Abstract
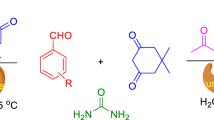
Bovine serum albumin (BSA) promoted simple and efficient one-pot procedure was developed for the direct synthesis of 3,4-dihydropyrimidin-2(1H)-ones including potent mitotic kinesin Eg5 inhibitor monastrol under mild reaction conditions. The catalyst recyclability and gram scale synthesis have also been demonstrated to enhance the practical utility of process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The considerable interest in employing environmentally acceptable processes has motivated the use of biocatalysts for the production of different chemical structures. Thus, many researchers are utilizing these natural catalysts for the smart insertion of chirality and selectivity with regard to regio- and stereo-chemistry for producing optically active, higher value molecules under milder reaction conditions (Santacoloma et al. 2011; Hudlicky and Reed 2009; Pollard and Woodley 2007; Schmid et al. 2001; Clouthier and Pelletier 2012; Patel 2008; Van Rantwijk and Sheldon 2007).The Biginelli reaction (Biginelli 1893) involving condensation of aldehyde, β-ketoester and urea ranks as one of the most recognized multicomponent reaction (Dömling 2006; Zhu and Bienaymé 2005; Wipf et al. 2003; Biggs-Houck et al. 2010) and widely employed reaction for the preparation of 3,4-dihydropyrimidin-2-(1H)-ones (DHPMs). DHPM derivatives exhibit a wide range of biological and pharmacological properties such as antiviral, antimitotic, anticarcinogenic, antihypertensive and most importantly, as calcium channel modulators (Kappe 1998, 2000; Yarim et al. 2003; Kidwai et al. 2005; Kumar et al. 2009; Jain et al. 2008; Mayer et al. 1999). Additionally, the biological activity of potent HIV gp-120-CD4 inhibitor Batzelladine A and B has also been attributed to DHPM moiety (Hojati et al. 2010).
In the past decade, a series of procedures has been developed (Gore et al. 2011; Ramalingan et al. 2010; Shen et al. 2010; Tamaddon et al. 2010; Chitra and Pandiarajan 2009; Lannou et al. 2008; Chari et al. 2009; Suzuki et al. 2008; Polshettiwar and Varma 2007; Yu et al. 2007; Debache et al. 2006, 2008; Bhosale et al. 2004; Rafiee and Jafari 2006; Banik et al. 2007; Bose et al. 2004; Li et al. 2003; Saha and Moorthy 2011) to overcome the relatively harsh acidic conditions of the original Biginelli reaction. However, in spite of their potential utility, most of these methods involve expensive reagents, stoichiometric amount of catalyst, longer reaction times, unsatisfactory yields and generation of waste materials besides cumbersome product isolation procedures and incompatibility with certain functional groups, thus are not closer to the principles of green chemistry. We envisioned that an improvement addressing these issues might be achieved by the use of a biocatalyst particularly for the asymmetric version.
Exploiting the promiscuous activity of a biocatalyst to achieve multicomponent reaction has always remained challenging (Znabet et al. 2010a, b; Wang et al. 2011) and in this case too, only few reports are there where enzymatic systems have been used for the resolution of DHPM esters (Prasad et al. 2009; Schnell et al. 2000) or towards the synthesis of racemic DHPMs (Borse et al. 2012; Kumar and Maurya 2007). Following our recent report on role of amino acid based ionic liquid (Sharma et al. 2012a) in DHPMs synthesis and exploitation of biocatalyst for various organic transformations (Sharma et al. 2009, 2011a, b, 2012b; Kasana et al. 2007), we targeted the waste-free synthesis of DHPMs involving a biocatalytic procedure where water ought to be the only by-product.
