on heats of formation for tetrazole derivatives with density functional theory B3LYP method. J Phys Chem A 103(40):8062–8066
Ju X-H, Li Y-M, Xiao H-M (2005) Theoretical studies on the heats of formation and the interactions among the difluoroamino groups in polydifluoroaminocubanes. J Phys Chem A 109(5):934–938
Xu X-J, Xiao H-M, Ju X-H, Gong X-D, Zhu W-H (2006) Computational studies on polynitrohexaazaadmantanes as potential high energy density materials. J Phys Chem A 110(17):5929–5933
Curtiss LA, Raghavachari K, Trucks GW, Pople JA (1991) Gaussian-2 theory for molecular energies of first-and second-row compounds. J Chem Phys 94(11):7221–7230
Ochterski JW, Petersson GA, Montgomery Jr JA (1996) A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J Chem Phys 104(7):2598–2619
Curtiss LA, Raghavachari K, Redfern PC, Pople JA (1997) Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J Chem Phys 106(3):1063–1079
Jursic B (2000) Computing the heat of formation for cubane and tetrahedrane with density functional theory and complete basis set ab initio methods. J Mol Struct THEOCHEM 499(1–3):137–140
Jursic BS (1998) Computing heats of formation from CBS-Q total energies and the experimental heats of vaporization of graphite and rhombic sulfur. J Mol Struct THEOCHEM 429:161–164
Politzer P, Murray JS, Edward Grice M, Desalvo M, Miller E (1997) Calculation of heats of sublimation and solid phase heats of formation. Mol Phys 91(5):923–928
Byrd EF, Rice BM (2006) Improved prediction of heats of formation of energetic materials using quantum mechanical calculations. J Phys Chem A 110(3):1005–1013
Kamlet MJ, Jacobs S (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J Chem Phys 48(1):23–35
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107(19):2095–2101
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2011) Sensitivity and the available free space per molecule in the unit cell. J Mol Model 17(10):2569–2574
Politzer P, Murray JS (2014) Impact sensitivity and crystal lattice compressibility/free space. J Mol Model 20(5):2223
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) A possible crystal volume factor in the impact sensitivities of some energetic compounds. J Mol Model 16(5):895–901
Boudart M (1977) Thermochemical kinetics, Sidney W. Benson, Wiley Interscience, 320 pp., $22.50, New York, 1976. AICHE J 23(4):613–613
Dechant J (1988) Quantities, units and symbols in physical chemistry. Compiled for the Commission on Physicochemical Symbols, Terminology, and Units, Physical Chemistry Division, International Union of Pure and Applied Chemistry, by I. MILLS, T. CVITAŠ, K. HOMANN, N. KALLAY and K. KUCHITSU. ISBN 0-632-01773-2. Oxford: Blackwell Scientific Publications 1988. IX, 134 pp., cloth,£ 19.95. Acta Polymerica 39(10):598–598
Blanksby SJ, Ellison GB (2003) Bond dissociation energies of organic molecules. Acc Chem Res 36(4):255–263
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09 package. Pittsburgh PA: Gaussian Inc
Curtiss LA, Raghavachari K, Redfern PC, Stefanov BB (1998) Assessment of complete basis set methods for calculation of enthalpies of formation. J Chem Phys 108(2):692–697
Zhang X, Zhu W, Xiao H (2010) Theoretical studies on heats of formation, detonation properties, and bond dissociation energies of monofurazan derivatives. Int J Quantum Chem 110(8):1549–1558
Huynh MHV, Hiskey MA, Chavez DE, Naud DL, Gilardi RD (2005) Synthesis, characterization, and energetic properties of diazido heteroaromatic high-nitrogen C− N compound. J Am Chem Soc 127(36):12537–12543
Gálvez-Ruiz JC, Holl G, Karaghiosoff K, Klapötke TM, Löhnwitz K, Mayer P, Nöth H, Polborn K, Rohbogner CJ, Suter M (2005) Derivatives of 1, 5-diamino-1 H-tetrazole: a new family of energetic heterocyclic-based salts. Inorg Chem 44(12):4237–4253
Smith MW, Cliff MD (1999) NTO-based explosive formulations: a technology review. DEFENCE SCIENCE AND TECHNOLOGY ORGANISATION CANBERRA (AUSTRALIA),
Türker L, Atalar T, Gümüş S, Çamur Y (2009) A DFT study on nitrotriazines. J Hazard Mater 167(1–3):440–448
Politzer P, Murray JS (2015) Impact sensitivity and the maximum heat of detonation. J Mol Model 21(10):262
Politzer P, Murray JS (2016) High performance, low sensitivity: conflicting or compatible? Propellants, Explosives, Pyrotechnics 41(3):414–425
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 21773119) and Science Challenging Program (No. TZ2016001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 40 kb)
Rights and permissions
About this article
Cite this article
Khan, R.U., Zhu, S. & Zhu, W. DFT studies on nitrogen-rich pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine–based high–energy density compounds. J Mol Model 25, 283 (2019). https://doi.org/10.1007/s00894-019-4167-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4167-4