Abstract
The present study undertook the design of nitro and dinitroamino compounds from the skeleton of isomeric N-oxides of octaazanaphthalene, using computational methods to predict their degradation and explosive characteristics. The atom equivalent method was employed to evaluate the gas phase heats of formation of the designed species. Condensed phase heats of formation were also determined and found to be in the range of 220–286 kcal mol−1. Crystal densities of all the designed molecules were calculated and found to be in the range of 1.91–1.98 g cm−3. Detonation pressure (P) and detonation velocity (D) determined using the Kamlet-Jacobs equation showed that the performance of nitro-substituted compounds was comparable to that of RDX while that of dinitroamino compounds (P ≈ 43.4–43.7 GPa; D ≈ 9.6–9.7 km s−1) showed their superiority over HMX (P ≈ 39.3 GPa and D ≈ 9.10 km s−1). Impact sensitivity (h 50) of the designed molecules was compared with nitro- and nitramino-based commercial explosives on the basis of the available free space (∆V) per molecule in their crystal lattice estimated using wave function analysis. The study showed that dinitroamino compounds were more sensitive compared to their nitro analogs. Reactivity or chemical stability of the designed molecules were measured in terms of charge distribution, molecular electrostatic potential and frontier molecular orbital energy. The nitro compounds of N-oxides of octaazaanthracene were found to be more stable than their dinitroamino analogs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous efforts are made in the search for high energy materials (HEMs), not only for explosives and pyrotechnics but also for components of pyrophobic compositions. In this regard, N-heterocyclic compounds with increased N-content have attracted considerable attention owing to their relatively high density, high positive heat of formation and good thermal stability [1–5]. In order to increase the energetic characteristics of a compound, the introduction of energy-enhancing functional groups (explosophores) such as nitro, nitroamino and azido has been a commonly used method [6, 7]. This fact is well reflected in the design and synthesis of a variety of nitrated high energy materials viz., 2,4,6-trinitrotoulene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocin (HMX), hexanitrohexaazaiso-wurtzitane (CL-20) and many more.
N-oxide functionalization of N-heterocycles is a recently visualized concept to design a new class of energetic materials [8–11]. Compounds having N-oxide linkage are thought to be highly energetic because the additional oxygen atom provides a greater density and a better oxygen balance [12, 13]. Trifluoroperacid, potassiumperoxomonosulphate and hypofluorous acid are a few versatile oxidizing reagents that have been used for introduction of the N-oxide linkage. In this context, two isomeric N-oxides of octaazanaphthalene [also known as di-1,2,3,4-tetrazinetetraoxide (DTTO) and iso-DTTO as shown in Fig. 1] have drawn considerable attention due to their predicted high loading density, high heat of formation and better detonation performance parameters [14–17].
The structural features of DTTO and iso-DTTO are such that synthesis of these two compounds is plausible. It is presumed that the cyclic nitrogen catenation in DTTO and iso-DTTO forming octaazaanthracene N-oxide (OAANO) and its isomer iso-OAANO is stabilized by the diminution of the repulsive effect of alternate nitrogen lone pair electrons owing to their involvement in the formation of coordinate covalent bonds with oxygen atoms. Recently, these compounds were revisited by Politzer et al. [18, 19], who employed computational procedures to explore their potential for use as HEMs.
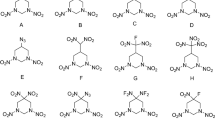
The present study deals with the design of a new class of N-oxide compounds by introducing a benzene ring substituted with nitro and dinitroamino groups into the skeleton of DTTO and iso-DTTO. Thus, four compounds viz., 9,10-dinitro-1,2,3,4,5,6,7,8-octaazaantharacene-1,3,6,8,-tetraoxide (A1), 9,10-dinitro-1,2,3,4,5,6,7,8-octaazaantharacene-1,3,5,7-tetraoxide (A2), 9,10-bis (dinitroamino)-1,2,3,4,5,6,7,8-octaazaantharacene-1,3,6,8,-tetraoxide (B1) and 9,10-bis (dinitroamino)-1,2,3,4,5,6,7,8-octaazaantharacene-1,3,5,7-tetraoxide (B2) as shown in Fig. 2 were designed. In designing these molecules, due care was taken to protect all the features of DTTO, i.e., nitrogen content and stabilization of nitrogen catenation by means of N-oxide linkages were kept intact.