Initially, lipases (Table 1; entries 1–6) were screened for obtaining enantiomeric pure DHPM (1b) by cyclocondensation of 0.25 mmol of benzaldehyde (1a), ethyl acetoacetate (2) (1 equiv.) and urea (3) (1 equiv.) in EtOH at 28 °C for 6 days. Among all, Candida antarctica lipase-B (CAL-B) and porcine pancreas lipase (PPL) provided 1b in 28 % yields (entries 1 and 3) with inferior enantioselectivity (5 % ee) at room temperature. Even the use of different solvents with CAL-B as catalyst, did not improve the yield and % ee of chiral 1b (Table 1; entries 7–13). Thereafter, the effect of temperature was probed to stir the reaction in desired direction. It was observed that though increase in temperature from 28 °C to 60 °C brought about a linear change on the yield (62 %), yet it could not exert any positive effect on % ee (Table 1; entry 1 vs 14, 15). In order to exclude background activity, we decided to perform some control experiments where denatured CAL-B or bovine serum albumin (BSA) was used to mediate the desired transformation (Table 1; entry 16, 17).

Surprisingly, 52 % conversion was reached with denatured CAL-B (entry 16) and a very high conversion value (80 %) using BSA (entry 17). With these results in hand (lack of enantioselectivity together with considerable yield observed in case of both denatured lipase and BSA), we hypothesized the role of protein catalysis instead of specific promiscuous catalysis by enzymes which is in contrast to some recent reports (Borse et al. 2012; Kumar and Maurya 2007) where the possibility of protein catalysis has not been considered. Moreover, in comparison to enzyme CAL-B (Walker 2005), BSA have a rich diversity of surface amino acids which might account for the enhanced yield of 1b. Replacing the BSA with porcine albumin and egg albumin also provided the desired DHPM (1b) in 77 % and 74 % yield (entries 18, 19), respectively whereas denatured BSA (Norberto et al. 2012) provided 1b in 28 % yield (entry 20).To validate the above hypothesis, further optimization of reaction conditions using BSA as catalyst was carried out. The effect of solvent, temperature, reaction time and the amount of catalyst on the yield of 1b was examined (Table 2). Best results were obtained when the reaction of 1a was carried out with 50 mg BSA in EtOH at 60 °C with shorter reaction time of 8 h in 82 % yield (Table 2, entry13).

Afterwards, to extend the substrate scope of BSA triggered Biginelli reaction, various benzaldehydes 1a–15a were treated with urea and 1,3 dicarbonyl compounds for the synthesis of dihydropyrimidones 1b–15b (Table 3, entries 1–15). All the obtained products were characterized by NMR and HRMS data (see supporting information). Thiourea was also used with similar success to provide the corresponding dihydropyrimidine-(2H)-thiones (Table 3, entries 16, 17), which are also of much interest with regard to their biological activity (e.g. monastrol Table 3, entry 17). As shown in Table 3 (entries 1–17), yields of this one-pot protocol following recrystallization from ethanol were of the order 69–83 %, which is quite favourable. In particular, the catalyst exhibited remarkable activity for Cl, Br, OH, NO2 and OCH3 functional group containing compounds. In order to check the standard deviation (SD), three independent experiments were conducted for the substrate with electron neutral, withdrawing and releasing groups (Table 3, entries 1, 10 and 13) where deviation in the yield of the products were 83 ± 3, 78 ± 5 and 78 ± 2 percent, respectively.

A plausible mechanism (Scheme 1) suggested the probable role of amino acid side chain in BSA as the catalytic base for the reaction where basic character of the amino group might be responsible for the catalysis as per prior reports (Taylor et al. 1975; Riva et al. 1998; Hollfelder et al. 1996; Boucher et al. 2005; Klein and Reymond 1998; Reetz et al. 2007; Strohmeier et al. 2009). Firstly, the urea attacks on the aldehyde group of substrate leading to the formation of an iminium ion (Kappe 1997; De Souza et al. 2009) followed by the removal of proton from active methylene group of ethylacetoacetate (Sharma et al. 2011b) by the free basic amino group of BSA which subsequently condensed with iminium ion to give desired product as shown in scheme 1. As expected, use of acetylated BSA drastically reduced the formation of 1b in 7 % yield only (Table 1, entry 21) which clearly indicates the importance of free amino group present in BSA indispensable for synthesis of DHPM.
