Abstract
Multi-domain cognitive impairment (CI) has been frequently described in patients with essential tremor (ET). However, the exact neuroanatomical basis for this impairment is uncertain. This study aims to ascertain the role of the hippocampal formation in cognitive impairment in ET. Forty patients with ET and 40 age, gender and education matched healthy controls (HC) were enrolled. Cognition was assessed using a structured neuropsychological battery and patients were categorized as ET with CI (ETCI) and ET without CI (ETNCI). Automatic segmentation of hippocampal subfields was performed using FreeSurfer 6.0. The obtained volumes were correlated with scores of neuropsychological tests. Significant atrophy of the left subiculum, CA4, granule-cell layer of dentate gyrus, right molecular layer, and hypertrophy of bilateral parasubiculum, right hippocampus-amygdala-transition-area, bilateral hippocampal tail (HT) and widening of right hippocampal fissure was observed in ET. Trends toward atrophy of right subiculum, and widening of left HF was also observed. Comparison of HC and ETCI revealed atrophy of right subiculum, hypertrophy of bilateral parasubiculum, HT, and widening of left HF. ETCI showed a trend toward widening of right HF. ETNCI had isolated left parasubicular hypertrophy and in comparison, to ETNCI the ETCI subgroup had atrophy of bilateral fimbria. Significant correlations were observed between the volumes of HT, HF, fimbria and scores of tests for executive function, working and verbal memory. Patients with ET have significant volumetric abnormalities of several hippocampal subfields and these abnormalities may be important contributors for some forms of cognitive impairment observed in ET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential tremor (ET) is a highly prevalent movement disorder classically considered to be a benign monosymptomatic tremor disorder. However, this concept has been challenged by several studies which have demonstrated the presence of a variety of motor and non-motor symptoms other than the classical action tremor (Chandran and Pal 2012; Louis 2016). In addition to tremor, patients with ET have been reported to experience subtle neurological motor deficits such as impaired balance and gait, ocular-motor abnormalities, impaired hand-eye co-ordination, dysarthria, dystonia and bradykinesia (Benito-Leon and Labiano-Fontcuberta 2016; Deuschl and Elble 2009; Prasad et al. 2018b). Non-motor symptoms such as cognitive impairment, mood disturbances, sensory deficits and sleep dysregulation have also been reported (Bermejo-Pareja 2011; Chandran and Pal 2012). The presence of this wide array of symptoms suggests that ET may be a slowly progressive neurodegenerative disorder dominated by cerebellar dysfunction (Bermejo-Pareja 2011).
Patients with ET exhibit a wide range of mild cognitive deficits which are progressive in nature and they are at an increased risk for developing mild cognitive impairment and dementia. Studies have consistently demonstrated multi-domain deficits encompassing the domains of attention, concentration, working memory, executive functions, verbal fluency, memory- both short-term and delayed, and occasionally visuospatial dysfunction (Benito-Leon et al. 2006; Bermejo-Pareja 2011; Gasparini et al. 2001; Janicki et al. 2013; Kim et al. 2009; Lombardi et al. 2001; Sahin et al. 2006). Although cerebellar cognitive syndrome (Manto and Marien 2015) is reported in literature, the cerebellar dysfunction observed in ET cannot be entirely implicated for the extent of cognitive impairment and the increased risk of dementia observed in ET (Bermejo-Pareja et al. 2007; Thawani et al. 2009). Neuroimaging studies in patients of ET with cognitive dysfunction have reported cortical volume loss and white matter abnormalities in several regions associated with cognitive processing including the cerebellum (Benito-Leon et al. 2017; Bhalsing et al. 2014, 2015). Aberrant connectivity of resting-state networks involved in cognitive processing has also been reported in ET (Fang et al. 2015). In addition to these observations, neuropathological studies have suggested the role of a tau pathology in the genesis of cognitive dysfunction in patients with ET (Farrell et al. 2019; Pan et al. 2014).
