Abstract
Essential tremor (ET) is considered to be a neurodegenerative disorder and it is plausible that the observed motor and non-motor symptoms may be attributable to functional alterations secondary to abnormalities of subcortical nuclei. This study aims to compare the volumes of subcortical nuclei in patients with ET to ascertain neuroimaging correlates of motor and non-motor features of ET. Forty patients of ET and 40 age- and gender-matched healthy controls (HC) were enrolled in this study. Tremor severity was quantified with the Fahn–Tolosa–Marin tremor rating scale. Patients of ET with and without a rest tremor were also compared. Structural imaging was performed on a 3T scanner, and volumes of subcortical structures were obtained using Freesurfer. There was no difference in total brain volume between ET and HC. However, compared to HC, significantly lower volumes of bilateral thalamus, hippocampus, and ventral diencephalon were observed in patients with ET. A significantly higher volume was observed in the right caudate nucleus, pallidum, amygdala, and bilateral putamen, and nucleus accumbens. No difference was observed between patients of ET with and without a rest tremor. Patients with ET have significant alterations in volumes of subcortical nuclei, which are not limited to the motor domain and include structures involved in cognitive and behavioral functions. These results add to the growing concept of a neurodegenerative pathophysiology of ET with abnormalities extending beyond the cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential tremor (ET) is a highly prevalent movement disorder (Louis and Ferreira 2010) characterized by an action tremor of upper limbs, with an occasional involvement of the head, legs and trunk. ET was initially considered to be a relatively benign monosymptomatic disorder with pathology limited to the cerebellum. However, there has been a paradigm shift in this notion, owing to several studies which have demonstrated the presence of non-motor symptoms encompassing the domains of cognitive impairment, psychiatric abnormalities, sensory and sleep disturbances in patients with ET (Chandran and Pal 2012; Louis 2016b). Additionally, the motor spectrum of ET has also expanded with focus on rest tremors (RT) and gait and balance abnormalities (Louis et al. 2015; Prasad et al. 2018b). Patients of ET with a RT (ETR) form a unique subgroup of ET owing to their phenotypic similarities with tremor-dominant Parkinson’s disease, and several studies have attempted to delineate the pathophysiology of ET with an isolated postural tremor (ETP) and ETR (Caligiuri et al. 2017; Nicoletti et al. 2015; Novellino et al. 2016). ET has been reported to have a genetic predisposition and it is uncertain if there is a difference in the pathophysiology of ET with positive family history (ETFHP) and ET with negative family history (ETFHN).
Neuroimaging studies in ET have demonstrated abnormalities extending beyond the cerebellum, with significant aberrations in cerebral gray matter and subcortical structures (Bagepally et al. 2012; Benito-Leon et al. 2009; Bhalsing et al. 2014; Buijink et al. 2015; Cameron et al. 2018; Cerasa et al. 2009; Choi et al. 2015; Daniels et al. 2006; Lin et al. 2013; Nicoletti et al. 2015; Quattrone et al. 2008). However, reports pertaining to the exact nature of cerebral involvement are incongruent, and range from no abnormality to widespread abnormalities. Furthermore, although the atrophy of basal ganglia structures has been demonstrated earlier (Lin et al. 2013), the possible cause and implications of these observations have not been adequately discussed. Previously, voxel-based morphometry (VBM) has been utilized to explored the brain volume in ET and atrophy of several cortical regions and the cerebellum has been reported (Bagepally et al. 2012; Bhalsing et al. 2014).
ET may be considered to be a neurodegenerative disorder (Benito-Leon 2014; Louis et al. 2014a), and several post-mortem studies have supported this hypothesis by demonstrating the presence of Purkinje cell abnormalities(Babij et al. 2013; Lin et al. 2014; Louis 2016a; Louis et al. 2011, 2014b). It is plausible that to compensate for loss of function which occurs secondary to atrophy, several brain regions within the same functional network may show an increase in size. This study aims to evaluate the volumes of subcortical structures in patients with ET by automated segmentation of subcortical structures, and ascertain the presence of differences, if any, between ET and healthy controls, ETP and ETR, ETFHP and ETFHN.
