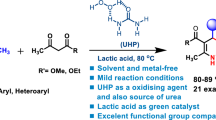
Abstract
Urease, a nickel-dependent enzyme, has a powerful catalytic activity to decompose urea into ammonia via hydrolysis reaction under mild condition. In the present work, urease was employed for the synthesis of two series of polyhydroquinoline and polyhydroacridine derivatives via one-pot condensation of the ammonia generated in situ from urea, aryl aldehydes, and dimedone or ethyl acetoacetate (i.e., Hantzsch-type reaction) in water under mild green condition. The valuable features of this enzymatic method are mild reaction conditions, short reaction times, wide substrate toleration, and high yield of products. The present work provides a novel enzymatic catalysis to synthesize polyhydroquinolines and polyhydroacridines and expands the application of urease in organic synthesis.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen-containing heterocycles have attracted considerable attention because they pose diverse pharmacological and biological activities. Among various nitrogen-containing heterocycles, polyhydroquinolines (PHQs) and polyhydroacridines (PHAs) occupied a unique position in medicinal chemistry due to their wide range of biological applications. In general, PHQs and PHAs are potential therapeutic agents such as antitumor, cardiovascular, and hypertension. For example, amlodipine, felodipine, isradipine, lacidipine, and nifedipine are prominent drugs in the treatment of cardiovascular and hypertension diseases [1,2,3]. In view of their tremendous significance, for the synthesis of PHQs and PHAs, a lot of catalysts have been recently used to produce these kinds of compounds via Hantzsch reaction [4, 5]. Among the documented works, multicomponent reaction (MCR) [6], one-pot processes in which highly functionalized target molecules, merging a large majority of atoms in starting materials, are constructed from three or more readily available reactants, is the most powerful method. In general, a great number of catalysts have been developed for one-pot four-component reactions (4CRs) between aryl aldehydes, dimedone, ethyl acetoacetate, and ammonium acetate for the synthesis of PHQs. Some of the developed catalysts include polyethylene glycol (PEG) [1], 3-methyl-1-sulfonic acid imidazolium chloride ([Msim]Cl) [2], magnetic Fe3O4/SiO2-OSO3H [3], amine-functionalized graphene oxide nanosheets (AFGONs) [7], RuIII@CMC/Fe3O4 [8], cobalt complex immobilized on SBA-15 [9], N-rich porous organic polymer (TrzMOP) [10], Fe/porphyrin complex (ILOS@Fe/TSPP) [11], sulfamic acid anchored on cellulose (Cell–Pr-NHSO3H) [12], Fe3O4@SiO2–PEG/NH2 [13], PMO-ICSPrSO3H [14], N-sulfonated Bronsted acidic catalyst (NS-[C4(DABCO-SO3H)2]·4Cl) [15], Co3O4–CNTs [16], and so on. For the synthesis of PHAs, a variety of catalyst such as AFGONs [7], Fe3+/4 Å molecular sieve [17], Fe3O4@SiO2–PEG/NH2 [13], β-CD-mono-SO3H [18], Fe3O4/HT-SMTU-ZnII [19], and nanocrystalline TiO2 [20] have been developed in the one-pot pseudo-4CRs between aryl aldehydes, dimedone, and ammonium acetate. However, despite of their own merits, most of these methods often suffer from one or more disadvantages such as use of expensive catalyst, longer reaction times, unsatisfactory yields, tedious operation, and incompatibility with certain functional groups. Therefore, there is still a great demand for more efficient, high-yielding, and cost-effective methods using new and efficient catalysts for the synthesis of PHQs and PHAs.
These defected issues might be addressed by use of a biocatalyst particularly for the enzymatic catalysis. Recently, enzymatic synthesis as an efficient, green, and mild catalytic method has flourished in organic synthesis due to its simple processing requirements, high catalytical performance, and good selectivity [21]. Urease is a binuclear Ni-containing hydrolase with high substrate specify for dissociation of urea to ammonia which in conjugation with urea can be considered as a biosource of ammonia instead of the risky odorous ones. In this way, F. Tamaddon et al. [22] reported the use of free urease as a superior biocompatible catalyst for the synthesis of 1,4-dihydropyridines (1,4-DHPs) and continued the use of the immobilized urease as a reusable catalyst for the synthesis of tetrahydropyrazolopyridines (THPPs) [23] in water. Recently, Martínez et al. [24] have reported the synthesis of 1,4-DHPs using immobilized urease as catalyst. Encouraged by this urease catalytic procedure, we envisage that an alternative route to synthesis of PHQs and PHAs employing urea as a potential surrogate for hygroscopic ammonium salts or risky odorous ammonia in the presence of urease to accelerate one-pot 4CRs under mild conditions is reasonable and desirable. In continuation of our previous research on the biocatalyst catalyzed MCRs, herein, for the first time, we have developed urease-catalyzed one-pot 4CRs for the synthesis of PHQs and PHAs in water under fully biocompatible conditions (Scheme 1).