The hippocampal formation composed of the hippocampus proper, dentate gyrus and subicular complex, plays an integral role in learning, memory, attention, executive functions, and visuo-spatial functions, with specific functions ascribed to each component of this formation (Mueller et al. 2011). The association of hippocampal subfield abnormalities and cognitive impairment has been well established in Alzheimer’s disease (La Joie et al. 2013), vascular dementia (Li et al. 2016) and other disorders associated with cognitive impairment (Lenka et al. 2018). Based on the existing evidence suggestive of cognitive impairment in ET, and the possibility of ET being a neurodegenerative disorder, it is plausible to expect hippocampal abnormalities in patients of ET with cognitive impairment. Till date, all neuroimaging studies in ET have examined the hippocampus as a single structure and there are no studies which have evaluated the structural integrity of hippocampal subfields in patients with ET.
This study aims to evaluate the cognitive functions and volume of hippocampal subfields in ET, and explore correlations between cognitive dysfunction and volume changes of the subfields. We hypothesize that patients with ET will demonstrate abnormalities in hippocampal subfields and these subfield abnormalities will correlate with scores of neuropsychological tests associated with functions of the subfield.
Methodology
Subject recruitment and clinical evaluation
This study included 40 patients with ET and 40 age, gender and education matched healthy controls (HC). Patients were recruited over a period of 2.5 years (January 2012–July 2014) from the general neurology outpatient clinic and movement disorder services at the National Institute of Mental Health and Neurosciences, Bangalore, India. ET was diagnosed based on the Consensus statement of the Movement Disorder Society on tremor (Deuschl et al. 1998) and confirmed by a trained movement disorder specialist. All patients underwent a detailed neurological examination and were evaluated for the presence of a persistent, bilateral, largely symmetric action tremor of the hands. In addition to this, other abnormal neurological signs, significant bradykinesia, rigidity and dystonia were specifically looked for to ensure the absence of parkinsonism or a dystonic tremor. HC were recruited from the hospital staff or from healthy caregivers of other patients admitted in the neurology wards. Only HC without a family history of ET, parkinsonism or any other movement disorder were recruited. All subjects were screened with the mini-mental-status examination (MMSE) and were included only if the score was ≥ 25.
Demographic details such as gender, age, age at onset and duration of illness were recorded. Additionally, medication history at time of evaluation was also recorded. The upper limbs, lower limbs, head, trunk and voice were evaluated for the presence of tremor. The Fahn–Tolosa–Marin tremor rating scale (FTMRS) was utilized to quantify disease severity (Fahn et al. 1993). The Hamilton anxiety rating scale (HAM-A) and Hamilton depression rating scale (HAM-D) were administered to the patient group to evaluate the presence and severity of anxiety and depression. The modified mini screen was applied to the control group to ensure the absence of a psychiatric disorder. All subjects underwent thorough neuropsychological evaluation, details of which are mentioned in the following section. This study was approved by the institute ethics committee and all subjects provided informed consent prior to recruitment. Subjects included in this study have been part of previous studies from our group (Bhalsing et al. 2014, 2015; Prasad et al. 2018a).
Neuropsychological evaluation
All subjects underwent a detailed neuropsychological evaluation with tests focusing on the domains of executive functions, attention, visuo-spatial functions and memory, which are relevant to hippocampal functioning (Wicking et al. 2014). Executive functions were tested using the Wisconsin Card Sorting Test (WCST) for evaluating set-shifting and Stroop test for evaluating response inhibition. WCST is evaluated based on categories completed, preservative response and preservative errors. The Color Trails Test I and II (CTT I, CTT II) were used to test attention. Visuo-spatial function was assessed using Benton’s Judgement of Line Orientation Test (LOT). Weschler Memory Scale III Spatial Span Test (WMS-SST) was used to evaluate working memory. Visual memory was assessed by the WMS III Face Recognition Test (WMS-FRT) and verbal memory was tested using Rey Auditory Verbal Learning Test (RAVLT). Both immediate and delayed recall were tested for FRT and RAVLT.
For the whole ET group i.e. 40 subjects, the mean scores were compared with the mean scores of HC to ascertain domains with abnormalities. To enable further classification of the ET group into ET with cognitive impairment (ETCI) and ET without cognitive impairment (ETNCI), scores of individual tests were compared and a test was considered as abnormal if the score was 1.5 standard deviations below or above the mean of HC. A patient was categorized as ETCI only if ≥ 3 neuropsychological tests were abnormal (Bhalsing et al. 2015).