Structures of the designed molecules. The numbers on the atoms correspond to the optimized structures shown in Fig. 3
The vulnerability of energetic compounds is of prime concern and is a deciding factor in gauging their potentiality. In fact, the sensitivity of an energetic material is a complex phenomenon involving several factors such as the physical state of the compound, its molecular and crystal properties and the nature of the stimulus. A large number of predictive correlations have been established between sensitivity and a variety of external stimuli and molecular properties such as electrostatic potentials, atomic charges and electronic energy levels [20–24]. Recently, Politzer and Murray [25] have shown that the impact sensitivity of nitro and nitramino compounds can be predicted on the basis of the amount of available free space (ΔV) in the crystal lattice of the compound determined at the 0.003 a.u. (electrons/bohr3) contour of the electron density space.
In order to explore the possibility of using these designed compounds as HEMs, attempts have been made to estimate their thermodynamic and detonation properties. A study of natural charge distribution through NBO analysis [26, 27] has been performed to assess the chemical reactivity of the designed molecules. Additionally, a detailed analysis of the charge distribution has been made by mapping the molecular electrostatic potential (MEP) [28]. The HOMO–LUMO energy gap has also been determined in order to explore the reactivity of the designed molecules [29, 30].
Computational details
The Gaussian 09 software package [31] was employed to execute all electronic structure calculations during the present investigation. Reliable estimation of a heat of formation of a newly designed energetic molecule is an arduous task. Of the several computational methods that have been shown to predict reliable values, the atom equivalent method for the estimation of gas phase heat of formation of CHNO compounds proposed by Byrd and Rice [32] was used during the present study. In brief, the gas phase heat of formation of a compound using the atom equivalent method can be computed as:
where E (g) is the electronic energy of the molecule at 0 K, n j is the number of atoms of element j present in the molecule and ε j is its atom equivalent energy. The values of ε j were determined by Byrd and Rice [32] by making a least-square fit of Eq. 1 between the experimentally determined ΔH f (g) of a series of CHNO compounds and their E (g) values calculated at density functional theory (DFT) using a dual-level procedure B3LYP/6–311++G (2df,2p)//B3LYP/6–31G (d) in which energy was determined at B3LYP/6–311++G (2df,2p) using the geometry optimized at B3LYP/6–31G (d) levels of theory. We utilized the values of ε j given by Byrd and Rice [32] to estimate the heats of formation of designed molecules by performing the calculation at the same level of theory. From the viewpoint of practical applications, estimation of solid phase heat of formation, ΔH f (s) of an energetic material is more worthwhile; in this study, it was obtained from the gas phase heat of formation using Hess’s law as follows:
where all the enthalpy values were taken at 298 K. The heat of sublimation used in Eq. 2 is a macroscopic property and would depend heavily on the non-covalent molecular interactions present in the condensed phase. On the basis of statistical analysis, Politzer et al. [33] correlated it to the computed molecular properties related to electrostatic potential and proposed the following empirical expression:
where A is the surface area of the 0.001 electrons/bohr3 isosurface of the electronic density of the molecule. σ2 total is the sum of the squares of positive (σ+) and negative (σ−) variances, and v is the balance parameter that describes the degree of balance between positive and negative potentials on the isosurface. These two quantities are given by following equations:
Furthermore, σ 2+ and σ 2− are defined as:
In the above equations, m and n are the number of surface point at which V s(r) is positive and negative, respectively. \( {\overline{V}}_s^{+} \) and \( {\overline{V}}_s^{-} \) are the average of positive and negative surface potentials. In many of their studies, Politzer and Murray [34, 35] have shown that σ2 total is an effective measure of a molecule’s tendency for non-covalent interactions. A self-compiled wave function analysis (WFA) program developed by Bulat et al. [36] was utilized to evaluate the values of v and σ2 total for the designed molecules. A rigorous parameterization of Eq. 3 was made by Byrd and Rice [32] and the values of a, b and c used in Eq. 3 were determined and listed as 0.000267, 1.650087 and 2.966078, respectively. These values were used as such in evaluating the heats of sublimation of the designed molecules in the present study. In doing so, due care was taken to perform the calculations at the same level of theory as that used by Byrd and Rice [32] during the parameterization.