Materials and methods
Subject recruitment and clinical evaluation
This study included 40 patients with ET and 40 age- and gender-matched healthy controls. Patients were recruited from the general neurology outpatient clinic and movement disorder services of the National Institute of Mental Health and Neurosciences, Bangalore, India. The diagnosis of ET was based on the Consensus statement of the Movement Disorder Society on tremor (Deuschl et al. 1998) and confirmed by a trained movement disorder specialist. Demographic details such as gender, age at onset, age at evaluation and the presence of family history were recorded. Handedness as established by the Edinburgh Handedness Inventory (Oldfield 1971) was also recorded. Additionally, Mini-Mental Status Examination was performed to ascertain the presence of cognitive impairment. Tremor of the upper limbs was assessed at rest, i.e., while sitting with hands placed supine on the lap, standing and while walking, with arms outstretched, flexed at the elbow and while performing the finger–nose test. In addition, the head, voice, trunk and lower limbs were also evaluated for tremor. Tremor severity was quantified by the Fahn–Tolosa–Marin tremor rating scale (FTMRS) (Fahn et al. 1993). All patients were evaluated at least 2 weeks after the last dose of medication to ensure a complete elimination of medications. Patients were classified as ETR if the score of any RT question on the FTMRS, i.e., head, trunk, upper or lower limb, was at least one. Patients with ET were also thoroughly evaluated to rule out the presence of bradykinesia, rigidity and dystonia. Some of the subjects included in this study have been part of previous studies from our group (Bhalsing et al. 2014, 2015; Prasad et al. 2018a, 2019).
This study was approved by the Institutional Ethics Committee and all subjects provided informed consent prior to recruitment.
Imaging protocol
All MRI scans were performed in a 3T Philips Achieva MRI Scanner. A 16-channel head coil was used to acquire 3D T1-weighted inversion recovery fast gradient echo images. The acquisition parameters were as follows: repetition time: 8.2 ms; echo time: 3.8 ms; flip angle: 8°; slice thickness: 1 mm; number of slices: 165; acquisition matrix: 256 × 256 mm.
Fluid-attenuated inversion recovery images were also acquired. Images were screened by a neuroradiologist for structural abnormalities.
Image analysis
Freesurfer 6.0 was employed for pre-processing and segmentation of the sub-cortical regions (http://surfer.nmr.mgh.harvard.edu). Pre-processing involved skull stripping, bias correction and non-linear registration to MNI305 atlas. Segmentation of sub-cortical structures was based on a Bayesian probabilistic approach and estimates were made using a manually labeled training dataset. The classification of each voxel was achieved by finding the segmentation that maximizes the probability, given the prior probabilities from the training dataset (Fischl et al. 2002, 2004). Absolute volumes of the following subcortical structures were acquired: thalamus, caudate, putamen, pallidum, hippocampus, amygdala, nucleus accumbens, and ventral diencephalon (composed of the hypothalamus, mammillary body, subthalamic nuclei, substantia nigra, red nucleus, lateral geniculate nucleus, and medial geniculate nucleus) and were mapped into the subject space. Manual inspection of the automated segmentations was performed to ensure goodness of fit. All acquired volumes were normalized to the intracranial volume of each subject prior to statistical analysis.
Statistical analysis
Subjects were categorized as (a) ET and (b) healthy controls. Patients with ET were further subdivided into ETP and ETR, and ETFHP and ETFHN. Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as frequency and percentage. The volumes of subcortical structures were compared using age and disease duration as covariates with Bonferroni correction to account for multiple comparisons. The association between the volumes of subcortical structures, and demographic and clinical variables (age, age at onset, duration of illness, MMSE, total FTMRS score, motor FTMRS score (A + B), right-sided FTMRS score and left-sided FTMRS score) was explored by performing Pearson’s correlation. Statistical significance was set at p < 0.05.