Imaging protocol
All MRI scans were acquired using a 3T Philips Achieva MRI Scanner. A 16-channel head coil was used to acquire 3D T1-weighted inversion recovery fast gradient echo images. The acquisition parameters were as follows: repetition time: 8.2 ms; echo time: 3.8 ms; flip angle: 8°; slice thickness: 1 mm; number of slices: 165; acquisition matrix: 256 × 256 mm; image resolution: 1 × 1 × 1 mm.
Fluid attenuated inversion recovery images were also acquired and all images were screened by a neuroradiologist for structural abnormalities prior to analysis.
Image analysis
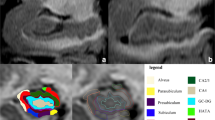
Pre-processing of images and automatic segmentation of hippocampal subfields was performed using FreeSurfer 6.0 (Fischl 2012). Pre-processing involved bias correction, automated transformation to the Talairach reference space, intensity normalization and removal of non-brain tissue. Following this, segmentation of subcortical white matter and deep gray matter nuclei, tessellation of gray and white matter boundary, automated topology correction, and surface deformation for the optimal placement of gray/white and gray/cerebrospinal fluid boundaries was performed. Automated hippocampal subfield segmentation was carried out using a Bayesian interference technique which employed a probabilistic atlas of the hippocampal formation which was trained on a hybrid data set consisting of in-vivo ultra-high resolution (0.1 mm) MRI and ex-vivo autopsied brain MRI of multiple subjects. Each hippocampal formation (left and right) was segmented into 12 subfields: parasubiculum, presubiculum, subiculum, cornu ammonis (CA)1, CA2–3, CA4 (composed of CA4, molecular layer and polymorphic layer of dentate gyrus), granule cell layer of dentate gyrus (GC-DG), molecular layer (ML) (composed of the molecular layers of CA 1, 2, 3, 4, and subiculum), hippocampal fissure (HF), hippocampal tail (HT), hippocampus amygdala transition area (HATA) and fimbria. The total hippocampus volume was considered as region 13. The detailed methodology for segmentation and description of each region is provided elsewhere (Iglesias et al. 2015). Figure 1 is a representative image demonstrating the automated segmentation with labelled regions in a subject from this study. Following automated subfield segmentation, volumes of each subfield was computed and normalized to total brain volume of each subject.
Statistical analysis
Statistical analysis of demographic variables was performed using a standard analysis of variance. Analysis of FTMRS scores, neuropsychological test scores and volumes of hippocampal subfields was performed between HC, ET, ETCI and ETNCI using multi-variate analysis of covariance where age and gender were used as nuisance variables. Group analysis was carried out between HC vs ET, HC vs ETCI, HC vs ETNCI, and ETCI vs ETNCI. Multiple comparisons were accounted for by employing false discovery rates (FDR) with a significance level was set at 95%. The significant hippocampal subfield volumes were correlated with clinical parameters that included age at onset, duration of illness, total FTMRS score and scores of individual neuropsychological tests. Residuals obtained after removing the effects of age and gender were employed for correlations. The level of significance was set at p < 0.05.
Results
Demographics and clinical profile
Based on the neuropsychological evaluation, 25 patients were categorized as ETCI and 15 were categorized as ETNCI. Details pertaining to the demographics, clinical and neuropsychological profile are provided in Tables 1 and 2. Patients of ETCI were significantly older in comparison to the ETNCI subgroup. There were no differences between ETCI and ETNCI with regard to the age at onset, duration of illness, FTMRS score, HAM-A, or HAM-D scores. The HC group had higher MMSE scores in comparison to ET and ETCI. The ETNCI subgroup had significantly higher MMSE scores in comparison to ETCI. Evaluation of treatment history revealed no significant differences between the treatment profiles of patients with ETCI and ETNCI.
Neuropsychological profile
Comparison of the results of neuropsychological tests between HC and the complete ET cohort revealed abnormalities in executive function, attention, visuo-spatial abilities, working, visual and verbal memory (Table 2). Patients with ET performed poorly in all domains of the WCST. They completed significantly fewer categories and had a higher number of perseverative responses and errors in comparison to HC. In comparison to HC, patients with ET also took more time to complete the Stroop test, CTT-I and CTT-II. Patients with ET also had lower scores in the WMS-SST, WMS-FRT immediate recall and RAVLT- immediate and delayed recall.