Results and discussion
Initially, to optimize the reaction conditions for access to the desired products, the effect of the amount of catalyst and temperature on the product yield was examined using the reaction of p-chlorobenzaldehyde, dimedone, ethyl acetoacetate, and urea in water as a model (Table 1). The selection of water as the sole solvent in this model reaction is attributed to that the water unlike organic solvents has no apparent negative impact on the configuration of the enzyme. To determine the catalytic role of urease, the control experiments were conducted. The results revealed that only trace product was observed in the blank experiments (Table 1, entry 1), which demonstrated that urease plays a crucial role in this catalytic transformation. The urease hydrolyzes the urea in situ to release ammonia to participate in the model reaction. The amount of urease was screened from 0 to 300U. The results showed that the highest yield was achieved with 200U of urease (Table 1, entry 10), which may be attributed to the accumulation of urease when its concentration reaches high. Further increasing the amount of urease has no significant improvement on the product yields (Table 1, entry 11). Thus, further enlarging the scope of the reaction was carried out at 200U urease loading. The reaction temperature was one of the most important parameters in enzymatic catalysis. Thereafter, the impact of temperature was evaluated. The results showed that the yields went up from trace to 51% with increasing temperature from 40 to 65 °C (Table 1, entries 2–5). When the temperature continues to rise to 70 °C, the yield decreased to 38% (Table 1, entry 6). This may be attributed to the partial deactivation of urease at a higher temperature. It was observed that the product yield reached its maximum at 65 °C, which was selected as the suitable temperature for the model reaction. The molar ratio of the substrates also has influence on the reaction. The molar ratio of aldehyde, demidone, 1,3-dicarbonyl compound, and urea is 1:1:1:3 that affords the best result (Table 1, entry 10). Therefore, it was concluded that the optimal condition involved p-chlorobenzaldehyde (1 mmol), dimedone (1 mmol), ethyl acetoacetate (1 mmol), urea (3 mmol), and urease (200U) in water at 65 °C.

Afterward, to extend the scope of substrates, various benzaldehydes were treated with ethyl acetoacetate or dimedone, urea and catalytic amount urease for the synthesis of PHAs and PHQs under the obtained optimal condition (Table 2). As can be seen from Table 2, the o-nitrobenzaldehyde and o-bromobenzaldehyde afforded the trace amounts of products even after 10 h, respectively, which illustrate that the structure and steric effect had a significant influence on the urease activity (Table 2, entries 10–11 and 22–23). The aldehydes bearing para- and meta-substituent on aromatic ring proceeded quite well with yields in the range of 75–97%.
The possible pathway for the urease-catalyzed one-pot multicomponent synthesis of PHQs and PHAs is depicted in Scheme 2 based on the previously reported mechanism [12]. Aryl aldehyde is attacked by dimedone or ethyl acetoacetate to produce the intermediate (A) or (B), respectively, through Knoevenagel condensation. Dimedone or ethyl acetoacetate is attacked by ammonia formed in situ from urea catalyzed by urease to afford enamine (C) or (D). Michael addition of (A) to (C) gives the desired PHAs and (B) to (C) or (A) to (D) produces the desired PHQs through deprotonation and cyclodehydration.
In order to evaluate the efficiency of urease biocatalyst with the previously reported catalysts, we have summarized several results for the synthesis of PHQ and PHA in Table 3. As can be comprehended that the present enzymic protocol is indeed an equal or more profitable catalyst in terms of biocompatible procedure, mild condition, reaction time, catalytic efficacy, product yields, the use of water as a green solvent, avoiding the use of acidic catalysts, and basic catalysts as well as toxic complicated metallic catalysts.
Experimental
Urease (CSA: 9002-13-5) was purchased from Macklin CO., Ltd. Other chemicals and reagents were used as received. 1H NMR and 13C NMR spectra were recorded on a Bruker-300 Avance Spectrometer with CDCl3 as solvent using TMS as an internal standard at 300 MHz and 75 MHz, respectively.