Besides the heat of formation, estimation of crystal density of a newly designed molecule is also part and parcel of the design of novel HEMs because of its effective contribution to detonation performance. In the present study, the crystal densities of the designed molecules were predicted using the following equation proposed by Politzer et al. [37].
where M is the molecular mass (g/molecule) and V0.001 is the molecular volume (cm3/molecule) of the 0.001 electrons/bohr3 contour of electronic density of the molecule. The quantities α, β and γ are the regression coefficients determined by Politzer et al. [37] as 0.9183, 0.0028 and 0.0443, respectively and these values were used as such in the present study. In order to use the above parameteric constants, the structures optimized at B3PW91/6-31G (d,p) level of theory were used to determine the molecular volume of all the designed molecules as used by Politzer et al. [37] during the parameterization of Eq. 8.
The explosive characteristics of the designed molecules were evaluated in terms of detonation pressure (P) and detonation velocity (D) using Kamlet-Jacobs equations [38] given as follows:
The initial loading density (ρo) used in the above equations was calculated using Eq. 8. The other parameters, viz., N, M and Q, defined as the number of moles of gaseous detonation products per gram of explosive, average molecular weight of the gaseous detonation products in grams per mole and the heat released during detonation in calories per gram, respectively were determined using the “most exothermic principle” in which it is presumed that all N atoms end up in N2 molecules and chemically available O atoms initially oxidize H atoms to form H2O (g) before being used to oxidize C atoms to form CO2 (g). If the number of O atoms is insufficient for complete oxidation of C and H atoms together, then the remaining carbons end up as C (solid) otherwise the excess O atoms end up as O2 (g). In order to calculate the values of N, M and Q, the following two stoichiometric reactions were devised:
(Series A compounds)
(Series B compounds)
and the values of N,M and Q were calculated as follows:
A recent approach put forward by Politzer et al. [25] based on the available free space per molecule, (∆V) in the crystal lattice determined at 0.003 au (electrons/bohr3) contour of electronic density was taken into account to estimate the impact sensitivity. According to the latter authors, the relationship between molecular compressibility during an external impact and available free space (∆V) in the crystal can be utilized for a rough estimate of the impact sensitivity. They defined ∆V as,
where V int is the intrinsic gas phase molecular volume obtained at 0.003 au contour of electronic density space assessed through the WFA–Surface Analysis Suite [36]. This was estimated using the optimized structure of the designed molecules at B3PW91/6–31G (d,p) level of theory. V eff is the hypothetical molecular volume corresponding to the unit cell being completely filled. The latter can be determined as:
where M is the molecular mass and d is the crystal density of designed molecules estimated with the help of Eq. 8.
Results and discussion
Heat of formation
The heat of formation of an energetic molecule is the most important factor that affects the heat evolved during a detonation process. Compounds containing positive heat of formation are always preferred in designing a high energetic material. However, experimental determination of heat of formation of such compounds is difficult due to their instability and unknown detonation characteristics. Therefore, theoretical methods have always been a preferred choice [32, 33]. Theoretically evaluated thermodynamic and molecular properties of the designed molecules considered during the present investigation are listed in Table 1. Results show that the solid phase heats of formation of the designed molecules are in the range of 220 to 286 kcal mol−1. This high positive heat of formation indicates that the designed molecules have the potential to find a place in the class of HEMs. The heats of formation of dinitroamino compounds B1 and B2 are superior to those of nitro compounds A1 and A2. This is probably due to relatively greater N and O contents in compounds B1 and B2. Amongst A1 and A2, the heat of formation of compound A2 is slightly greater than that of A1. This may be due to the difference in the position of the N-oxide linkage in molecules A1 and A2. The same may also be held to account for a slight difference in the heat of formation of B1 and B2.