Subsequently, we studied the recyclability of the BSA for the above reaction. Complete conversion was observed up to third reaction cycle with benzaldehyde (1a), ethyl acetoacetate (2) and urea (3) as reaction substrates. However, slight loss of activity (74 %) was observed after fourth catalytic cycle onwards. Further, to demonstrate the practical applicability of the developed method, preparative scale reaction (1 g batch) of 3-hydroxybenzaldehyde (17a) with ethyl acetoacetate and thiourea was effectively accomplished using BSA as catalyst leading to the formation of a potent mitotic kinesin Eg5 inhibitor monastrol (Mayer et al. 1999) (17b) in good yield (Scheme 2).
Conclusion
In conclusion, we have successfully developed a simple, inexpensive and waste-free methodology for the synthesis of bioactive 3,4-dihydropyrimidin-2(1H)-ones using “off-the shelf” protein- bovine serum albumin (BSA) while exploring the promiscuous lipase catalysed system for the asymmetric version of Biginelli reaction. Moreover, catalyst recyclability and gram scale synthesis have also been demonstrated to enhance the practical utility of process.
Experimental Section
General Procedure for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones (1b–17b) from substituted benzaldehydes (Table 3, entries 1–17 and Scheme 2)
Substituted benzaldehyde (1a–17a, 0.25 mmol), ethyl acetoacetate (1 equiv.), urea or thiourea (1 equiv.) and BSA (50 mg) was taken in 3 mL ethanol in a round bottom flask and the reaction mixture was incubated in a orbital shaker at 60 °C for 8–12 h. Afterwards, the reaction mixture was worked up and analyzed with the HPLC. Further, the crude product was purified by recrystallization from water–ethanol mixture giving an isolated yield of 1b–17b in the range of 69–83 %. 1H and 13C NMR spectra were recorded and matched with reported values and further confirmed by HRMS/MS.
Abbreviations
- BSA:
-
Bovine serum albumin
- CAL-B:
-
Candida antarctica lipase-B
- DHPMs:
-
3,4-dihydropyrimidin-2(1H)-ones
- HIV:
-
Human immunodeficiency virus
- HPLC:
-
High-performance liquid chromatography
- PPL:
-
Porcine pancreas lipase
References
Banik BK, Reddy AT, Datta A, Mukhopadhyay C (2007) Microwave-induced bismuth nitrate-catalyzed synthesis of dihydropyrimidones via Biginelli condensation under solventless conditions. Tetrahedron Lett 48:7392–7394
Bhosale RS, Bhosale SV, Bhosale SV, Wang T, Zubaidha PK (2004) An efficient, high yield protocol for the one-pot synthesis of dihydropyrimidin-2(1H)-ones catalyzed by iodine. Tetrahedron Lett 45:9111–9113
Biggs-Houck JE, Younai A, Shaw JT (2010) Recent advances in multicomponent reactions for diversity- oriented synthesis. Curr Opin Chem Biol 14:371–382
Biginelli P (1893) Aldehyde-urea derivatives of aceto- and oxaloacetic acids. Gazz Chim Ital 23:360-413
Borse BN, Borude VS, Shukla SR (2012) Synthesis of novel dihydropyrimidin-2(1H)-ones derivatives using lipase and their antimicrobial activity. Curr Chem Lett 1:59–68
Bose AK, Pednekar S, Ganguly SN, Chakraborty G, Manhas MS (2004) A simplified green chemistry approach to the Biginelli reaction using ‘Grindstone Chemistry’. Tetrahedron Lett 45:8351–8353
Boucher G, Sylvain R, Fargeas V, Dintinger T, Mathe-Allainmat M, Lebreton J, Tellier C (2005) Serum albumin-catalyzed trigger system by using a tandem kemp elimination/b-elimination reaction. ChemBioChem 6:807–810
