Abstract
The study of the postmortem changes in essential tremor (ET) is in its infancy, although recent evidence points to a central role of the cerebellum, where Purkinje cell axonal swellings (“torpedoes”) are significantly more common in ET than control brains. Yet, all existing studies have been confined to the cerebellar hemispheres, and whether there is a more widely distributed cerebellar problem is presently unknown. Our aims were to address whether: (1) ET cases have greater numbers of torpedoes in the vermis than controls, (2) there a correlation between the extent of vermal torpedo pathology and hemispheric torpedo pathology, and (3) vermal torpedo pathology is correlated with clinical features of the disease. A parasagittal neocerebellar block and a vermal block were harvested from 24 ET and 10 control brains. Paraffin sections (7 μm) were stained with Luxol fast blue/hematoxylin and eosin, and torpedoes were quantified. All torpedo counts were corrected for Purkinje cell layer length. Vermal corrected torpedo count (VermTc) was higher in ET cases than controls (7.1 ± 6.8 [median, 4.3] vs. 2.6 ± 2.5 [median, 2]), p = 0.002). The VermTc and the hemispheric corrected torpedo count (HemTc) were correlated with one another (Spearman’s r = 0.54, p = 0.002). ET cases with neck, voice, and jaw tremors had the highest VermTc (p = 0.046). The abundance of torpedoes in the ET brain is not confined to the ponto- or neocerebellum but is more broadly distributed, also involving the spino- or paleocerebellum. These data further confirm the central role of the cerebellum in the underlying pathophysiology of this common neurological disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential tremor (ET), a progressive, age-associated disease, is among the most common neurological disorders [1–3] and is the most common tremor disorder [2]. Although individuals of all age groups, including children [4] and adults [5], may develop ET, the incidence and prevalence rise with age [6, 7]. The prevalence attains values of 6–7% by the seventh decade and is greater than 20% among the oldest old (≥95 years) [2]. Although ET shares several important clinical features with the neurodegenerative diseases [8, 9], historically, there had been few postmortem examinations. Hence, the localization of pathological changes was unknown [10, 11]. With recent, intensive efforts to perform detailed postmortems, an understanding of the basic anatomic pathology is beginning to emerge [10–12]. In the majority of ET brains, changes have been demonstrated in the cerebellum, with the most consistent of these being an increase in the number of damaged Purkinje cell axons (i.e., Purkinje cell axonal swellings or “torpedoes”) [11]. These new postmortem data converge with a wealth of existing clinical and neuroimaging data linking ET to a problem in the cerebellum [11]. Despite recent efforts, the study of the postmortem changes in ET is in its infancy. Thus, the pathological changes associated with this disease have not been fully catalogued and its links with the cerebellum, not completely established.
The cerebellum is neither anatomically nor functionally homogeneous. Our existing studies on torpedoes in ET have been confined to the cerebellar hemisphere (i.e., pontocerebellum or neocerebellum). That is, we have thus far restricted our analyses to one sagittal section of the cerebellar hemisphere; the differential vulnerability of other Purkinje cell subsets to axonal pathology remains unclear. Our goal here was to explore the regional distribution of pathological changes within the cerebellum and, more specifically, to determine whether Purkinje cell axonal pathology in ET extends to the other functional/anatomic cerebellar regions than the part of the neocerebellum evaluated so far. ET is defined by the presence of a 4–14-Hz kinetic tremor in the hands and arms. Hence, that there are changes in the neocerebellum (i.e., cerebellar hemispheres) is not surprising. Given the changes in gait, balance, and postural control, more recently described in ET [13, 14], as well as the presence of midline tremors (head [i.e., neck], voice, jaw tremors) in large subsets of patients [14–17], we questioned whether the cerebellar vermis might also exhibit increased torpedo pathology.
Using brain samples from the Essential Tremor Centralized Brain Repository (ETCBR), which holds the largest collection of ET brains worldwide, we addressed the following aims: (1) Do ET cases have greater torpedo pathology in the vermis than controls? (2) Is there a correlation between the extent of vermal torpedo pathology and the extent of hemispheric torpedo pathology, suggesting that the postmortem changes thus far observed in the cerebellar hemispheres in ET are more broadly indicative of a wider cerebellar systems problem? (3) Is the extent of vermal torpedo pathology correlated with clinical features of the disease (presence of neck, voice, jaw tremors)?