An identical pattern of cognitive abnormality was observed in the comparison between HC and ETCI. There were no significant differences between HC and ETNCI. A comparison between ETCI and ETNCI revealed specific abnormalities limited to executive dysfunction- WCST and Stroop test, attention—CTT-I, and verbal memory—RAVLT-immediate recall.
Volumes of hippocampal subfields
Comparison of the volumes of hippocampal subfields between HC, ET, ETCI and ETNCI revealed a combination of atrophy and hypertrophy of hippocampal subfields (Table 3). HC vs ET.
In comparison to HC, patients with ET had hypertrophy of bilateral parasubiculum, bilateral HT, right HATA, and widening of right HF. A trend toward significant widening of the left HF was also observed in the ET group. Atrophy of left subiculum, CA4, GC-DG and right ML was observed in patients of ET in comparison to HC. The right subiculum showed a trend toward significant atrophy in patients with ET. There were no significant differences in total hippocampal volume between patients with ET and HC.
HC versus ETCI, ETNCI and ETCI versus ETNCI
A comparison between HC and ETCI revealed hypertrophy of bilateral parasubiculum, bilateral HT and widening of left HF. A trend toward significant widening of the right HF was observed in the ETCI subgroup. Atrophy of the right subiculum was observed in ETCI. In comparison to HC, the ETNCI group had isolated hypertrophy of the left parasubiculum. Comparison of subfield volumes between ETCI and ETNCI revealed isolated atrophy of bilateral fimbria in the ETCI subgroup. There were no significant differences in total hippocampal volume between any of the above compared subgroups.
Correlations
Several significant correlations were observed between volumes of significantly different hippocampal subfields and scores of neuropsychological tests (Table 4). Significant negative correlations were observed between the volumes of bilateral HT and cognitive tests-MMSE score and WCST-categories completed. The volume of right HT also showed a significant negative correlation with WMS SST scores. Significant positive correlations were observed between the HAM-A score and left parasubicular volume, WMS FRT-immediate recall scores correlated with the volumes of left HP tail and right HP fissure, and RAVLT-immediate recall scores correlated with volume of the left fimbria. No significant correlations were observed between age at onset, duration of illness, FTMRS scores and the volumes of hippocampal subfields.
Discussion
Multi-domain cognitive impairment has been frequently described in patients with ET and several neuroimaging studies have reported the presence of abnormalities of regions involved in cognitive processing (Benito-Leon et al. 2017; Bhalsing et al. 2014, 2015; Duane and Vermilion 2002; Fang et al. 2015; Gasparini et al. 2001; Higginson et al. 2008; Kim et al. 2009; Lacritz et al. 2002; Lombardi et al. 2001; Sahin et al. 2006). To the best of our knowledge, no study has attempted to explore the structural integrity of the hippocampal formation in patients with ET and ascertain the influence of this abnormality in the development of cognitive impairment in ET. In the present study, we explored the cognitive profile and volumes of the hippocampal subfields in ET. Furthermore, we attempted to ascertain the presence of abnormalities specific to cognitive impairment in ET by comparing patients of ETCI and ETNCI.
Overview of hippocampal pathways
The hippocampal formation is known to play a critical role in cognition specifically in the domains of learning, memory, attention, executive function, and visuo-spatial abilities (Duvernoy et al. 2013). The main pathways described in conjecture with functions of the hippocampal formation are the polysynaptic intrahippocampal pathway (PIP) and the direct intrahippocampal pathway (DIP) (Duvernoy et al. 2013). These intrahippocampal pathways play a critical role in all aspects of memory and are part of the Papez circuit. A brief overview of these pathways is crucial to understand the functional implications and correlates of the subfield abnormalities observed in the present study.