Chari MA, Mano A, Selvan ST, Mukkanti K, Vinu A (2009) Synthesis of 3,4-dihydropyrimidin-2-ones (DHPMs) using mesoporous aluminosilicate (AlKIT-5) catalyst with cage type pore structure. Tetrahedron 65:10608–10611
Chitra S, Pandiarajan K (2009) Calcium fluoride: an efficient and reusable catalyst for the synthesis of 3,4- dihydropyrimidin-2(1H)-ones and their corresponding 2(1H)thione: an improved high yielding protocol for the Biginelli reaction. Tetrahedron Lett 50:2222–2224
Clouthier CM, Pelletier JN (2012) Expanding the organic toolbox: a guide to integrating biocatalysis in synthesis. Chem Soc Rev 41:1585–1605
Debache A, Amimour M, Belfaitah A, Rhouati S, Carboni B (2008) A one-pot Biginelli synthesis of 3,4- dihydropyrimidin-2-(1H)-ones/thiones catalyzed by triphenylphosphine as Lewis base. Tetrahedron Lett 49:6119–6121
Debache A, Boumoud B, Amimour M, Belfaitah A, Rhouati S, Carboni B (2006) Phenylboronic acid as a mild and efficient catalyst for Biginelli reaction. Tetrahedron Lett 47:5697–5699
De Souza ROMA, da Penha ET, Milagre HMS, Garden SJ, Esteves PM, Eberlin MN, Antunes OAC (2009) The three-component Biginelli reaction: a combined experimental and theoretical mechanistic investigation. Chem Eur J 15:9799–9804
Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89
Gore S, Baskaran S, Koenig B (2011) Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid–urea mixtures. Green Chem 13:1009–1013
Hojati SF, Gholizadeh M, Haghdoust M, Shafiezadeh F (2010) 1,3-Dichloro-5,5-dimethylhydantoin as a novel and efficient homogeneous catalyst in Biginelli reaction. Bull Kor Chem Soc 31:3238–3240
Hollfelder F, Kirby AJ, Tawfik DS (1996) Off-the-shelf proteins that rival tailor-made antibodies as catalysts. Nature 383:60–63
Hudlicky T, Reed JW (2009) Applications of biotransformations and biocatalysis to complexity generation in organic synthesis. Chem Soc Rev 38:3117–3132
Jain KS, Bariwal JB, Kathiravan MK, Phoujdar MS, Sahne RS, Chauhan BS, Shah AK, Yadav MR (2008) Recent advances in selective a1-adrenoreceptor antagonists as antihypertensive agents. Bioorg Med Chem 16:4759–4800
Kappe CO (1997) A re-examination of the mechanism of the Biginelli dihydropyrimidine synthesis. Support for an N-acyliminium ion intermediate. J Org Chem 62:7201–7204
Kappe CO (1998) 4-Aryldihydropyrimidines via the Biginelli condensation: aza analogs of nifedipine-type calcium channel modulators. Molecules 3:1–9
Kappe CO (2000) Biologically active dihydropyrimidones of the Biginelli-type—a literature survey. Eur J Med Chem 35:1043–1052
Kasana RC, Sharma UK, Sharma N, Sinha AK (2007) Isolation and identification of a novel strain of Pseudomonas chlororaphis capable of transforming isoeugenol to vanillin. Curr Microbiol 54:457–461
Kidwai M, Saxena S, Khan MKR, Thukral SS (2005) Synthesis of 4-aryl-7,7-dimethyl-1,2,3,4,5,6,7,8- octahydroquinazoline-2-one/thione-5-one derivatives and evaluation as antibacterials. Eur J Med Chem 40:816–819
Klein G, Reymond JM (1998) An enantioselective fluorimetric assay for alcohol dehydrogenases using albumin-catalyzed-elimination of umbelliferone. Bioorg Med Chem Lett 8:1113–1116
Kumar A, Maurya RA (2007) An efficient bakers’ yeast catalyzed synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Tetrahedron Lett 48:4569–4571
Kumar P, Sankar BR, Nasir G, Baig RB, Chandrashekaran S (2009) Novel Biginelli dihydropyrimidines with potential anticancer activity: a parallel synthesis and CoMSIA study. Eur J Med Chem 44:4192–4198