Explosive properties
Detonation velocity (D) and detonation pressure (P) are the two most important parameters determining the brisance of explosive materials. Based on the estimated solid phase heat of formation and crystal density, the values of D and P calculated using Kamlet-Jacobs equation (Eqs. 9, 10) are listed in Table 2. The data show that the heat of detonation, crystal density, detonation velocity and detonation pressure of nitro compounds A1 and A2 are lower as compared to dinitroamino compounds B1 and B2. This may be due to an increase in the N and O contents of the latter molecules. Although the detonation parameters of the nitro compounds A1 (D = 8.98, P = 37.15) and A2 (D = 8.75, P = 35.40) are lower than those of the dinitroamino compounds B1 (D = 9.66, P = 43.7) and B2 (D = 9.64, P = 43.40) yet their performance is seen to be better than that of commercially used explosive RDX (D = 8.75, P = 34.0). A close examination of the data recorded in Table 2 show that, although the heat of detonation of A2 is 5 cal/g more than that of A1, a slight decrease of about 0.5 % in the loading density of compound A2 resulted in a decrease of about 2.6 % in D and 4.7 % in P. This shows that loading density has a very strong influence on the detonation parameter. The results further show that performance parameters of designed molecules B1 and B2 are superior to the powerful explosive HMX and comparable to those of CL-20. Furthermore, the results also show that the designed molecules B1 and B2 are superior to DTTO and iso-DTTO.
Electronic structure
All the designed molecules, their structural frameworks OAANO and isomer iso-OAANO were optimized at B3LYP/6–31G (d) level of theory; the optimized structures are shown in Fig. 3. A few important parameters that undergo major changes in comparison to the structural frameworks are also listed along the optimized structures. The results show that internal bond lengths and bond angles of all the designed molecules remain almost the same as those of the frameworks. The detailed optimized parameters of all the designed molecules are given in the supporting material as Tables S1–S6. However, the substitution of nitro and dinitroamino groups in place of H15 and H16 in OAANO and iso-OAANO leads to significant deviations in some of the bond angles. Additionally, nitro and dinitroamino groups place themselves out of the plane of the molecule. In all the designed molecules, the oxygen atoms of nitro and dinitroamino groups are almost perpendicular to the plane of the ring. This may be due to repulsion from the nearby oxygen atoms of the N-oxide linkage as well as from the lone pair electrons of nitrogen atoms of the adjacent tetrazine rings. This repulsion is symmetrical in molecules A1 and B1 whereas the opposite is true in the case of A2 and B2 because of the position of the N-oxide linkage in the corresponding tetrazine rings. This interaction is reflected by the bond angle deviations as listed on the optimized structures of the designed molecules as shown in Fig. 3. In the case of A1, the bond angles around C9 [C11–C9–N15 (120.9) and C14–C9– N15 (121.0)] and C10 [C12–C10–N16 (119.2) and C13–C10–N16 (119.2)] are almost the same as that in the OAANO molecule. This is probably due to the repulsive forces provided by O atoms and lone pair of electrons of N-atoms of the tetrazine ring operating symmetrically on each side of the nitro groups placed at the C9 and C10 carbon atoms. These repulsive forces are also responsible for the oxygen atoms of the nitro groups placing themselves almost perpendicular to the plane of the ring. In the case of the molecule A2, the repulsion experienced by nitro groups at C9 and C10 is not symmetrical. Repulsion by the oxygen of N-oxide from one side and by the lone pair of nitrogens of the tetrazine ring from the other side are not equivalent; as a result there is a deviation in the bond angles in comparison to that of the parent iso-OAANO molecule. Thus, in A2 the angles around C9 [C11–C9–N15 (124.0) and C14–C9–N15 (116.0)] and the same around C10 [C13–C10–N16 (121.2) and C12–C10–N16 (116.6)] are not equal and in fact deviated significantly from the parent angles in the iso-OAANO molecule. This may be attributed to the fact that these repulsions are asymmetric in nature. This could explain the non-planarity of the oxygen atom of the nitro groups. In the case of molecule B1, the repulsion from oxygen atoms of the N-oxide linkage and nitrogen lone pair electrons of the neighboring tetrazine ring is symmetrical but neither of the angles around C9 and C10 are the same as that of their parent OAANO molecule. This is probably due to the additional steric hindrance provided by the bulkier dinitroamino groups. The combined effect of symmetrical repulsion and steric-hindrance may force the dinitroamino group to adopt a non-planer orientation with a slight variation in the bond angles. A similar argument can also be made for molecule B2, where deviations in the angles C11–C9–N15 (125.6) and C14–C9–N15 (116.2) are significant compared to their parent angles C11–C9–H15 (121.2) and C14–C9–N15 (120.7), also due to the asymmetrical repulsion and steric hindrance.