Methods
Selection of Cases
The ETCBR [12, 18, 19], established in 2003, is a centralized repository for the prospective collection of ET brains from donors throughout the USA. Cases are ascertained through advertisements in patient-centered organizations as well as a repository website (www.essentialtremor.us). Control brains were obtained through the New York Brain Bank (Columbia University Medical Center); these were individuals who during life remained free of a diagnosis of ET, Parkinson’s disease (PD), or Alzheimer’s disease (AD) and whose brains were without diagnostic abnormalities on thorough, standardized neuropathological evaluation. In July 2009, the ETCBR database was queried regarding the availability of vermal tissue blocks; blocks were available on 24 ET cases and 10 normal controls (including 4 ET cases and 5 controls on whom data on cerebellar hemispheric torpedo count were previously reported) [12].
Clinical Assessment
The study procedures were approved by the Columbia University Ethics Committee, and signed, written, informed consent was obtained from all prospective donors during life. During life, demographic and clinical data were collected through a series of semi-structured questionnaires. Heavy ethanol use was defined previously as consumption of an average of four or more standard drinks (15 ml of absolute ethanol) per day for a man, or three or more per day for a woman, at any point in their lives [20]. Data on lifetime exposure to medications known to cause cerebellar damage (e.g., lithium, diphenylhydantoin) were collected. Most ET cases also underwent a standardized videotaped neurological examination, which included an assessment of postural tremor (sustained arm extension) and five tests of kinetic tremor (pouring, drinking, using spoon, finger–nose–finger maneuver, and drawing spirals). Each of these six tests was performed with each arm (12 tests total). Videotaped action tremor was rated by a senior neurologist specializing in movement disorders (EDL) during each test using a scale from 0 (no tremor) to 3 (large amplitude tremor) [21], resulting in a total tremor score (range, 0–36). On videotaped examination, jaw and voice tremors were coded as present or absent while cases were seated facing the camera. Jaw tremor was assessed while the mouth was stationary (closed), while the mouth was slightly open, during sustained phonation, and during speech [16]. Voice tremor was assessed during sustained phonation, while reading a prepared paragraph, and during speech. Neck tremor in ET was coded as present or absent and was distinguished from dystonic tremor by the absence of twisting or tilting movements of the neck, jerk-like or sustained neck deviation, or hypertrophy of neck muscles; it was distinguished from titubation by its faster speed and the absence of accompanying truncal titubation or ataxia while seated or standing. A cranial tremor score (range, 0–3) was calculated for each subject based on the number of locations (neck, jaw, voice) in which tremor was present on examination [14]. The clinical diagnosis of ET, initially assigned by treating neurologists, was confirmed (EDL) using ETCBR clinical criteria, as described [12].
Neuropathological Studies
All brains had a complete neuropathological assessment (J-PGV) as described [12]. Each brain had standardized measurement of brain weight (grams), and postmortem interval (hours between death and placement of brain in a cold room or upon ice) was recorded [12]. Tissue was examined microscopically by a senior neuropathologist (J-PGV) blinded to clinical information.
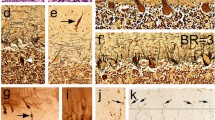
A standard 3 × 20 × 25-mm parasagittal neocerebellar block was harvested from the same region of each brain; the block included the cerebellar cortex, white matter, and dentate nucleus [12]. Paraffin sections (7-μm thick) were stained with Luxol fast blue/hematoxylin and eosin (LH&E) as described previously [12]. Using the standard cerebellar section, torpedoes in the entire LH&E-stained section were counted. We have shown that counts on one neocerebellar section correlate robustly with counts on other neocerebellar sections from the same cases (r = 0.86, p < 0.001), indicating that repeat sectioning and counting is likely to yield similar results [22].
The vermis was harvested in each brain from a 3-mm sagittal slice just lateral to the cerebellar midline. In most brains, the blocks were obtained from the superior vermis (including the lingula, central lobule, culmen, declive, and folium), although in some brains, the nodulus, uvula, pyramis, and tuber were represented. Paraffin sections (7-μm thick) were stained with LH&E.
