Abstract
In order to seek the potential high energy density compounds (HEDCs) with excellent performance and satisfactory safety, some combination rules are presented and 15 HEDCs are designed and sifted, and followed by the properties predicting. From the results, HEDC-3, HEDC-4, HEDC-9, HEDC-10, HEDC-11, HEDC-12, HEDC-13, and HEDC-14 have good comprehensive properties. They are furoxan, fused ring or cage-type compounds, whose frame is composed of some single ring by single (double or multi) point addition. Their densities are over 1.95 g cm−3, and detonation velocities are over 9500 m s−1. Their BDEs are over 85 kJ mol−1, and the values of available free space (∆V) are lower than the ∆V of β-CL20 (∆V = 86). In view of the synthesis feasibility, the synthesis routes of HEDC-4, HEDC-9, HEDC-10, HEDC-12, HEDC-13, and HEDC-14 have been designed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
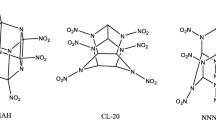
The three most important reasons for the development of energetic materials for military purposes today are the needs for increased performance, increased safety and certain tailored properties. This is important for warhead, propulsion, and launch applications [1]. High energy density compounds (HEDCs), which can increase performance, have been investigated for many years [1–10]. Molecules with furoxan, nitrogen-containing polycyclic or three-dimensional cage-type frame have great potential as HEDCs. Such as, hexanitrohexaazaisowurtzitane (CL-20) has a density of 2.04 g cm−3, and detonation velocity of 9380 m s−1 [11]. Dinitrodiazenofuroxan (DNAOF) based on density 2.02 g cm−3, and detonation velocity 10,000 m s−1 is the highest energy density compound synthesized [12]. Octanitrocubane (ONC) which was predicted to possess detonation velocity 10,100 m s−1 when theoretical density is 2.1 g cm−3, but the experimental density is lower (1.979 g cm−3) [10]. Cis-syn-cis-2,6-dioxo-1,3,4,5,7,8-hexanitrodecahydro-1H, 5H-diimidazo [4,5-b:4′, 5′-e] pyrazine (HHTDD) was supposed to be the explosive with the best performance ever prepared [13]. 2,4,6,8-Tetranitro-1,3,5,7-tetraaza-cubane, the calculated performance is 1.4 times that of HMX [1–4]. Tetrazino [5,6-e] [1–4] tetrazine-1,3,5,7-tetraoxide (TTTO) has not been prepared but has the calculated properties with density 2.38 g cm−3, and relative performance is 2.2 times as large as HMX [1]. However, so far, only CL-20 has good applications. Both DNAOF and HHTDD have the weakness of high sensitivity and chemical instability. ONC is limited to its high cost of the reaction agents and the severe preparation condition.
For the purpose of finding HEDCs with detonation velocity, sensitivity, and stability close to or exceeding CL-20, some new compounds with furoxan, nitrogen-containing polycyclic or three-dimensional cage-type frame were designed by the combination principle. Fifteen high energy density compounds were sifted firstly according to their oxygen balances, densities, and detonation velocities. Then, seven HEDCs were selected based on the stability and sensitivity analysis. Finally, the synthetic routes of six compounds were designed by the inverse synthetic analysis.
Calculation details
Design of HEDCs
Nitrogen-rich heterocycles (such as tetrazine, piperazine, imidazole, furoxan, and so on) have become the ideal building blocks for HEDCs owing to their novel properties, such as high density, high positive heat of formation, high thermal stability, low sensitivity, and so on [14–21]. In the present work, some combination rules were presented to build new HEDCs. The rules can be classified as four types. We assumed that there are two nitrogen-rich heterocycles, A and B. The first type can be named simple single-point addition. That is, there is only one point in A that can be connected directly to one point in B (see Fig. 1). In the second type named simple double-points addition, there are two points in A that can be connected directly to two points in B (see Fig. 2). The third type can be named complicated double-points addition. Firstly, B is divided into two parts from two nonconsecutive points, and secondly the two points in each part can be connected to two points in A (see Fig. 3). In the fourth type, there are multi-points in A, and they can be connected to multi-points in B (see Fig. 4). This type can be named complicated multi-points addition. The first and second rules are also fit for groups. The combined frame can also be new building blocks to design HEDCs according to the rules. All the rules are achieved by computers with C++ self-program.