Charge distribution and MEP
Natural charge distributions on the designed molecules were investigated by natural bond orbital (NBO) analysis and the results are given in Table 3. The data show that same kind of atoms have similar charge distributions. In the compound A1, atoms N1 and N8, N2 and N7, N3 and N6 and N4 and N5, C11 and C14, and C12 and C13 are of the same kind. The same is true for compound B1. On the other hand, in compound A2, atoms N1 and N5, N2 and N6, N3 and N7, N4 and N8, C11 and C13, and C12 and C14 are of the same kind, which is also true for compound B2. Results recorded in Table 3 show that, in all the designed molecules, the N atoms associated with nitro groups are positive—a characteristic feature of nitro groups from the viewpoint of valance bond theory. In the dinitroamino group, nitrogen atoms N15 and N16 are negatively charged in all the designed molecules. This may be ascribed to the fact that the N atom is more electronegative in comparison to the C to which it is bonded and, therefore, pulls the bond pair electrons and acquires negative charge. The N atoms involved in N-oxide linkage are positively charged because of electron donation for the formation of the co-ordinate linkage. In all cases, N atoms adjacent to N-oxide linkages (N2, N4, N5, N7 in molecule A1 and B1 and N2, N4, N6, N8 in molecules A2 and B2) are negatively charged. This could be due to the delocalization of negative charge on O atoms of N-oxide linkages, as shown schematically for molecule A1 in Scheme 1. The same reasoning could be given for the rest of the designed molecules. Carbon atoms C9 and C10, which are linked directly to electron-withdrawing nitro and dinitroamino groups have positive charge of varying amount because of the different environments existing in all four of the designed molecules. All other C atoms (C11, C12, C13 and C14) in all the designed molecules also carry a positive charge because they are attached to more electronegative N atoms.
Although charge distribution is an electronic structure property that depends on the distribution of electrons in the molecule, it does not have any physical basis. On the other hand, MEP is a physical observable that arises due to interaction between nuclei and electronic charges. It has been established earlier that MEP gives substantive insight into charge distribution [39]. Therefore, in order to explain the variation in the electronic charge on atoms, we computed the molecular electrostatic potential (MEP) on the 0.001 a.u. (electrons/bohr3) surface. The results are shown in Fig. 4, where the extent of the charge distribution is shown by the color intensity. Intense blue and red colors represent the most positive and negative potentials. In all the designed molecules, positive MEP is seen over the central portion of the molecule, which is a characteristic property of energetic compounds [20], whereas negative MEP is visualized around the periphery of the designed molecules, which is basically due to the presence of negatively charged O atoms. In nitramine compounds, B1 and B2, the positive potential is extended somewhat towards dinitroamino explosophores. This may be due to the fact that N atoms of the nitro groups of dinitroamino group are positively charged as listed in Table 3. The MEPs shown in Fig. 4 for all the designed molecules show local polarity due to charge density distribution that could be related to molecular reactivity [40]. It is envisaged that the central portion of all the designed molecules will be prone to electrophilic reactivity while the nucleophilic ability would be restricted mostly to the peripheral region.
Impact sensitivity
Impact sensitivity (h 50) measured in terms of centimeters is an important consideration in the synthesis and design of new HEMs. Minimizing impact sensitivity and maximizing detonation performance have always been a primary objective for newly designed energetic molecules. The experimental measurement of h50 refers to the height from which a hammer of standard weight falling upon the explosive gives a 50 % probability of detonation. The greater the value of h 50 , the lower the lesser impact sensitivity. However, experimental measurement of h 50 does not always yield reliable values due to the nature of the test and also due to the fact that detonation is a complex phenomenon that involves many physico-chemical processes occurring simultaneously.