To standardize the torpedo count (i.e., adjust for any variance in the length of the Purkinje cell layer from case to case), we measured the length of the Purkinje cell layer in each section. The length of the Purkinje cell layer (micrometer) was traced continuously along folia of the entire slide using a Zeiss Imager M2 microscope under a FLUAR 5× (NA = 0.25) dry objective with a Neurolucida software system (MBF Bioscience, Williston, VT, USA).
Each brain received a Braak and Braak Alzheimer’s disease (AD) stage for neurofibrillary tangles (NFTs) [23] and Consortium to Establish a Registry for AD (CERAD) ratings for neuritic plaques [24]. Using alpha-synuclein and LH&E stains, sections were examined at two to four levels of the pons with the locus ceruleus, the medulla (with dorsal vagal nucleus, nucleus raphé magnus, gigantocellular reticular nucleus), hippocampus, cingulate gyrus, temporal cortex, prefrontal cortex, and motor cortex. Lewy bodies and Lewy neurites were assessed using the previously noted semiquantitative scale [12] and Braak PD stage was also assigned [25].
Statistical Analyses
Analyses were performed in SPSS (version 18.0). Raw torpedo counts were presented. We also corrected the torpedo count for the length of the Purkinje cell layer (i.e., torpedo count ÷ Purkinje cell layer length). Because some values for the torpedo count were 0, we needed to add the value 1 to the numerator in all cases and controls to allow for mathematical division; we also multiplied the numerator by 105 in order to avoid the presentation of data with multiple decimal places (e.g., 2.1 rather than 0.000021). Hence, the final formula for corrected torpedo count = 105 (raw value + 1)/Purkinje layer length. Raw and corrected values are reported initially, and then in subsequent analyses, we used only the corrected values. As these torpedo counts were not normally distributed, we used nonparametric tests (Mann–Whitney test, Spearman’s r, Kruskal–Wallis test) when assessing these variables. To consider the effects of possible confounding by age, postmortem interval, and Braak and Braak AD stage for NFTs, a multivariate linear regression model was performed in which the log-transformed vermal corrected torpedo count was the outcome variable, and case–control status, age, postmortem interval, and Braak and Braak AD stage for NFTs were included in the model. To assess whether vermal torpedo counts were associated with midline tremors, we used a non-parametric approach (Kruskal–Wallis test).
Results
ET cases were older and had a shorter postmortem interval than controls (Table 1). ET cases had higher Braak and Braak AD stage for NFTs but similar CERAD ratings (Table 1). All cases and controls had a Braak PD stage of 0. No ET cases were heavy ethanol users; the median number of alcoholic beverages consumed was <1 per week. None was exposed to medications known to cause cerebellar damage (e.g., lithium, diphenylhydantoin).
There was no correlation between vermal corrected torpedo counts (VermTc) and age in controls (Spearman’s r = 0.21, p = 0.57) or cases (Spearman’s r = 0.29, p = 0.16). Similarly, there was no correlation between VermTc and postmortem interval in controls (Spearman’s r = 0.22, p = 0.53) or cases (Spearman’s r = −0.13, p = 0.56). Braak and Braak AD stage for NFTs was not correlated with VermTc (Kruskal–Wallis test = 5.13, p = 0.27) either. In cases, VermTc were similar in men and women (Mann–Whitney z = 0.62, p = 0.58).
Torpedoes were abundant in the vermis in ET cases (Figs. 1 and 2), with mean and median values being approximately two to four times greater in ET cases than controls (Table 1, Fig. 2). The hemispheric corrected Torpedo counts (HemTc) were similarly higher in ET cases than controls (Table 1).
Age was not correlated with torpedo count; hence, it could not have confounded the association between vermal torpedo count and case–control status. Nevertheless, we took several additional steps to consider the effects of possible confounding by age. First, a multivariate linear regression model was performed in which the log-transformed VermTc was the outcome variable, and case–control status, age, postmortem interval, and Braak and Braak AD stage for NFTs were included in the model. In that model, case–control status was independently associated with the log-transformed VermTc (beta = 0.41, p = 0.002). Second, we removed the youngest controls and the oldest ET cases in order to achieve case and control groups that were more similar in age (83.3 ± 6.5 vs. 75.5 ± 7.4 years, p > 0.05); the VermTc remained higher in the remaining eight ET cases than six controls (10.1 ± 9.7 [median, 6.3] vs. 2.9 ± 2.6 [median, 2.0], Mann–Whitney z = 2.07, p = 0.039).