Calculation methods of properties for HEDCs
DFT B3LYP method (Becke’s three-parameter hybrid function [22] with the non-local correlation of Lee et al. [23]) with a 6-31G* basis set has been proved [24–30] to be able to give quite reliable energies, molecular structures, and other properties after fully optimizing the molecular geometry. So B3LYP/6-31G* were carried out for investigating the designed compounds, using Gaussian 09 program [31]. The geometries of all compounds were fully optimized, and the frequency calculations were performed, which indicated that these geometries correspond to be minima (no imaginary frequency) in the potential energy surfaces.
The density and enthalpy of formation play a key role during the design and estimate of energetic materials. In the work, densities of the designed compounds were predicted by Eq. 1, which was first introduced by Politzer and coworkers [32]. Their molar volumes [V m ], which were defined as inside a contour of 0.001 electrons/Bohr3 densities were evaluated using a Monte Carlo integration [33, 34]. Their solid phase enthalpies of formation [ΔH f (solid)] can be obtained from the gas phase enthalpies of formation using Hess’ law of constant heat summation (see Eq. 2) [35]. The gas phase enthalpies of formation [ΔH f (gas)] can be calculated at B3LYP/6-31G* level with the help of the following reactions:
With the calculated enthalpies of all species and the experimental sublimation enthalpy of graphite, it is easy to obtain the gas phase heat of formations of 15 HEDCs. The enthalpies of sublimation [ΔH sub] can be calculated by Eqs. 3, 4, 5, 6, and 7 first presented by Politzer and coworkers [36].
Where A is the molecular surface area on the specified isosurface, ν is balance parameter, V (r) is electrostatic potential, V(r i ) is the value of V (r) at any point r i on the surface, V +(r i ) and V −(r j ) represent the positive and negative value of V (r) on the surface, \( {\overline{V}}_{\mathrm{S}}^{+} \) and \( {\overline{V}}_{\mathrm{S}}^{-} \) are their averages, σ 2 tot is the total variance, and a, b, c, a’, b’, c’ are coefficients. In this paper, A, V +(r i ) and V −(r j ) of the designed compounds were calculated at B3LYP/6-31G* level.
The detonation properties can be predicted according to enthalpy of formation and density by the formulae of Kamlet-Jacobs [37], VLW [38], and BKW [39] etc. In the present work, the detonation velocities (D) and detonation pressures (P) of the designed compounds were estimated by the formula of Kamlet-Jacobs.
Bond dissociation energies (BDEs) can be used to evaluate the thermal stabilities of energetic materials [15, 40, 41]. The BDEs of trigger linkages for the designed HEDCs are computed (see Eq. 8) [15]. On the basis of these results, the thermal stabilities of the HEDCs are discussed.
Impact sensibility is one of the most important parameters to evaluate HEDCs’ security. Pospíšil et al. [42–44] proposed a method that permitted a rough estimate of the impact sensitivity based on the correlation between the available free space per molecule (ΔV) in the unit cell of the energetic solid and the impact sensitivity of the corresponding molecule. They defined ΔV as:
where V int is the intrinsic gas-phase molecular volume, which obtained at the 0.003 electrons/bohr3 isodensity surface is the best [44]. V eff is the effective volume of the molecule, which corresponds to 100 % packing of the unit cell. The optimized structure obtained at the B3PW91/6-31G** level of theory was used to predict the impact sensitivity of each molecule using Eq. 9.
Design method of synthesis route for HEDCs
In order to obtain a promising synthesis route, the inverse synthesis analysis was performed, and it is one widely used method of designing synthesis route.