The PIP which is primarily associated with episodic and spatial memory links all parts of the hippocampus by a long neuronal chain whereas the DIP which is associated with semantic memory is a shorter more direct pathway (Fig. 2). The PIP is composed of the entorhinal cortex, the dentate gyrus, CA and the subiculum. It receives inputs from the temporal cortex, occipital cortex, and posterior parietal cortex via the entorhinal cortex. The perforant pathway which arises from layer II of the entorhinal cortex passes through the subiculum to connect to the dentate gyrus. Within the molecular layer of the dentate gyrus, these fibers synapse with dendrites of granular cells, which in turn via the mossy fibers stimulate the dendrites of CA3 and CA4. The axons of CA3 and CA4 prior to entering the alveus emit Schaffer collaterals which communicate with CA1, and the axons of CA1 emit collaterals to the subiculum prior to entering the alveus. Fibers from the subiculum also enter the alveus, which in turn communicates with the fimbria which is the main output of the PIP. The output from the PIP travels through the mamillary bodies and anterior thalamic to reach the retrosplenial cortex, posterior and anterior cingulate cortex. In the DIP, input fibers from the inferior temporal association cortex directly reach the CA1 via layer III of the entorhinal cortex. The CA1 neurons project to the subiculum, the axons of which return to deep layers of the entorhinal cortex, from where output fibers reach the temporal pole, prefrontal cortex and inferior temporal association cortex.
Neuropsychological profile of patients with ET
In the present study, the comparison between HC and ET, and between HC and ETCI, revealed cognitive abnormalities encompassing the domains of executive function-set shifting and response inhibition, attention, visuo-spatial abilities and memory-working, verbal and visual (Table 2). A comparison of ETCI and ETNCI revealed cognitive impairment restricted to executive dysfunction, attention and verbal memory. These results are similar to reports from previous studies (Benito-Leon et al. 2017; Gasparini et al. 2001; Higginson et al. 2008; Kim et al. 2009; Lacritz et al. 2002; Lombardi et al. 2001; Sahin et al. 2006; Troster et al. 2002).
Volumetric differences observed in ET, ETCI and ETNCI and the possible implications of these alterations on cognitive impairment
The observed differences in volumes of hippocampal subfields either atrophy or hypertrophy may be important contributors for some forms of cognitive impairment observed in ET. In patients with ET we observed atrophy of bilateral subiculum (left subiculum, p < 0.01 and trend in right subiculum, p = 0.06), CA4, GC-DG and right ML (Table 3). Comparison of HC and ETCI revealed isolated atrophy of right subiculum and comparison of ETCI and ETNCI revealed atrophy of bilateral fimbria (Table 3). The executive dysfunction in ET may be associated with atrophy of the ML of CA1, and subiculum which could lead to abnormal output to the prefrontal cortex (Yuan and Raz 2014). Abnormal memory may also be attributable to involvement of the above structures since the PIP is involved in episodic and spatial memory. The fimbria is a crucial outflow structure and the atrophy observed in ETCI subgroup in comparison to ETNCI could suggest a role of the fimbria in cognitive processing. The significant positive correlation observed between volume of the fimbria and verbal memory i.e. RAVLT-immediate recall score lends support to the role of this structure in the observed cognitive impairment.
We also observed widening of bilateral HF (right HF, p < 0.01 and trend in left HF, p = 0.06), right HATA and hypertrophy of bilateral HT and parasubiculum in ET, and widening of bilateral HF (right HF, p = 0.06), hypertrophy of bilateral HT and parasubiculum in ETCI (Table 3). Widening of the HF has been reported to correlate with a decrease in overall hippocampal volume and has been considered as a radiological marker for hippocampal atrophy (Bastos-Leite et al. 2006; Li et al. 2018). Hence, the observation of widening of bilateral HF in ET and ETCI in comparison to controls may be suggestive of impending atrophy of the whole hippocampus. Widening of the HATA, which is the zone between the hippocampus and amygdala may not have any direct functional implications, rather this may also be an indirect indicator of hippocampal atrophy. The HT is a small structure at the posterior part of the hippocampal arc and is composed of the hippocampal fissure, fimbria, CA1, CA3 and DG (Duvernoy 2005) and we observed several significant negative correlations between volume of the HT and scores of tests associated with executive function (WCST-categories completed), working memory (WMS-SST) and MMSE (Table 4). These correlations could suggest a contributory role of the HT to cognitive impairment in ET.