The VermTc and the HemTc were correlated with one another (Spearman’s r = 0.54, p = 0.002) such that higher scores of one were accompanied by higher counts of the other. In an analysis restricted to ET cases, the correlation between the VermTc and the HemTc remained robust (Spearman’s r = 0.41, p = 0.047).
The VermTc was correlated with the cranial tremor score such that ET cases with neck, voice, and jaw tremors had the highest VermTc (p = 0.046, Table 2). The VermTc was not correlated with the total tremor score, which was a measure of limb tremor (Spearman’s r = −0.18, p = 0.55).
Discussion
We demonstrated that ET cases had more vermal torpedoes than did controls, with cases demonstrating, on average, a two- to fourfold amplification of the torpedo count compared to controls. Up until now, there have been no published data on the extent of vermal torpedo pathology in ET. Our prior studies of torpedo pathology in ET have been confined to the cerebellar hemisphere (neocerebellum) [11, 12, 18, 22], and there are no published data from other brain banks. The cerebellum is neither anatomically nor functionally homogeneous, and there are a number of examples of toxic or disease processes that do not affect the cerebellum in a uniform manner. For example, the cerebellar hemispheres are relatively less involved than the vermis in chronic alcoholics [26–28]; conversely, some forms of cerebellar olivary degeneration are manifest by relative preservation of the vermis in contrast to the cerebellar hemispheres [29]. The sine qua non of ET is the presence of hand tremor [30] and, on that basis, one would expect that the postmortem changes would involve the cerebellar hemispheres, which are physiologically responsible with limb coordination. Given that cranial tremors and gait difficulty are less common in ET [14, 15], there is less of an a priori expectation that the vermis is involved in the disease. Nonetheless, the current study demonstrates that axonal swellings in the ET brain involve both the cerebellar hemispheres as well as the cerebellar midline.
We found that there is a correlation between the extent of vermal torpedo pathology and the extent of hemispheric torpedo pathology, suggesting that these postmortem changes are not independent, but rather, that they likely reflect the presence of a common, underlying (i.e., broader) cerebellar system problem. There is a long-standing clinical literature that supports the concept that a dysfunction of the cerebellum causes ET, including the presence in ET patients of intention tremor [31, 32], gait ataxia [13, 33], oculomotor abnormalities [34], and problems with dysrhythmia and motor learning [35–39]. These findings often occur in patients with cerebellar diseases (e.g., spinocerebellar ataxia) [37, 40]. Deep brain stimulation surgery in ET targets the specific thalamic nucleus (i.e., ventral intermediate) that is the cerebellar receiving area, providing further support for this notion [41]. Many neuroimaging studies, including functional MRI [42], positron emission tomography [43–45], [1H] magnetic resonance spectroscopic imaging [46, 47], diffusion tensor imaging [48–50], voxel-based morphometry [51, 52], and studies using other automated volumetric methods [53], indicate the presence of functional, metabolic, and structural abnormalities of both gray and white matter in the cerebellum in ET. Although other brain regions (esp. red nucleus) variably show abnormalities in select imaging studies [44, 48, 51], it is the cerebellum that is consistently implicated in these numerous imaging studies. Future studies are needed in ET in order to establish links between (1) clinical features (e.g., intention tremor, gait ataxia, dysrhythmia), (2) laterality, location, and severity of neuroimaging abnormalities, and (3) distribution and nature of underlying microscopic changes in the cerebellum. Such studies are important in terms of expanding our understanding of the underlying pathophysiology and in developing clinical biomarkers, of which there are currently none.
There was a tendency in this study for greater cranial tremor to be associated with more vermal torpedo pathology, with the highest vermal torpedo counts being evident in ET cases with voice, jaw, and neck tremors and the lowest in controls. Of interest is that a recent volumetric and voxel-based morphometry magnetic resonance imaging study showed that ET patients with head tremor demonstrated cerebellar vermal atrophy compared with controls, whereas ET patients who did not have head tremor did not demonstrate this pattern [52]. Those findings as well as our present findings are in agreement with current data on the somatotopic organization of the cerebellum, which show that head and neck regions are mainly located in the vermis, whereas limbs are represented in adjacent regions in the hemispheres [52, 54, 55].