Results and discussion
Structures
Fifteen novel HEDCs (see Fig. 5), whose predicted densities and detonation velocities are over 1.95 g/cm3 and 9500 m/s respectively, have been obtained through the combination of energetic group with single cycle, double cycle, fused ring, and cage. It is shown that most of these compounds have fused rings or cage-type frames. For example, four energetic groups -N (NO2)2 and an energetic frame constitute the HEDC-1 through the single-point addition, and the energetic frame is composed of two 1,3,5-triazinane molecules through the multi-points addition. Similar to HEDC-1, HEDC-2′s frame is composed of one 1,3,5-triazinane and one planar N4 through the multi-points addition. The structure of HEDC-3 is composed of 1,2,4,5-tetrazine, ligand oxygen, -N = N-, and > N-NO2 by single-point addition. The fused rings of HEDC-4 to HEDC-12 are composed by double-point addition. For example, 1,2,3,4-tetrazine-1,3-dioxide, pyrazinyl-1-oxide constituted the frame of HEDC-4, and the energetic groups -NH-NO2 and  combine with the frame to form HEDC-4. HEDC-5 and HEDC-6 are composed of 1,2,3,4-tetrazine-1,3-dioxide, piperazine, imidazolidine, and different energetic groups. HEDC-8 and HEDC-9 have the same frame (imidazolidine). In HEDC-10, HEDC-11, and HEDC-12, the same frame is composed of furozan, piperazine, and imidazolidine, and the different groups are combined to form them. However, HEDC-13 and HEDC-14 are chain compounds composed of furoxan and energetic groups by single-point addition. HEDC-15 is a cage-type compound through the multi-points addition of 1,3,5,7-tetrazocane, followed by the single-point addition of the nitro group.
combine with the frame to form HEDC-4. HEDC-5 and HEDC-6 are composed of 1,2,3,4-tetrazine-1,3-dioxide, piperazine, imidazolidine, and different energetic groups. HEDC-8 and HEDC-9 have the same frame (imidazolidine). In HEDC-10, HEDC-11, and HEDC-12, the same frame is composed of furozan, piperazine, and imidazolidine, and the different groups are combined to form them. However, HEDC-13 and HEDC-14 are chain compounds composed of furoxan and energetic groups by single-point addition. HEDC-15 is a cage-type compound through the multi-points addition of 1,3,5,7-tetrazocane, followed by the single-point addition of the nitro group.
Properties
Densities and detonation properties
The oxygen balances, nitrogen contents, predicted densities, enthalpies of formation, and detonation properties of 15 HEDCs are listed in Table 1. In 15 HEDCs, there are 11 compounds having positive or zero oxygen balances (the maximum is 10.57 %, and the minimum is -18.35 %). The densities of all the compounds are in the range of 1.97 g cm−1 to 2.07 g cm−1. The nitrogen contents of all the compounds are over 30 %, and the maximum for their enthalpy of formation is up to 1858 kJ mol−1. Under the joint actions of large density and enthalpy, all compounds have obvious detonation properties. The structures of HEDC-1, HEDC-2, and HEDC-15 are similar with CL-20 that has the most excellent comprehensive performance up to now. The predicted densities of three compounds excel or are close to that of CL-20 [1, 11], and the predicted detonation properties are better than CL-20 [1, 11, 45]. HEDC-7 has the same frame, a similar density, and a higher enthalpy of formation than HHTDD [1], and its predicted detonation properties are slightly better than those of HHTDD evaluated by the same method. It is indicated that the contribution of =N-NO2 group to the energy is larger than that of =O group. The frame structures of HEDC-8 and HEDC-9 are the same as 1,3,4,6-tetranitroglycouril (TNGU). Their predicted densities and detonation velocities are larger than TNGU [46], and the predicted properties of HEDC-9 are better than those of HEDC-8. It indicates that the contributions of =N-NO2 and =C(NO2)2 groups to the energy and density are larger than that of =O group.