Finally, we observed bilateral parasubicular hypertrophy in patients with ET, ETCI and interestingly even in the ETNCI subgroup. The parasubiculum receives visuo-spatial information from the retrosplenial cortex, parahippocampal cortex and posterior cingulate cortex and plays a significant role in spatial processing and scene-based cognition (Dalton and Maguire 2017). The retrosplenial cortex plays a crucial role in the generation of egocentric and allocentric reference frames (Dalton and Maguire 2017), and it has been hypothesized that a tremor perturbs feedback regarding self-motion, owing to which the integration of this information is hampered (Daniels et al. 2006). This change may lead to increased demands of structures involved in these processing pathways, and parasubicular hypertrophy may be attributed to the adaptive reorganization that occurs as a result of these aberrations in processing. A comparable task-induced expansion in gray matter was reported in a study of young adults who acquired juggling skills, which required increased processing from structures involved in visuo-spatial processing (Draganski et al. 2004). The presence of this hypertrophy even in the ETNCI group lends support to this theory. Furthermore, it is also possible that in ET and ETCI, the hypertrophy is also influenced by a compensatory increase in parasubicular dendrites due to a reduction in the excitatory input from the atrophied subiculum.
Although not observed in this study cohort, patients with ET are known to develop mood disturbances specifically anxiety and depression. Apart from the above described functions, the hippocampus is also involved in emotion processing. Hence, alterations in volumes of hippocampal subfields may contribute to the development of these symptoms.
Limitations
There are several limitations to this study, the sample size of the ETCI and ETNCI subgroups is relatively small and larger sample sizes of these groups are necessary to validate the observed results. The ages of these two subgroups were significantly different and since hippocampal volume is known to decrease with age, better age-matched subgroups would have aided in excluding this confounding factor. The ET cohort was broadly sub-grouped into ETCI, and ETNCI; further stratification of the ETCI group into those with and without dementia would have provided a complete perspective of the cognitive spectrum in ET. The positive correlation was observed between left HT volume and WMS FRT-immediate recall score, right HF volume and WMS-FRT immediate recall score and volume of left parasubiculum and HAM-A scores cannot be entirely explained since these correlations are contrary to expected patterns of volume change and function. With the exception of cognition, anxiety and depression we did not investigate the presence of other non-motor symptoms such as sleep disturbances, etc which may have a bearing on cognitive functioning. Finally, the resolution of the images is 1 mm isotropic which may not provide high accuracy in hippocampal subfield segmentation. Despite this limitation we feel the observed results hold definite merit, however, volumes of the internal subfields (especially GC-DG, CA4 and ML) need to be evaluated in future studies with sub-millimeter voxel size to validate our results.
Conclusions
Patients with essential tremor have significant volumetric abnormalities of several hippocampal subfields and these abnormalities may be important contributors for some forms of cognitive impairment observed in essential tremor. The observations of the present study provide novel insights into the expanding concept of pathogenesis of cognitive dysfunction in essential tremor.
References
Bastos-Leite AJ, van Waesberghe JH, Oen AL, van der Flier WM, Scheltens P, Barkhof F (2006) Hippocampal sulcus width and cavities: comparison between patients with Alzheimer disease and nondemented elderly subjects. AJNR Am J Neuroradiol 27:2141–2145
Benito-Leon J, Labiano-Fontcuberta A (2016) Linking essential tremor to the cerebellum. Clin Evid Cereb 15:253–262. https://doi.org/10.1007/s12311-015-0741-1