Prior studies have demonstrated that ET cases have greater cerebellar hemispheric torpedo pathology than do controls [11, 12]. While patients with other neurodegenerative diseases, including PD and AD, also have increased numbers of cerebellar torpedoes, the numbers in ET are several fold higher than seen on those diseases [22]. There are no data on whether cerebellar torpedo counts in PD cases correlate with the extent or severity of action tremor that can occur in that disorder.
This study had several limitations. First, ET cases were older than controls. Nevertheless, we previously demonstrated in a group of controls spanning a wide age range (6–93 years) that torpedoes do not accumulate with age [56], and again in the current sample, did not find an association between the number of torpedoes and age, indicating that age could not have been a confounder in our case–control comparison. To further ensure this, we (1) adjusted for age in a multivariate linear regression model and, (2) in a sub-analysis, only included cases and controls of similar age, finding that the case–control difference in vermal torpedo count remained robust in both analyses. Second, data on presence vs. absence of neck, voice, and jaw tremor were available, but we were not able to present data on the severity of those tremors. Third, although it would have been interesting to correlate tandem gait difficulty with the extent of vermal torpedo formation, we did not have quantitative data on tandem gait difficulty in most of these cases. This study also had a number of strengths. This is the first systematic attempt to examine the extent of vermal torpedo formation in ET cases, comparing them to a group of controls. All counts (i.e., raw data) were corrected for the underlying Purkinje cell layer length. Furthermore, our analyses considered a large number of potential confounding factors, which were considered in our analyses. Finally, the analyses drew upon the resources of the ETCBR, which houses the largest collection of ET brains worldwide.
In summary, the abundance of axonal swellings in the ET brain is not confined to a single region, but is a more broadly distributed finding involving both the cerebellar midline as well as the hemispheres. These data further confirm the central role of the cerebellum in the underlying pathophysiology of this common neurological disorder.
References
Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology. 2009;32:208–14.
Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41.
Zesiewicz TA, Chari A, Jahan I, Miller AM, Sullivan KL. Overview of essential tremor. Neuropsychiatr Dis Treat. 2010;6:401–8.
Louis ED, Dure LS, Pullman S. Essential tremor in childhood: a series of nineteen cases. Mov Disord. 2001;16:921–3.
Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, et al. Prevalence of essential tremor: door-to-door neurologic exams in Mersin province, Turkey. Neurology. 2003;61:1804–6.
Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–94.
Benito-Leon J, Bermejo-Pareja F, Louis ED. Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64:1721–5.
Louis ED. Essential tremors: a family of neurodegenerative disorders? Arch Neurol. 2009;66:1202–8.
Benito-Leon J. Essential tremor: one of the most common neurodegenerative diseases? Neuroepidemiology. 2011;36:77–8.
Louis ED, Vonsattel JP. The emerging neuropathology of essential tremor. Mov Disord. 2007;23:174–82.
Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9:613–22.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307.
Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9:193–6.
Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord. 2010;25:1633–8.
Louis ED, Ford B, Frucht S. Factors associated with increased risk of head tremor in essential tremor: a community-based study in northern Manhattan. Mov Disord. 2003;18:432–6.
Louis ED, Rios E, Applegate LM, Hernandez NC, Andrews HF. Jaw tremor: prevalence and clinical correlates in three essential tremor case samples. Mov Disord. 2006;21:1872–8.
Sulica L, Louis ED. Clinical characteristics of essential voice tremor: a study of 34 cases. Laryngoscope. 2010;120:516–28.
Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66:1756–9.
Louis ED, Borden S, Moskowitz CB. Essential tremor centralized brain repository: diagnostic validity and clinical characteristics of a highly selected group of essential tremor cases. Mov Disord. 2005;20:1361–5.
Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–35.
Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–6.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Pahwa R, et al. Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord. 2009;24:1600–5.
Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S85–8.
Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18:S91–4.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Yokota O, Tsuchiya K, Terada S, Oshima K, Ishizu H, Matsushita M, et al. Alcoholic cerebellar degeneration: a clinicopathological study of six Japanese autopsy cases and proposed potential progression pattern in the cerebellar lesion. Neuropathology. 2007;27:99–113.
Karhunen PJ, Erkinjuntti T, Laippala P. Moderate alcohol consumption and loss of cerebellar Purkinje cells. BMJ. 1994;308:1663–7.
Torvik A, Torp S. The prevalence of alcoholic cerebellar atrophy. A morphometric and histological study of an autopsy material. J Neurol Sci. 1986;75:43–51.