Results
Demographic and clinical data
Forty patients with ET were included in this study, of which 27 were definite ET, 6 were probable ET and 7 were possible ET. Details of demographic and clinical features are provided in Table 1. Men outnumbered women in all the groups. There were no significant differences between the age of patients with ET and healthy controls, and all subjects were right handed. There was no significant difference between ETP and ETR with respect to the mean age at presentation (44.85 ± 12.74 vs 45.05 ± 12.84, p > 0.05) and the mean age at onset (33.52 ± 13.87 vs 35.47 ± 15.31, p > 0.05). Furthermore, the duration of illness was also similar between the two subgroups (9.00 ± 7.18 vs 9.57 ± 8.52, p > 0.05). Both subgroups reported a similar prevalence of family history. Patients with ET had a significantly lower MMSE score in comparison with HC. There were no differences between ETFHP and ETFHN for any of the above parameters.
The total and part A + B score of the FTMRS was significantly higher in the ETR subgroup in comparison with the ETP subgroup. The difference in tremor severity between ET and ETR persisted even after scores pertaining to RT were excluded while calculating the total FTMRS score (29.00 ± 8.41 vs 36.76 ± 14.70, p < 0.05). No differences were observed when sub-scores of part C were compared. There were no differences between the right and left FTMRS part A + B scores for ET, ETP or ETR. Additionally, no significant differences were observed between ETFHP and ETFHN for the FTMRS total score or sub-scores.
Volumes of subcortical structures
There were no differences in the total intracranial volume between patients with ET and controls (1,289,486.52 ± 130,601.96 mm3 vs 1,307,825.58 ± 99,719.26 mm3, p = 0.48) (Table 2), ETP and ETR (1,294,127.46 ± 131,420.77 mm3 vs 1,284,357.06 ± 129,497.57 mm3, p = 0.81) (Table 2), and ETFHP and ETFHN (1,285,966.72 ± 149,331.07 mm3 vs 1,294,766.23 ± 95,635.07 mm3, p = 0.83) (Table 2).
Patients with ET versus healthy controls (Table 2)
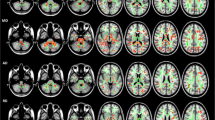
Comparison of the volumes of subcortical structures in patients with ET and healthy controls revealed a significant atrophy of bilateral thalamus, hippocampus, and ventral diencephalon. A significant increase in volume in patients with ET was observed in the right caudate nucleus, pallidum, amygdala, and bilateral putamen, and nucleus accumbens (Fig. 1).
Abnormal subcortical volumes in ET. a Axial view showing: atrophy of bilateral thalamus, hippocampus and right pallidum, and hypertrophy of right caudate, bilateral putamen. b Coronal view showing hypertrophy of bilateral putamen, nucleus accumbens and right caudate. c Coronal view showing atrophy of bilateral thalamus, ventral diencephalon, and hippocampus, and hypertrophy of bilateral putamen, right caudate and right amygdala
Patients with ETP versus ETR (Table 2)
Comparison of the volumes of subcortical structures in patients with ETP and ETR did not reveal any significant differences between the two subgroups.
Patients with ETFHP versus ETFHN (Table 2)
Comparison of the volumes of subcortical structures in patients with ETFHP and ETFHN did not reveal any significant differences between the two subgroups.
Correlations (Table 3)
Significant negative correlations were observed between the age of patients with ET and the volumes of bilateral thalamus, putamen, pallidum, nucleus accumbens and ventral diencephalon. The total FTMRS score negatively correlated with volumes of the left putamen and bilateral hippocampus. The part A + B score of the FTMRS score was found to have a negative correlation with volumes of the left putamen and hippocampus. Right FTMRS scores negatively correlated with left putaminal volume and the left FTMRS scores negatively correlated with bilateral hippocampal and left putaminal volumes. No significant correlations were observed between the MMSE score and volumes of subcortical structures.