Benito-Leon J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain Study G (2006) Population-based case–control study of cognitive function in essential tremor. Neurology 66:69–74. https://doi.org/10.1212/01.wnl.0000192393.05850.ec
Benito-Leon J et al (2017) White matter microstructural changes are related to cognitive dysfunction in essential tremor. Sci Rep 7:2978. https://doi.org/10.1038/s41598-017-02596-1
Bermejo-Pareja F (2011) Essential tremor—a neurodegenerative disorder associated with cognitive defects? Nat Rev Neurol 7:273–282. https://doi.org/10.1038/nrneurol.2011.44
Bermejo-Pareja F, Louis ED, Benito-Leon J, Neurological Disorders in Central Spain Study G (2007) Risk of incident dementia in essential tremor: a population-based study. Mov Disord 22:1573–1580. https://doi.org/10.1002/mds.21553
Bhalsing KS, Upadhyay N, Kumar KJ, Saini J, Yadav R, Gupta AK, Pal PK (2014) Association between cortical volume loss and cognitive impairments in essential tremor. Eur J Neurol 21:874–883. https://doi.org/10.1111/ene.12399
Bhalsing KS, Kumar KJ, Saini J, Yadav R, Gupta AK, Pal PK (2015) White matter correlates of cognitive impairment in essential tremor. AJNR Am J Neuroradiol 36:448–453. https://doi.org/10.3174/ajnr.A4138
Chandran V, Pal PK (2012) Essential tremor: beyond the motor features. Parkinsonism Relat Disord 18:407–413. https://doi.org/10.1016/j.parkreldis.2011.12.003
Dalton MA, Maguire EA (2017) The pre/parasubiculum: a hippocampal hub for scene-based cognition? Curr Opin Behav Sci 17:34–40. https://doi.org/10.1016/j.cobeha.2017.06.001
Daniels C et al (2006) Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology 67:1452–1456. https://doi.org/10.1212/01.wnl.0000240130.94408.99
Deuschl G, Elble R (2009) Essential tremor—neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord 24:2033–2041. https://doi.org/10.1002/mds.22755
Deuschl G, Bain P, Brin M (1998) Consensus statement of the movement disorder society on tremor. Ad Hoc Sci Committee Mov Disord 13(Suppl 3):2–23
Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004) Neuroplasticity: changes in grey matter induced by training. Nature 427:311–312. https://doi.org/10.1038/427311a
Duane DD, Vermilion KJ (2002) Cognitive deficits in patients with essential tremor. Neurology 58:1706 (author reply 1706)
Duvernoy HM (2005) The human hippocampus functional anatomy, vascularization and serial sections with MRI, 3rd edn. Springer, Berlin
Duvernoy H, Cattin F, Risold P-Y (2013) Structure, functions, and connections. In: The human hippocampus. Springer, pp 5–38
Fahn S, Tolosa E, Marín C (1993) Clinical rating scale for tremor Parkinson’s disease and movement disorders. Williams and Wilkins, Baltimore, pp 271–280
Fang W et al (2015) Multiple resting-state networks are associated with tremors and cognitive features in essential tremor. Mov Disord 30:1926–1936. https://doi.org/10.1002/mds.26375
Farrell K et al (2019) Quantitative assessment of pathological tau burden in essential tremor: a postmortem study. J Neuropathol Exp Neurol 78:31–37. https://doi.org/10.1093/jnen/nly104
Fischl B (2012) FreeSurfer. Neuroimage 62:774–781
Gasparini M, Bonifati V, Fabrizio E, Fabbrini G, Brusa L, Lenzi GL, Meco G (2001) Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol 248:399–402
Higginson CI, Wheelock VL, Levine D, King DS, Pappas CT, Sigvardt KA (2008) Cognitive deficits in essential tremor consistent with frontosubcortical dysfunction. J Clin Exp Neuropsychol 30:760–765. https://doi.org/10.1080/13803390701754738
Iglesias JE et al (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo. MRI Neuroimage 115:117–137. https://doi.org/10.1016/j.neuroimage.2015.04.042
Janicki SC, Cosentino S, Louis ED (2013) The cognitive side of essential tremor: what are the therapeutic implications? Ther Adv Neurol Disord 6:353–368. https://doi.org/10.1177/1756285613489591
Kim JS, Song IU, Shim YS, Park JW, Yoo JY, Kim YI, Lee KS (2009) Cognitive impairment in essential tremor without dementia. J Clin Neurol 5:81–84. https://doi.org/10.3988/jcn.2009.5.2.81