Stability
The BDEs (correction factor is 0.96) of 15 HEDCs are calculated (Table 2). It is seen that the BDE values of HEDC-4, HEDC-11, HEDC-13, HEDC-14, and HEDC-15 are over 120 kJ mol−1, and their stabilities are good. The BDE values of HEDC-1, HEDC-2, HEDC-5, HEDC-6, and HEDC-8 are lower than 80 kJ mol−1, and their stabilities are poor. It indicates that they hardly can be applied. Remarkably, the BDE values of HEDC-10 and HEDC-12 are lower than that of HEDC-11 though they have the same frame. We can draw a conclusion that the contribution of the geminaldinitro group to the stability is larger than =N-NO2 and =C (NO2)2 groups.
Impact sensitivity
The ΔV values of 15 HEDCs along with their effective volumes permolecule (V eff) and intrinsic gas-phase molecular volumes (V int) calculated at the B3PW91/6-31G** level are listed in Table 2. Pospíšil et al. [42] suggested that there was a predictive and direct correlation between available free space (∆V) and impact sensitivity. The larger the value of ∆V is, the greater the impact sensitivity. HEDC-13 has the fewest number of nitro groups, and the ∆V of HEDC-13 is the lowest, and the value is similar to the ∆V of HMX (49.0 Å3). The ∆V values of HEDC-2 (59.9 Å3), HEDC-4 (61.9 Å3), and HEDC-8 (64.7 Å3) are higher than that of HEDC-13 and lower than the other HEDCs, which is similar to the ∆V of TNT (58.0 Å3). The ∆V values of HEDC-3, HEDC-5, HEDC-6, HEDC-9, HEDC-10, HEDC-11, HEDC-12, and HEDC-14 are in the range of 70.0-79.4 Å3, which are higher than the ∆V of TNT and lower than the ∆V of β-CL20 (86.0 Å3). HEDC-1, HEDC-7, and HEDC-15 have the most number of nitro groups, and the ∆V values of HEDC-1 (90.1 Å3), HEDC-7 (88.4 Å3), and HEDC-15 (88.5 Å3) are similar to the ∆V of β-CL20.
Synthesis route designing
According to the above calculation results, the comprehensive properties of HEDC-3, HEDC-4, HEDC-9, HEDC-10, HEDC-11, HEDC-12, HEDC-13, and HEDC-14 are good. In view of the synthesis feasibility, we designed the synthesis route of HEDC-4, HEDC-9, HEDC-10, HEDC-12, HEDC-13, and HEDC-14 (Schemes 1, 2, 3, 4, 5, and 6).
Conclusions
In the present work, 15 HEDCs which predicted densities and detonation velocities over 1.95 g cm−3 and 9500 m s−1 have been designed by some combination rules. Their densities, oxygen balances, nitrogen contents, enthalpies of formation, BDEs, impact sensitivities, and many detonation properties, including detonation velocity, detonation pressure, and detonation heat have been calculated. According to their BDEs and impact sensitivity values, we designed the synthesis route of six HEDCs, whose comprehensive properties are good. We can see from the result that the contributions of =N-NO2 and =C(NO2)2 groups to the energy and density are larger than that of =O group for the same frame. The stabilities of HEDC-1, HEDC-5, and HEDC-6 are poor. The contribution of geminal dinitro group to the stability is larger than =N-NO2 and =C(NO2)2 groups. The comprehensive properties of HEDC-3, HEDC-4, HEDC-9, HEDC-10, HEDC-11, HEDC-12, HEDC-13, and HEDC-14 are good.