Discussion
In this study, we investigated changes in volumes of subcortical structures in patients with ET using automated segmentation. Our analysis revealed several significant areas of atrophy and hypertrophy involving structures associated with motor and non-motor functions. We observed a significant atrophy of bilateral thalamus, hippocampus, and ventral diencephalon, and a significant hypertrophy of bilateral putamen and nucleus accumbens and right caudate, pallidum and amygdala. A review of literature reveals an incongruent picture of subcortical volume changes in ET, with reports ranging from no difference to regions of widespread changes (Supplementary Table 1). These differences may be due to variability in methods of evaluation and sample size. The observations in our study may aid in explaining the motor and non-motor symptoms observed in ET and provide further insights into the pathogenesis of ET.
Motor symptoms
ET has been considered to be a neurodegenerative disorder and the most favored pathophysiological hypothesis is of GABAergic deficit secondary to Purkinje cell pathology (Babij et al. 2013; Helmich et al. 2013; Louis 2016a; Louis et al. 2011, 2014b). Several recent studies have implicated the possible involvement of both the basal ganglia and cerebellar networks in the pathogenesis of ET, with the former observed in patients of ET with a RT (Caligiuri et al. 2017; Nicoletti et al. 2015). The role of both these networks in the generation and modulation of tremor has been previously described (Helmich et al. 2013). Hence, concurrent morphometric changes in the structures involved in these networks may be expected.
To explain the observed volume changes in the caudate, putamen and the globus pallidus interna (GPi), we postulate a model (Fig. 2) which suggests that these changes may occur secondary to Purkinje cell death. The initial GABAergic deficit due to Purkinje cell degeneration may lead to a reduction of the inhibitory output from the cerebellar deep nuclei to the ventral lateral posterior nucleus (VLp) (Fig. 2a). Owing to this, there is an increase in the excitatory output from the VLp to the motor cortex (Fig. 2b). This results in an increased cortical output from the motor cortex to the pontine nuclei, which in turn may produce hyperactivity of the cerebellar network (Fig. 2c). Furthermore, the increased excitatory input which the motor cortex receives from the VLp may lead to a sequential increase in the excitatory output from the motor cortex to the basal ganglia, i.e., the striatum (Fig. 2d). Increased excitation of the striatum can potentially increase the inhibitory output of the GPi (Fig. 2e), subsequently reducing the inhibitory output of the GPi to the ventral lateral anterior nucleus (VLa), and thereby increasing the excitatory output to the motor cortex (Fig. 2f, g). These changes may lead to hyperactivity of the basal ganglia network.
Proposed model for the mechanism of volume change in motor networks. Arrows in brackets indicate the observed or expected volume change. GPi globus pallidus interna, VLa ventral lateral anterior nucleus, VLp ventral lateral posterior nucleus. See “Discussion” for details
Although the observed caudate hypertrophy fits our postulated model, it varies from the report of atrophy by Lin et al. (2013). This discrepancy may be attributable to a significantly smaller sample size in comparison with our study and differences in method of volume estimation—VBM versus automated segmentation. Although we have observed thalamic atrophy in our study, it is possible that individual thalamic nuclei, i.e., the VLa and the VLp may be selectively hypertrophied in comparison with controls. Therefore, a further evaluation of the volumes of thalamic nuclei is necessary to confirm our findings.
A comparison between ETP and ETR subgroups revealed no differences, which is in concurrence with a previous report by Nicoletti et al. (2015). Although changes in pallidal volumes were anticipated based on the reports of GPi involvement in the genesis of rest tremor (Caligiuri et al. 2017; Nicoletti et al. 2015), we did not observe such a finding.