In the present study we estimated the impact sensitivity using the value of available free space (∆V) per molecule in the unit cell of all four molecules considered here. When an explosive solid is subjected to an impact, it undergoes rapid compression and the temperature of the system increases, which leads ultimately to the formation of “hot spots” [41, 42] resulting in the initiation of detonation. Politzer and Murray [25] estimated ∆V values of a number of highly energetic nintamines and aromatics and non-nitramines and non-aromatics. On the basis of their detailed analysis they suggested a general trend between ∆V in the unit cell of the crystal lattice and impact sensitivity. Accordingly, a higher value of free space leads to a lower value of h 50. However, they emphasized that ∆V may be only one of several factors that may govern reactivity and that the results could be used only for making comparisons. The ∆V values of the designed molecules taken during the present study along with their effective volume per molecule (Veff) and intrinsic gas phase molecular volume (Vint) are listed in Table 4. The results show that the ∆V of molecules A1 and A2 are in the range of 72–74 Å3. These values envisage that the designed nitro compounds A1 and A2 are less sensitive in comparison to CL-20, which has a ∆V value of 89 Å3 and an experimental h 50 value of 15 cm [25] but more sensitive when compared with RDX, HMX, and Tetryl [25]. On the other hand, compounds B1 and B2, having ∆V values of 93 and 95 Å3, respectively, are highly sensitive. This is in accordance with the observation made in an earlier study that nitramine compounds would be sensitive due to weak N–NO2 bonds (37–46 kcal mol−1) [22]. The calculations performed in this study are also in accord with the views proposed by Storm et al. [43] that about 80 % of nitramine compounds possess h 50 values lower than 40 cm. Therefore, we envisage that the presence of the N–NO2 bond may be one of the factors for the high impact sensitivity of designed molecules B1 and B2.
Frontier molecular orbital energies
Fukui et al. [29] were the first to notice that frontier molecular orbitals [FMO: highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO)] play an important role in governing the reactivity and/or stability of aromatic compounds. Several studies have also suggested that the HOMO–LUMO energy gap is an indicator of chemical stability [44, 45]. The smaller the HOMO–LUMO gap in the compound, the easier it would be for the HOMO electrons to cross this gap resulting in poor stability. The HOMO and LUMO of all the designed molecules obtained at B3LYP/6–31G (d) level of theory are shownpictorially in Fig. 5 along with their corresponding energy gaps. The results show that the energy gaps in A1 and A2 are greater than their corresponding dinitroamino analogs B1 and B2. Thus, it may be envisaged that nitro compounds A1 and A2 are more stable compared to the dinitroamino analogs B1 and B2. This is also supported by thermodynamic data, ΔH f (s) and impact sensitivity (h 50). The values listed in Table 1 show that ΔH f (s) values of A1 and A2 are about 64 kcal mol−1 less than their dinitroamino analogs B1 and B2, showing thereby that A1 and A2 are more stable. This is further supported by ∆V values of compounds as listed in Table 4, which show that the designed nitro compounds A1 and A2 are about 21 Å3 less than that of dinitroamino compounds B1 and B2, predicting the prior ones to have higher h 50 values as compared to B1 and B2. This further substantiates the finding that compounds A1 and A2 are more stable than their analogs B1 and B2.
Conclusions
In the present study we designed benzene-based molecules containing N-oxide linkages with two different explosophores, nitro and dinitroamino groups. Four different molecules were designed and their thermodynamic, explosive and electronic structure characteristics have been determined and the following conclusions drawn:
-
(1)
High positive solid phase heats of formation of designed compounds indicate that these have the potential to be characterized as HEMs.
-
(2)
Predicted values of detonation parameters of the nitro derivatives A1 and A2 are superior to those of RDX whereas dinitroamino derivatives B1 and B2 outperform RDX and HMX and have properties comparable to that of the most powerful explosive CL-20.
-
(3)
The impact sensitivity of molecules A1 and A2 was found to be better than that of B1 and B2. Compounds B1 and B2 can be classified as highly sensitive compounds.
-
(4)
FMO analysis shows that nitro compounds A1 and A2 are chemically more stable than their dinitroamino analogs B1 and B2.
In summary, we find that the designed molecules can be treated as reasonably stable and powerful explosives; our work may provide a route for further investigation into new high energy materials (Scheme 1).