Ota S, Tsuchiya K, Anno M, Niizato K, Akiyama H. Distribution of cerebello-olivary degeneration in idiopathic late cortical cerebellar atrophy: clinicopathological study of four autopsy cases. Neuropathology. 2008;28:43–50.
Louis ED. Clinical practice. Essential tremor. N Engl J Med. 2001;345:887–91.
Leegwater-Kim J, Louis ED, Pullman SL, Floyd AG, Borden S, Moskowitz CB, et al. Intention tremor of the head in patients with essential tremor. Mov Disord. 2006;21:2001–5.
Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: prevalence and association with disease duration. Mov Disord. 2009;24:626–7.
Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. 2001;124:2278–86.
Helmchen C, Hagenow A, Miesner J, Sprenger A, Rambold H, Wenzelburger R, et al. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126:1319–32.
Trillenberg P, Fuhrer J, Sprenger A, Hagenow A, Kompf D, Wenzelburger R, et al. Eye–hand coordination in essential tremor. Mov Disord. 2006;21:373–9.
Avanzino L, Bove M, Tacchino A, Ruggeri P, Giannini A, Trompetto C, et al. Cerebellar involvement in timing accuracy of rhythmic finger movements in essential tremor. Eur J Neurosci. 2009;30:1971–9.
Bares M, Lungu OV, Husarova I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–35.
Farkas Z, Szirmai I, Kamondi A. Impaired rhythm generation in essential tremor. Mov Disord. 2006;21:1196–9.
Kronenbuerger M, Gerwig M, Brol B, Block F, Timmann D. Eyeblink conditioning is impaired in subjects with essential tremor. Brain. 2007;130:1538–51.
Bares M, Lungu OV, Liu T, Waechter T, Gomez CM, Ashe J (2011) The neural substrate of predictive motor timing in spinocerebellar ataxia. Cerebellum (in press).
Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, et al. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62:1250–5.
Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol. 1997;41:32–40.
Jenkins I, Bain P, Colebatch J, Thompson P, Findley L, Frackowiak S, et al. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol. 1993;34:82–90.
Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann Neurol. 1994;36:636–42.
Colebatch JG, Findley LJ, Frackowiak RS, Marsden CD, Brooks DJ. Preliminary report: activation of the cerebellum in essential tremor. Lancet. 1990;336:1028–30.
Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. 2002;333:17–20.
Pagan FL, Butman JA, Dambrosia JM, Hallett M. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology. 2003;60:1344–7.
Shin DH, Han BS, Kim HS, Lee PH. Diffusion tensor imaging in patients with essential tremor. AJNR Am J Neuroradiol. 2008;29:151–3.
Klein JC, Lorenz B, Kang JS, Baudrexel S, Seifried C, van de Loo S, et al. Diffusion tensor imaging of white matter involvement in essential tremor. Hum Brain Mapp. 2011;32(6):896–904. doi:10.1002/hbm.21077.
Nicoletti G, Manners D, Novellino F, Condino F, Malucelli E, Barbiroli B, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74:988–94.
Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Louis ED. Brain structural changes in essential tremor: voxel-based morphometry at 3-tesla. J Neurol Sci. 2009;287:138–42.
Quattrone A, Cerasa A, Messina D, Nicoletti G, Hagberg GE, Lemieux L, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. Am J Neuroradiol. 2008;29:1692–7.
Cerasa A, Messina D, Nicoletti G, Novellino F, Lanza P, Condino F, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. Am J Neuroradiol. 2009;30:1240–3.
Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci. 2004;5:241–9.
Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13:55–73.
Louis ED, Faust PL, Vonsattel JP, Erickson-Davis C. Purkinje cell axonal torpedoes are unrelated to advanced aging and likely reflect cerebellar injury. Acta Neuropathol. 2009;117:719–21.
Acknowledgments
The authors would like to acknowledge funding from R01 NS42859 from the National Institutes of Health (Bethesda, MD) and the Parkinson’s Disease Foundation (New York, NY).
Disclosure
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Louis, E.D., Faust, P.L., Ma, K.J. et al. Torpedoes in the Cerebellar Vermis in Essential Tremor Cases vs. Controls. Cerebellum 10, 812–819 (2011). https://doi.org/10.1007/s12311-011-0291-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-011-0291-0