Significant correlations between the volume of the left putamen and disease severity as measured by the FTMRS were observed. This result contributes to the possible role of the putamen in the pathogenesis of ET. The correlation observed between age and thalamus, putamen and pallidum may be attributable to normal age-related changes rather than a disease process.
Non-motor symptoms
The motor features of ET are the main symptoms in ET; however, non-motor features are increasingly being recognized as a crucial component of ET. These symptoms may be classified into distinct domains such as cognitive, psychiatric, sensory and other (sleep disturbances) (Chandran and Pal 2012; Louis 2016b). The observed volume changes in the present study may account for several of these symptoms.
Cognitive non-motor symptoms
Numerous studies have documented cognitive deficits in patients with ET, and these deficits are not limited to older ET or older onset cases. Executive dysfunction and impairment of memory are the most commonly reported deficits (Louis 2016b). Although cerebellum plays a role in these cognitive processes, the observed deficits in these domains in patients with ET cannot be entirely implicated due to cerebellar dysfunction. Several neuroimaging studies have demonstrated the involvement of frontal and temporoparietal areas, which are involved in cognitive and visuospatial processing (Bagepally et al. 2012; Bhalsing et al. 2014, 2015; Daniels et al. 2006). Bhalsing et al. (2014) demonstrated significant correlations between neurocognitive deficits in ET and observed gray matter atrophy. We found a significant atrophy of the hippocampus in our cohort of ET patients, and this finding has not been previously reported in ET. Hippocampal volume loss has been frequently implicated in cognitive impairment (Peng et al. 2015). Although in the present cohort patients with ET had a lower MMSE in comparison with controls, we did not observe any significant correlations between MMSE scores and any subcortical volumes. Furthermore, the thalamic atrophy observed in the present study may also be contributory to cognitive impairment. The ventral anterior nucleus of the thalamus forms a crucial component of the Papez circuit (Dalgleish 2004), and it is possible that there is atrophy of this nucleus in ET, which may contribute to cognitive impairment.
Psychiatric non-motor symptoms
Depression, apathy, anxiety and personality characteristics specifically harm avoidance have been frequently reported in patients with ET (Chandran and Pal 2012; Louis 2016b). The amygdala and nucleus accumbens are key structures involved in emotional processing (Dalgleish 2004). We observed hypertrophy of both the amygdala and nucleus accumbens in patients with ET. This finding may provide an explanation for the reported psychiatric non-motor symptoms in ET. Lafo et al. (2017) reported an abnormal emotion modulation in patients with ET which may reflect an aberrant cerebellar input to the limbic circuitry. This report may be extrapolated to imply that psychiatric non-motor symptoms observed in ET may also be secondary Purkinje cell death which leads to abnormal cerebellar output.
Limitations
Although our sample size was larger than most studies of a similar nature, automated segmentation to evaluate volumes must be performed in larger cohorts to validate our findings. Several of the observed results demonstrate unilateral volume changes. The explanations we have provided for these observations are generalized and refer to bilateral structures, and do not account for the unilateral nature of these results. Evaluation of volumes of thalamic nuclei is necessary to further understand the basis of the observed thalamic atrophy. Owing to the lack of quantitative evaluation of non-motor symptoms, we were unable to provide correlations between the observed volume changes and these symptoms. Finally, this is a cross-sectional study and a prospective longitudinal study may be necessary to establish the neurodegenerative nature of the observations obtained in our study.
Conclusions
Patients with essential tremor have significant alterations in volumes of subcortical structures, which are not limited to the motor domain and include structures involved in cognitive and behavioral functions. Patterns of volume changes, both atrophy and hypertrophy, may be observed within functional networks. The widespread nature of these results adds to the ideology of essential tremor being a neurodegenerative disorder and lends support to the growing concept that the pathophysiology of essential tremor extends beyond the cerebellum.