References
Molchanova MS, Pivina TS, Arnautova EA, Zefirov NS (1999) Computer aided search for high-density energetic compounds among hydrogen-free heterocycles. J Mol Struct (THEOCHEM) 465:11–24
Chavez DE, Hiskey MA, Gilardi RD (2000) 3,3′-Azobis(6-amino-1,2,4,5-tetrazine): a novel high-nitrogen energetic material. Angew Chem Int Ed 39:1791–1793
Huynh MHV, Hiskey MA, Chavez DE, Naud DL, Gilardi RD (2005) Synthesis, characterization and energetic properties of diazidoheteroaromatic high-nitrogen C–N compound. J Am Chem Soc 127:12537–12543
Jones CB, Haiges R, Schroer T, Christe KO (2006) Oxygen-balanced energetic ionic liquid. Angew Chem Int Ed 45:4981–4984
Singh HJ, Upadhyay MK (2013) Nitro derivatives of 1,3,5-triazepine as potential high-energy materials. J Energ Mater 31:301–313
Xu XJ, Zhu WH, Xiao HM (2008) Theoretical predictions on the structures and properties for polynitrohexaazaadamantanes (PNHAAs) as potential high energy density compounds (HEDCs). J Mol Struct (THEOCHEM) 853:1–6
Wang GX, Gong XD, Liu Y, Du HC, Xu XJ, Xiao HM (2011) Looking for high energy density compounds applicable for propellant among the derivatives of DPO with –N3, –ONO2, and – NNO2 groups. J Comput Chem 32:943–952
Dippold AA, Klapotke TM (2013) A Study of dinitro-bis-1,2,4-triazole-1,1′-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N-oxides. J Am Chem Soc 135:9931–9938
Rong D, Phillips VA, Rubio RS, Castro MA, Wheelhouse RT (2008) A safe, convenient and efficient method for the preparation of heterocyclic N-oxides using urea hydrogen peroxide. Tetrahedr Lett 49:6933–6935
Harel T, Rozen S (2010) The tetrazole 3-N-oxide synthesis. J Org Chem 75:3141–3143
Göbel M, Karaghiosoff K, Klapötke TM, Piercey DG, Stierstorfer J (2010) Nitrotetrazolate-2N-oxides and the strategy of N-oxide introduction. J Am Chem Soc 132:17216–17226
Kalpotke TM, Piercey DG, Stierstorfer J (2011) The taming of CN7ˉ: the azidotetrazolate 2-oxide anion. Chem Eur J 17:13068–13077
Singh H, Mukherjee U, Saini RS (2012) Computational studies on nitro derivatives of 1-hydroxy-1,2,4-triazole. J Energ Mater 30:265–281
Rezchikova KI, Churakov AM, Shlyapochnikov VA, Tartakovsky VA (1999) A quantum-chemical study of 1,2,3,4,5,6,7,8-octaazanaphthalene and its N-oxides. Russ Chem Bull 48:870–872
Churakov AM, Tartakovsky VA (2004) Progress in 1,2,3,4-tetrazine chemistry. Chem Rev 104:2601–2616
Song X, Li J, Hou H, Wang B (2009) Extensive theoretical studies of a new energetic material: Tetrazino-tetrazine-tetraoxide (TTTO). J Comput Chem 30:1816–1820
Wilson KJ, Perera SA, Bartlett RJ, Watts JD (2001) Stabilization of the pseudo- benzene N6 ring with oxygen. J Phys Chem A 105:7693–7699
Politzer P, Lane P, Murray JS (2013) Computational analysis of relative stabilities of polyazine N-oxides. Struct Chem 24:1965–1974
Politzer P, Lane P, Murray JS (2013) Computational characterization of two di-1,2,3,4-tetrazine tetraoxides, DTTO and iso-DTTO, as potential energetic compounds. Centr Euro J Energ Mater 10:37–52
Rice BM, Hare JJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem A 106:1770–1783
Politzer P, Murray JS (1996) Relationship between dissociation energies and electrostatic potentials of C-NO2 bonds: applications to impact sensitivities. J Mol Mod 376:419–424
Murray JS, Concha MC, Politzer P (2009) Links between surface electrostatic potentials of energetic molecules, impact sensitivities and C-NO2/N-NO2 bond dissociation energies. Mol Phys 107:89–97
Zhang C, Shu Y, Huang Y, Zhao X, Dong H (2005) Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds. J Phys Chem B 109:8978–8982
Zhi C, Cheng X (2010) The correlation between electric spark sensitivity of polynitroaromatic compounds and their molecular electronic properties. Propell Explo Pyrotech 35:555–560