General procedure for the synthesis of polyhydroquinolines (PHQs) and polyhydroacridines (PHAs)
A mixture of an aldehyde (1 mmol), dimedone (1 mmol), dimedone or ethyl acetoacetate (1 mmol), urea (3 mmol), and urease (200U, 100 mg) was successively added into a 5-ml sealed tube containing 0.5 ml deionized water. The mixture was gently stirred at 65 °C for appropriate times indicated in Table 2 to the end of the reaction monitored by TLC (2:5, n-hexane/ethyl acetate). Upon the completion of the reaction, the mixture was extracted for several times with ethyl acetate, washed with brine, and dried over anhydrous Na2SO4. After removal of the solvent, the pure product was obtained by recrystallization from ethanol. 1H and 13C NMR spectra were recorded and matched with reported data in the literature.
A similar reaction procedure is followed using dimedone (2 mmol) in place of ethyl acetoacetate for the synthesis of PHAs.
Conclusions
In conclusion, a simple and efficient method for the synthesis of polyhydroquinolines and polyhydroacridines via urease-triggered multicomponent reaction of various aldehyde, dimedone, 1,3-dicarbonyl compounds, and urea is described here. Two series of polyhydroquinoline and polyhydroacridine derivatives were obtained in good to excellent yields. The high catalytic activity of the obtained catalyst can be attributed to the unique inherent property of urease. High catalytic activity, low cost, simple operation, short reaction time, mild condition, and tolerance of wide scope of substrates are the salient features of this enzymatic catalytical process.
References
Siddaiah V, Basha GM, Rao GP, Prasad UV, Rao RS (2012) PEG-mediated catalyst-free synthesis of hantzsch 1,4-dihydropyridines and polyhydroquinoline derivatives. Synth Commun 42:627–634. https://doi.org/10.1080/00397911.2010.528289
Jadhvar SC, Kasraliker HM, Goswami SV, Chakrawar AV, Bhusare SR (2017) One-pot synthesis and evaluation of anticancer activity of polyhydroquinoline derivatives catalyzed by [Msim]Cl. Res Chem Intermed 43:7211–7221. https://doi.org/10.1007/s11164-017-3069-2
Maleki A, Akbarzade AR, Bhat AR (2017) Green synthesis of polyhydroquinolines via MCR using Fe3O4/SiO2–OSO3H nanostructure catalyst and prediction of their pharmacological and biological activities by PASS. J Nanostruct Chem 7:309–316. https://doi.org/10.1007/s40097-017-0240-7
Hantzsch A (1881) Condensationsprodukte aus Aldehydammoniak und ketonartigen Verbindungen. Ber Dtsch Chem Ges 14:1637–1638
Hantzsch A (1882) Ueber die synthese pyridinartiger verbindungen aus acotessigäther und aldehydainmcniak. Justus Liebigs Ann Chem 215:1–82. https://doi.org/10.1002/jlac.18822150102
Ruijter E, Scheffelaar R, Orru RVA (2011) Multicomponent reaction design in the quest for molecular complexity and diversity. Angew Chemie Int Ed 50:6234–6246. https://doi.org/10.1002/anie.201006515
Choudhury P, Ghosh P, Basu B (2020) Amine-functionalized graphene oxide nanosheets (AFGONs): an efficient bifunctional catalyst for selective formation of 1,4-dihydropyridines, acridinediones and polyhydroquinolines. Mol Divers 24:283–294. https://doi.org/10.1007/s11030-019-09949-0
Chen Y, Zhang Z, Jiang W, Zhang M, Li Y (2019) RuIII@CMC/Fe3O4 hybrid: an efficient, magnetic, retrievable, self-organized nanocatalyst for green synthesis of pyranopyrazole and polyhydroquinoline derivatives. Mol Divers 23:421–442. https://doi.org/10.1007/s11030-018-9887-3
Ghorbani-Choghamarani A, Mohammadi M, Tamoradi T, Ghadermazi M (2019) Covalent immobilization of Co complex on the surface of SBA-15: green, novel and efficient catalyst for the oxidation of sulfides and synthesis of polyhydroquinoline derivatives in green condition. Polyhedron 158:25–35. https://doi.org/10.1016/j.poly.2018.10.054
Das SK, Mondal S, Chatterjee S, Bhaumik A (2018) N-rich porous organic polymer as heterogeneous organocatalyst for the one-pot synthesis of polyhydroquinoline derivatives through the hantzsch condensation reaction. ChemCatChem 10:2488–2495. https://doi.org/10.1002/cctc.201702013