References
Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED (2013) Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain 136:3051–3061. https://doi.org/10.1093/brain/awt238
Bagepally BS et al (2012) Decrease in cerebral and cerebellar gray matter in essential tremor: a voxel-based morphometric analysis under 3T MRI. J Neuroimaging 22:275–278. https://doi.org/10.1111/j.1552-6569.2011.00598.x
Benito-Leon J (2014) Essential tremor: a neurodegenerative disease? Tremor Other Hyperkinet Mov (N Y) 4:252. https://doi.org/10.7916/D8765CG0
Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Louis ED (2009) Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. J Neurol Sci 287:138–142. https://doi.org/10.1016/j.jns.2009.08.037
Bhalsing KS, Upadhyay N, Kumar KJ, Saini J, Yadav R, Gupta AK, Pal PK (2014) Association between cortical volume loss and cognitive impairments in essential tremor. Eur J Neurol 21:874–883. https://doi.org/10.1111/ene.12399
Bhalsing KS, Kumar KJ, Saini J, Yadav R, Gupta AK, Pal PK (2015) White matter correlates of cognitive impairment in essential tremor. AJNR Am J Neuroradiol 36:448–453. https://doi.org/10.3174/ajnr.A4138
Buijink AW et al (2015) Motor network disruption in essential tremor: a functional and effective connectivity study. Brain 138:2934–2947. https://doi.org/10.1093/brain/awv225
Caligiuri ME et al (2017) Structural connectivity differences in essential tremor with and without resting tremor. J Neurol 264:1865–1874. https://doi.org/10.1007/s00415-017-8553-5
Cameron E, Dyke JP, Hernandez N, Louis ED, Dydak U (2018) Cerebral gray matter volume losses in essential tremor: a case-control study using high resolution tissue probability maps. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2018.03.008
Cerasa A et al (2009) Cerebellar atrophy in essential tremor using an automated segmentation method. AJNR Am J Neuroradiol 30:1240–1243. https://doi.org/10.3174/ajnr.A1544
Chandran V, Pal PK (2012) Essential tremor: beyond the motor features. Parkinsonism Relat Disord 18:407–413. https://doi.org/10.1016/j.parkreldis.2011.12.003
Choi SM et al (2015) Comparison of the brain volume in essential tremor and Parkinson’s disease tremor using an automated segmentation method. Eur Neurol 73:303–309. https://doi.org/10.1159/000381708
Dalgleish T (2004) The emotional brain. Nat Rev Neurosci 5:583–589. https://doi.org/10.1038/nrn1432
Daniels C et al (2006) Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology 67:1452–1456. https://doi.org/10.1212/01.wnl.0000240130.94408.99
Deuschl G, Bain P, Brin M (1998) Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Sci Comm Mov Disord 13(Suppl 3):2–23
Fahn S, Tolosa E, Marín C (1993) Clinical rating scale for tremor Parkinson’s disease and movement disorders. Williams and Wilkins, Baltimore, pp 271–280
Fischl B et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Fischl B et al (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22
Helmich RC, Toni I, Deuschl G, Bloem BR (2013) The pathophysiology of essential tremor and Parkinson’s tremor. Curr Neurol Neurosci Rep 13:378. https://doi.org/10.1007/s11910-013-0378-8
Lafo JA, Mikos A, Mangal PC, Scott BM, Trifilio E, Okun MS, Bowers D (2017) Emotion modulation of the startle reflex in essential tremor: blunted reactivity to unpleasant and pleasant pictures. Parkinsonism Relat Disord 34:54–58. https://doi.org/10.1016/j.parkreldis.2016.11.003
Lin CH, Chen CM, Lu MK, Tsai CH, Chiou JC, Liao JR, Duann JR (2013) VBM reveals brain volume differences between Parkinson’s disease and essential tremor patients. Front Hum Neurosci 7:247. https://doi.org/10.3389/fnhum.2013.00247