Lannou MI, Hélion F, Namy JL (2008) Applications of lanthanide trichloride hydrates, prepared from mischmetall, in the Biginelli reaction. Synlett 105-107
Li JT, Han JF, Yang JH, Li TS (2003) An efficient synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by NH2SO3H under ultrasound irradiation. Ultrasound Sonochem 10:119–122
Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286:971–974
Norberto DR, Vieira JM, de Souza AR, Bispo JA, Bonafe CFS (2012) Pressure & urea-induced denaturation of bovine serum albumin: considerations about protein heterogeneity. Open J Biophys 2:4–14
Patel RN (2008) Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord Chem Rev 252:659–701
Pollard DJ, Woodley JM (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25:66–73
Polshettiwar V, Varma RS (2007) Biginelli reaction in aqueous medium: a greener and sustainable approach to substituted 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett 48:7343–7346
Prasad AK, Arya P, Bhatia S, Sharma RK, Singh R, Singh BK, Van der Eycken E, Singh R, Olsen CE, Parmar VS (2009) Synthesis and lipase-catalysed enantioselective acylation studies on Ethyl-4-aryl-3,4- dihydropyrimidin-2(1H)-ones. Ind J Chem 48B:1738–1748
Rafiee E, Jafari H (2006) A practical and green approach towards synthesis of dihydropyrimidinones: using heteropoly acids as efficient catalysts. Bioorg Med Chem Lett 16:2463–2466
Ramalingan C, Park S-J, Lee I-S, Kwak Y-W (2010) A piperidinium triflate catalyzed Biginelli reaction. Tetrahedron 66:2987–2994
Reetz MT, Mondire R, Carballeira JD (2007) Enzyme promiscuity: first protein-catalyzed Morita–Baylis–Hillman reaction. Tetrahedron Lett 48:1679–1681
Riva S, Mendozza M, Carrea G, Chattopadhay P, Tramontano A (1998) Comparison of antibody and albumin catalyzed hydrolysis of steroidal p-Nitrophenylcarbonates. Appl Biochem Biotechnol 75:33–44
Saha S, Moorthy JN (2011) Enantioselective organocatalytic Biginelli reaction: dependence of the catalyst on Sterics, hydrogen bonding, and reinforced chirality. J Org Chem 76:396–402
Santacoloma PA, Sin G, Gernaey KV, Woodley JM (2011) Multienzyme-catalyzed processes: next- generation biocatalysis. Org Process Res Develop 15:203–212
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409:258–268
Schnell B, Krenn W, Faber K, Kappe CO (2000) Synthesis and reactions of Biginelli-compounds. Part 23. chemoenzymatic syntheses of enantiomerically pure 4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J Chem Soc Perkin Trans 1:4382–4389
Sharma N, Sharma UK, Salwan R, Kasana RC, Sinha AK (2011a) A synergic blend of newly isolated Pseudomonas mandelii KJLPB5 and [hmim]Br for chemoselective 2º aryl alcohol oxidation in H2O2: synthesis of aryl ketone or aldehydes via sequential dehydration-oxidative C = C cleavage. Catal Lett 141:616–622
Sharma N, Sharma UK, Kumar R, Kumar R, Katoch N, Sinha AK (2011b) First bovine serum albumin- promoted synthesis of enones, cinnamic acids and coumarins in ionic liquid: an insight into the role of protein impurities in porcine pancreas lipase for olefinic bond formation. Adv Synth Catal 353:871–878
Sharma N, Sharma UK, Kumar R, Richa AK, Sinha AK (2012a) Green and recyclable glycine nitrate (GlyNO3) ionic liquid triggered multicomponent Biginelli reaction for the efficient synthesis of dihydropyrimidinones. RSC Adv 2:10648–10651
Sharma UK, Sharma N, Kumar R, Kumar R, Sinha AK (2009) Biocatalytic promiscuity of lipase in chemoselective oxidation of aryl alcohols/acetates: a unique synergism of CAL-B and [hmim]Br for the metal-free H2O2 activation. Org Lett 11:4846–4848