Elhamifar D, Badin P, Karimipoor G (2017) Alkyl-imidazolium based organosilica supported Fe/porphyrin complex: as novel, highly efficient and reusable catalyst for the unsymmetrical Hantzsch reaction. J Colloid Interface Sci 499:120–127. https://doi.org/10.1016/j.jcis.2017.03.084
Karhale S, Bhenki C, Rashinkar G, Helavi V (2017) Covalently anchored sulfamic acid on cellulose as heterogeneous solid acid catalyst for the synthesis of structurally symmetrical and unsymmetrical 1,4-dihydropyridine derivatives. New J Chem 41:5133–5141. https://doi.org/10.1039/c7nj00685c
Kardooni R, Kiasat AR, Motamedi H (2017) Designing of a novel dual-function silica-iron oxide hybrid based nanocomposite, Fe3O4@SiO2–PEG/NH2, and its application as an eco-catalyst for the solvent-free synthesis of polyhydroacridines and polyhydroquinolines. J Taiwan Inst Chem Eng 81:373–382. https://doi.org/10.1016/j.jtice.2017.10.013
Yaghoubi A, Dekamin MG, Karimi B (2017) Propylsulfonic acid-anchored isocyanurate-based periodic mesoporous organosilica (PMO-ICS-PrSO3H): a highly efficient and recoverable nanoporous catalyst for the one-pot synthesis of substituted polyhydroquinolines. Catal Lett 147:2656–2663. https://doi.org/10.1007/s10562-017-2159-5
Goli-Jolodar O, Shirini F, Seddighi M (2016) Introduction of a novel nanosized N-sulfonated Brönsted acidic catalyst for the promotion of the synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent-free conditions. RSC Adv 6:26026–26037. https://doi.org/10.1039/c6ra04148e
Zarnegar Z, Safari J, Kafroudi ZM (2015) Co3O4-CNT nanocomposites: a powerful, reusable, and stable catalyst for sonochemical synthesis of polyhydroquinolines. New J Chem 39:1445–1451. https://doi.org/10.1039/c4nj01588f
Magyar Á, Hell Z (2019) An efficient one-pot four-component synthesis of 9-aryl-hexahydroacridine-1,8-dione derivatives in the presence of a molecular sieves supported iron catalyst. Catal Lett 149:2528–2534. https://doi.org/10.1007/s10562-019-02845-0
Madankumar N, Pitchumani K (2018) β-Cyclodextrin monosulphonic acid promoted multicomponent synthesis of 1,8-dioxodecahydroacridines in water. ChemistrySelect 3:10886–10891. https://doi.org/10.1002/slct.201802244
Zarei Z, Akhlaghinia B (2017) ZnII doped and immobilized on functionalized magnetic hydrotalcite (Fe3O4/HT-SMTU-ZnII): a novel, green and magnetically recyclable bifunctional nanocatalyst for the one-pot multi-component synthesis of acridinediones under solvent-free conditions. New J Chem 41:15485–15500. https://doi.org/10.1039/c7nj03281a
Eidi E, Kassaee MZ, Nasresfahani Z (2015) Nanocrystalline TiO2, via green combustion synthesis, as an efficient and reusable catalyst for the preparation of 1,8-dioxooctahydroxanthenes and 1,8-dioxodecahydroacridines. Appl Organomet Chem 29:793–797. https://doi.org/10.1002/aoc.3370
Wohlgemuth R (2010) Biocatalysis-key to sustainable industrial chemistry. Curr Opin Biotechnol 21:713–724. https://doi.org/10.1016/j.copbio.2010.09.016
Tamaddon F, Ghazi S (2015) Urease: a highly biocompatible catalyst for switchable Biginelli reactionand synthesis of 1,4-dihydropyridines from the in situ formed ammonia. Catal Commun 72:63–67. https://doi.org/10.1016/j.catcom.2015.09.006
Tamaddon F, Arab D (2019) Urease covalently immobilized on cotton-derived nanocellulose-dialdehyde for urea detection and urea-based multicomponent synthesis of tetrahydro-pyrazolopyridines in water. RSC Adv 9:41893–41902. https://doi.org/10.1039/c9ra05240b
Vargas AY, Rojas HA, Romanelli GP, Martínez JJ (2017) Synthesis of 1,4-dihydropyrimidines with immobilized urease: effect of method immobilization on magnetic supports. Green Process Synth 6:377–384. https://doi.org/10.1515/gps-2016-0143
Acknowledgements
We are grateful to the Natural Science Foundation of Guangdong Province (No. 2020A1515010399) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, G., Li, Y. Urease: a highly efficient biocatalyst for synthesis of polyhydroquinolines and polyhydroacridines from the ammonia formed in situ. Mol Divers 25, 2149–2159 (2021). https://doi.org/10.1007/s11030-020-10109-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10109-y