Politzer P, Murray JS (2014) Impact sensitivity and crystal lattice compressibility/free space. J Mol Model 20:2223–2230
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interaction froma natural bond orbital, donor- acceptor viewpoint. Chem Rev 88:899–926
Singh HJ, Mukherjee U (2013) A computational approach to design energetic ionic liquid. J Mol Model 19:2317–2327
Politzer P, Truhlar DG (eds) (1981) Chemical applications of molecular electrostatic potentials. Plenum, New York
Fukui F, Yonezawa T, Shingu HJ (1952) A molecular orbital theory of reactivity in aromatic hydrocarbons. J Chem Phys 20:722–725
Zhou Z, Parr RG, Garst JF (1988) Absolute hardness as a measure of aromaticity. Tetrahedr Lett 29:4843–4846
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Barone V, Mennucci B, Petersson GA, Carikato M, Li X, Nakatsuji H, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda J, Mol Model R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T Jr, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J (2009) Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian, Inc, Wallingford
Byrd EFC, Rice BM (2006) Improved prediction of heats of formation of energetic materials using quantum mechanical calculations. J Phys Chem A 110:1005–1013
Politzer P, Murray JS, Grice ME, DeSalvo M, Miller E (1997) Calculation of heats of sublimation and solid phase heats of formation. Mol Phys 91:923–928
Politzer P, Murray JS (1998) Statistical analysis of the molecular surface electrostatic potential: an approach to describing noncovalent interactions in condensed phase. J Mol Struct (THEOCHEM) 425:107–114
Politzer P, Murray JS (2002) The fundamental nature and role of the electrostatic potential in atom and molecules. Theor Chem Acc 108:134–142
Bulat FA, Toro-Labbe A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16:1679–1691
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107:2095–2101
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I A simple method for calculating detonation properties of CHNO explosives. J Chem Phys 48:23–35
Murray JS, Politzer P (2011) The electrostatic potential: an overview. WIREs Comp Mol Sci 1:153–163
Scrocco E, Tomasi J (1973) The electrostatic molecular potential as a tool for the interpretation of molecular properties. In: Topics in Current Chemistry. Springer, Berlin, pp 95–170
Dlott DD (2003) In: Politzer P, Murray JS (eds) Energetic materials Part 2. Detonation, combustion. Elsevier, Amsterdam, pp 125–191
Tarver CM, Urtiew PA, Tran TD (2005) Sensitivity of 2,6-diamino-3,5-dinitropyrozine-1-oxide. J Energ Mater 23:183–203
Storm CB, Stine JR, Kramer JF (1990) In: Chemistry and physics of energetic materials. Bulusu, SN (ed), Kluwer, Dordrecht
Du J, Guan L, Cheng B (2006) New route for stabilizing silicon fullerenes. J Phys Chem B 110:14619–14622
Kietzmann H, Rochow R, Ganteför G, Eberhardt W, Vietze K, Seifert G, Fowler PW (1998) Electronic structure of small fullerenes: evidence for the high stability of C32. Phys Rev Lett 81:5378–5381
Talawar MB, Sivabalan R, Mukundan T, Muthurajan H, Sikder AK, Gandhe BR, Rao AS (2009) Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater 161:589–607
Acknowledgments
One of the authors (M.K.U.) is grateful to UGC, New Delhi for providing a research fellowship under its DSA (BSR) program. The authors are also thankful to the UP State Government and UGC for providing financial assistance to establish a High Performance Computing Center in the Department of Chemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Upadhyay, M.K., Sengupta, S.K. & Singh, H.J. Nitro and dinitroamino N-oxides of octaazaanthracene as high energy materials. J Mol Model 21, 18 (2015). https://doi.org/10.1007/s00894-014-2565-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2565-1