Sharma UK, Sharma N, Salwan R, Kumar R, Kasana RC, Sinha AK (2012b) Efficient synthesis of hydroxystyrenes via biocatalytic decarboxylation/deacetylation of substituted cinnamic acids by newly isolated Pantoea agglomerans strains. J Sci Food Agric 92:610–616
Shen ZL, Xu XP, Ji SJ (2010) Brønsted base-catalyzed one-pot three-component Biginelli-type reaction: an efficient synthesis of 4,5,6-triaryl-3,4-dihydropyrimidin-2(1H)-one and mechanistic study. J Org Chem 75:1162–1167
Strohmeier GA, Sovic T, Steinkellner G, Hartner FS, Andryushkova A, Purkarthofer T, Glieder A, Gruber K, Griengl H (2009) Investigation of lipase-catalyzed Michael-type carbon–carbon bond formations. Tetrahedron 65:5663–5668
Suzuki I, Iwata Y, Takeda K (2008) Biginelli reactions catalyzed by hydrazine type organocatalyst. Tetrahedron Lett 49:3238–3241
Tamaddon F, Razmi Z, Jafari AA (2010) Synthesis of 3,4-dihydropyrimidin-2(1H)-ones and 1,4-dihydropyridines using ammonium carbonate in water. Tetrahedron Lett 51:1187–1189
Taylor RP, Chau V, Bryner C, Berga S (1975) Bovine serum albumin as a catalyst. II. Characterization of the kinetics. J Am Chem Soc 97:1934–1943
Van Rantwijk F, Sheldon RA (2007) Biocatalysis in ionic liquids. Chem Rev 107:2757–2785
Walker JM (ed) (2005) Protein identification and analysis tools on the ExPASy server. The proteomics protocols handbook. Humana press, Totowa, pp 571–607
Wang J-L, Liu B-K, Yin C, Wu Q, Lin X-F (2011) Candida antarctica lipase B-catalyzed the unprecedented three-component Hantzsch-type reaction of aldehyde with acetamide and 1,3-dicarbonyl compounds in non-aqueous solvent. Tetrahedron 67:2689–2692
Wipf P, Stephenson CRJ, Okumura K (2003) Transition-metal-mediated cascade reactions: C, C-dicyclopropylmethylamines by way of double C, C-σ-bond insertion into bicyclobutanes. J Am Chem Soc 125:14694–14695
Yarim M, Saraç S, Kiliç FS, Erol K (2003) Synthesis and in vitro calcium antagonist activity of 4-aryl-7,7-dimethyl/1,7,7-trimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione derivatives. Farmaco 58:17–24
Yu Y, Liu C, Luo G (2007) One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using chloroacetic acid as catalyst. Bioorg Med Chem Lett 17:3508–3510
Zhu J, Bienaymé H (2005) Multicomponent reactions. Wiley-VCH, Weinheim
Znabet A, Polak MM, Janssen E, de Kanter FJJ, Turner NJ, Orru RVA, Ruijter E (2010a) A highly efficient synthesis of telaprevir by strategic use of biocatalysis and multicomponent reactions. Chem Commun 46:7918–7920
Znabet A, Zonneveld J, Janssen E, de Kanter FJJ, Helliwell M, Turner NJ, Ruijter E, Orru RVA (2010b) Asymmetric synthesis of synthetic alkaloids by a tandem biocatalysis/Ugi/Pictet–Spengler-type cyclization sequence. Chem Commun 46:7706–7708
Acknowledgments
UKS, NS, RK are indebted to CSIR, New Delhi, for the award of research fellowships. The authors gratefully acknowledge the Director, IHBT Palampur, for his kind cooperation and encouragement as well as project MLP0025 for financial assistance. IHBT Communication No. 2371.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, U.K., Sharma, N., Kumar, R. et al. Biocatalysts for multicomponent Biginelli reaction: bovine serum albumin triggered waste-free synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Amino Acids 44, 1031–1037 (2013). https://doi.org/10.1007/s00726-012-1437-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1437-1