Lin C-Y, Louis ED, Faust PL, Koeppen AH, Vonsattel J-PG, Kuo S-H (2014) Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 137:3149–3159
Louis ED (2016a) Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum 15:235–242. https://doi.org/10.1007/s12311-015-0692-6
Louis ED (2016b) Non-motor symptoms in essential tremor: a review of the current data and state of the field. Parkinsonism Relat Disord 22(Suppl 1):S115–S118. https://doi.org/10.1016/j.parkreldis.2015.08.034
Louis ED, Ferreira JJ (2010) How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 25:534–541. https://doi.org/10.1002/mds.22838
Louis ED, Faust PL, Ma KJ, Yu M, Cortes E, Vonsattel JP (2011) Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum 10:812–819. https://doi.org/10.1007/s12311-011-0291-0
Louis ED, Huang CC, Dyke JP, Long Z, Dydak U (2014a) Neuroimaging studies of essential tremor: how well do these studies support/refute the neurodegenerative hypothesis? Tremor Other Hyperkinet Mov (N Y) 4:235. https://doi.org/10.7916/D8DF6PB8
Louis ED, Lee M, Babij R, Ma K, Cortes E, Vonsattel J-PG, Faust PL (2014b) Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 137:3142–3148
Louis ED, Hernandez N, Michalec M (2015) Prevalence and correlates of rest tremor in essential tremor: cross-sectional survey of 831 patients across four distinct cohorts. Eur J Neurol 22:927–932. https://doi.org/10.1111/ene.12683
Nicoletti V et al (2015) Morphometric and functional MRI changes in essential tremor with and without resting tremor. J Neurol 262:719–728. https://doi.org/10.1007/s00415-014-7626-y
Novellino F et al (2016) Cerebellar involvement in essential tremor with and without resting tremor: a diffusion tensor imaging study. Parkinsonism Relat Disord 27:61–66. https://doi.org/10.1016/j.parkreldis.2016.03.022
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Peng GP, Feng Z, He FP, Chen ZQ, Liu XY, Liu P, Luo BY (2015) Correlation of hippocampal volume and cognitive performances in patients with either mild cognitive impairment or Alzheimer’s disease. CNS Neurosci Ther 21:15–22. https://doi.org/10.1111/cns.12317
Prasad S et al (2018a) DTI in essential tremor with and without rest tremor: two sides of the same coin? Mov Disord. https://doi.org/10.1002/mds.27459
Prasad S, Velayutham SG, Reddam VR, Stezin A, Jhunjhunwala K, Pal PK (2018b) Shaky and unsteady: dynamic posturography in essential tremor. J Neurol Sci 385:12–16
Prasad S, Shah A, Bhalsing KS, Kumar KJ, Saini J, Ingalhalikar M, Pal PK (2019) Abnormal hippocampal subfields are associated with cognitive impairment in essential tremor. J Neural Transmission. https://doi.org/10.1007/s00702-019-01992-3
Quattrone A et al (2008) Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR Am J Neuroradiol 29:1692–1697. https://doi.org/10.3174/ajnr.A1190
Funding
Symbiosis International (Deemed) University has received partial support from DST SERB (ECR/2016/000808) for setting up the computing facility.
Author information
Authors and Affiliations
Contributions
(1) Research project: (A) conception: SP, JS, PKP, (B) organization: SP, KB, JS, PKP, (C) execution: SP, AS, KB, MI, PKP. (2) Statistical analysis: (A) design: SP, AS, KB, MI, JS, (B) execution: SP, AS, KB, MI, (C) review and critique: AS, KB, MI, JS, PKP. (3) Manuscript: (A) writing of the first draft: SP, (B) review and critique: AS, KB, MI, JS, PKP.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any financial disclosure to make or have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prasad, S., Shah, A., Bhalsing, K.S. et al. Clinical correlates of abnormal subcortical volumes in Essential Tremor. J Neural Transm 126, 569–576 (2019). https://doi.org/10.1007/s00702-019-02004-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02004-0