References
Östmark H (2006) High energy density materials (HEDM): overview, theory and synthetic efforts at FOI. New Trends in Research of Energetic Materials, Czech Republic, p 231
Zhang Y, Shreeve JM (2011) Angew Chem Int Ed 50:935
Goöbel M, Karaghiosoff K, Klapötke TM, Piercey DG, Stierstorfer J (2010) J Am Chem Soc 132:17216
Wang R, Gao H, Ye C, Twamley B, Shreeve JM (2007) Inorg Chem 46:932
Gao Y, Ye C, Twamley B, Shreeve JM (2006) Chem Eur J 12:9010
Xue H, Shreeve JM (2005) Adv Mater 17:2142
Xue H, Arritt SW, Twamley B, Shreeve JM (2004) Inorg Chem 43:7972
Hammerl A, Klapötke TM, Nöth H, Warchhold M (2001) Inorg Chem 40:3570
Zhang MX, Eaton PE, Gilardi R (2000) Angew Chem Int Ed 39:401
Eaton PE, Gilardi RL, Zhang MX (2000) Adv Mater 12:1143
Sikder AK, Maddala G, Agrawal JP, Singh H (2001) J Hazard Mater A84:1
Ovchinnikov IV, Makhova NN, Khmel’nitskii LI, Kuz’min VS, Akimova LN, Pepekin VI (1998) Dokl Akad Nauk 359:499
Vedachalam M, Ramakrishnan VT, Boyer JH (1991) J Org Chem 56:3413
Lian P, Lai WP, Wang BZ, Ge ZX, Zhu WL, Xue YQ (2009) Acta Chim Sinica 67:2343
Wei T, Zhu WL, Zhang XW, Li YF, Xiao HM (2009) J Phy Chem A 113:9404
Kerth J, Lobbecke S (2002) Propell Explos Pyrot 27:111
Chavez DE, Hiskey MA, Gilardi RD (2000) Angew Chem Int Ed 39:1791
Huynh MHV, Hiskey MA, Hartline EL, Montoya DP, Gilardi RD (2004) Angew Chem Int Ed 43:4924
Neutz J, Grosshardt O, Schaufele S, Schuppler H, Schweikert W (2003) Propell Explos Pyrot 28:181
Huynh MHV, Hiskey MA, Pollard CJ, Montoya DP, Hartline EL, Gilardi RD (2004) J Energy Mater 22:217
Churakov AM, Smirnov OY, Ioffe SL, Strelenko YA, Tartakovsky VA (2002) Eur J Org Chem 14:2342
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Xiao HM, Xu XJ, Qiu L (2008) Theoretical design of high energy density materials. Science Press, Beijing
Xiao HM (2004) Structures and properties of energetic compounds. National Defence Industry Press, Beijing
Chen ZX, Xiao JM, Xiao HM, Chiu YN (1999) J Phys Chem A 103:8062
Zhang J, Xiao HM (2002) J Chem Phys 116:10674
Xu XJ, Xiao HM, Ju XH, Gong XD, Zhu WH (2006) J Phys Chem A 110:5929
Wang GX, Gong XD, Xiao HM (2008) Chin J Chem 26:1357
Wang GX, Gong XD, Liu Y, Xiao HM (2010) Int J Quantum Chem 110:1691
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford, CT
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) Mol Phys 107:2095
Qiu L, Xiao HM, Gong XD, Ju XH, Zhu WH (2007) J Hazard Mater 141:280
Rice BM, Hare JJ, Byrd EFC (2007) J Phys Chem A 111:10874
Atkins PW (1982) Physical chemistry. Oxford University Press, Oxford
Politzer P, Murray JS, Grice ME, Desalvo M, Edward M (1997) Mol Phys 91:923
Kamlet MJ, Jacobs SJ (1968) J Chem Phys 48:23
Wu X (1986) In: Proceedings of the 8th Symposium (International) on Detonation. Albuquerque, p 796
Mader CL (1987) Technical Report ISPBKW
Owens FJ (1996) J Mol Struct THEOCHEM 370:11
Rice BM, Sahu S, Owens FJ (1996) J Mol Struct THEOCHEM 583:69
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2011) J Mol Mod 17:2569
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) J Mol Mod 16:895
Politzer P, Murray JS (2014) J Mol Mod 20:2223
Byrd EFC, Rice BM (2006) J Phys Chem A 110:1005
Meyer R (1987) Explosives, 3rd edn. Wiley-VCH, Weinheim, p 452
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, Wp., Lian, P., Liu, Yz. et al. Design and theoretical study of 15 novel high energy density compounds. J Mol Model 20, 2479 (2014). https://doi.org/10.1007/s00894-014-2479-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2479-y