La Joie R et al (2013) Hippocampal subfield volumetry in mild cognitive impairment. Alzheimer’s Dis Semant Dement Neuroimage Clin 3:155–162. https://doi.org/10.1016/j.nicl.2013.08.007
Lacritz LH, Dewey R Jr, Giller C, Cullum CM (2002) Cognitive functioning in individuals with “benign” essential tremor. J Int Neuropsychol Soc 8:125–129
Lenka A et al (2018) Hippocampal subfield atrophy in patients with Parkinson’s disease and psychosis. J Neural Transm (Vienna) 125:1361–1372. https://doi.org/10.1007/s00702-018-1891-3
Li X, Li D, Li Q, Li Y, Li K, Li S, Han Y (2016) Hippocampal subfield volumetry in patients with subcortical vascular mild cognitive impairment. Sci Rep 6:20873. https://doi.org/10.1038/srep20873
Li Y, Yan J, Zhu X, Zhu Y, Qin J, Zhang N, Ju S (2018) Increased hippocampal fissure width is a sensitive indicator of rat hippocampal atrophy. Brain Res Bull 137:91–97. https://doi.org/10.1016/j.brainresbull.2017.11.014
Lombardi WJ, Woolston DJ, Roberts JW, Gross RE (2001) Cognitive deficits in patients with. essential tremor. Neurology 57:785–790
Louis ED (2016) Non-motor symptoms in essential tremor: a review of the current data and state of the field. Parkinsonism Relat Disord 22(Suppl 1):S115-118 https://doi.org/10.1016/j.parkreldis.2015.08.034
Manto M, Marien P (2015) Schmahmann’s syndrome—identification of the third cornerstone of clinical ataxiology. Cereb Ataxias 2:2. https://doi.org/10.1186/s40673-015-0023-1
Mueller SG, Chao LL, Berman B, Weiner MW (2011) Evidence for functional specialization of hippocampal subfields detected by MR subfield volumetry on high resolution images at 4 T. Neuroimage 56:851–857. https://doi.org/10.1016/j.neuroimage.2011.03.028
Pan JJ, Lee M, Honig LS, Vonsattel JP, Faust PL, Louis ED (2014) Alzheimer’s-related changes in non-demented essential tremor patients vs. controls: links between tau and tremor? Parkinsonism Relat Disord 20:655–658. https://doi.org/10.1016/j.parkreldis.2014.03.003
Prasad S et al (2018a) DTI in essential tremor with and without rest tremor: two sides of the same coin? Mov Disord. https://doi.org/10.1002/mds.27459
Prasad S, Velayutham SG, Reddam VR, Stezin A, Jhunjhunwala K, Pal PK (2018b) Shaky and unsteady: dynamic posturography in essential tremor. J Neurol Sci 385:12–16
Sahin HA, Terzi M, Ucak S, Yapici O, Basoglu T, Onar M (2006) Frontal functions in young patients with essential tremor: a case comparison study. J Neuropsychiatry Clin Neurosci 18:64–72. https://doi.org/10.1176/jnp.18.1.64
Thawani SP, Schupf N, Louis ED (2009) Essential tremor is associated with dementia: prospective population-based study in New York. Neurology 73:621–625. https://doi.org/10.1212/WNL.0b013e3181b389f1
Troster AI, Woods SP, Fields JA, Lyons KE, Pahwa R, Higginson CI, Koller WC (2002) Neuropsychological deficits in essential tremor: an expression of cerebello-thalamo-cortical pathophysiology? Eur J Neurol 9:143–151
Wicking M, Nees F, Steiger F (2014) Neuropsychological measures of hippocampal function. Front Neurol Neurosci 34:60–70. https://doi.org/10.1159/000356425
Yuan P, Raz N (2014) Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev 42:180–192. https://doi.org/10.1016/j.neubiorev.2014.02.005
Funding
Symbiosis International University has received partial support from DST SERB (ECR/2016/000808) for setting up the computing facility.
Author information
Authors and Affiliations
Contributions
Research Project: conception: SP, JS, MI, PKP, organization: SP, KSB, KJK, JS, PKP, execution: SP, AS, KSB, MI, PKP. Statistical analysis: design: SP, AS, KSB, JS, MI, execution: SP, AS, KSB, MI, review and critique: AS, KSB, KJK, JS, MI, PKP. Manuscript: writing of the first draft: SP, review and critique: AS, KSB, KJK, JS, MI, PKP.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any financial disclosure to make or have any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prasad, S., Shah, A., Bhalsing, K.S. et al. Abnormal hippocampal subfields are associated with cognitive impairment in Essential Tremor. J Neural Transm 126, 597–606 (2019). https://doi.org/10.1007/s00702-019-01992-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-01992-3