Abstract
Objective
Essential tremor (ET) is the second most common movement disorder; however, the pathophysiological mechanism of ET is unclear. We aimed to investigate the microstructural degeneration of gray matter (GM) and white matter (WM) and their correlations with cognition and tremor in patients with ET.
Methods
The participants were 63 patients with ET and 63 matched healthy controls (HCs) who underwent 3D-T1 weighted and diffusion kurtosis images (DKI). Microstructural degeneration was measured using high-level diffusion parameters derived from DKI. A voxel-wise analysis of the means of the GM-based spatial statistics and tract-based spatial statistics were conducted to assess differences in diffusion parameters between the ET and HC groups. The volume differences between the two groups were also assessed, and tremor severity and multi-domain cognitive performance were evaluated. Finally, the relationship between microstructural degeneration and clinical characteristics were assessed.
Results
The ET group had significantly lower mean kurtosis of the temporal, parietal, and occipital lobes and the cerebellum and lower radial kurtosis in several tracts. These microstructural changes in GM and WM were correlated with tremor and cognitive scores. However, no significant difference in volume was found between the groups.
Conclusion
Our findings suggest that ET entails extensive GM and WM microstructural alterations, which support the neurodegenerative hypothesis of ET. Our study contributes to a better understanding of the mechanisms underlying tremor and cognitive impairment in ET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential tremor (ET) is the second most common movement disorder [1]. It is characterized by an upper extremity postural or action tremor, with or without tremor of the lower limbs, head, mouth, face or voice. Although ET was initially considered to be a benign motor disorder, reports are increasing that it is associated with cognitive disorders, mood disorders, and early mortality [2]. The pathophysiological mechanism of ET remains unclear, but neurodegeneration is one of the proposed hypotheses. Symptoms of ET are hypothesized to be localized in the cerebello-thalamo-motor cortical pathway [3]. Some postmortem studies have shown neuronal changes in the cerebellum, especially the cerebellum cortex, in patients with ET [4]. However, emerging evidence suggests that areas outside the cerebello-thalamo-motor cortical pathway are also involved in the pathology of ET, though this has not been fully elucidated.
To examine the pathology of cortical and subcortical structures in patients with ET, many studies have used morphological MRI techniques to explore cerebral cortical thickness [5, 6] and brain volume [7,8,9]. And many studies have employed diffusion tensor imaging (DTI) to explore the altered white matter (WM) structural integrity [3]. However, these studies have inconsistent results, which may be due to insufficient sample size or methodological limitations. DTI has intrinsic shortcomings, because it assumes that the diffusion of water in the brain is unrestricted and follows a Gaussian distribution [10, 11], which does not correspond to reality. To overcome this limitation, diffusion kurtosis imaging (DKI) was developed specifically for deep nucleus and cortical gray matter [12]. DKI, as an extension of DTI, can quantify the non-Gaussian distribution of water molecules and effectively quantify cortical and subcortical microstructural complexity [10, 11]. Evidence from neurodegenerative diseases suggests that DKI is a more sensitive technology than DTI for detecting brain damage [11, 13]. At present, there is no study that has used DKI to explore gray and white matter microstructure alterations in the same samples of patients with ET.
Therefore, we aimed to investigate possible GM and WM microstructure changes and their correlations with motor and non-motor symptoms of ET. In addition, we also assessed alteration of GM volume in patients with ET to test whether DKI parameters were more sensitive than volume measures to detect brain damage from ET.
Methods
Participants
Patients were recruited from the Second Affiliated Hospital of Zhejiang University School of Medicine between March 2019 and December 2021. This study was approved by The Medical Ethics Committee. The diagnosis of ET was made by experienced neurologists, based on the 2018 Consensus Statement by the task force on tremor of the International Parkinson and Movement Disorder Society (MDS) [14]. The inclusion criterion for the ET group was satisfying the MDS criteria for probable ET. The healthy controls (HCs) consisted of patients’ spouses and volunteers. The inclusion criteria for HCs were: (a) no neurological diseases; (b) no clinical dysthymic disorders; (c) no obvious cognitive disorders; and (d) no family history of ET. The exclusion criteria were: (a) serious nervous system diseases (excluding probable ET); (b) serious mental illness; (c) use of non-ET-related medication that influence brain function; (d) a history of head trauma. In the end, we recruited 63 patients with ET and 63 age- and sex-matched HCs. All the participants provided their informed consent.
Neuropsychological tests
All the patients completed multidimensional neuropsychological tests, including the Essential Tremor Rating Assessment Scale (TETRAS) to assess the severity of tremor and its impact on daily life, the Montreal Cognitive Assessment scale (MoCA), the Mini-Mental State Examination (MMSE), the Auditory Verbal Learning Test (AVLT), the Boston Naming Test (BNT), the Animal Fluency Test (AFT), the Trail Making Test, Part A, (TMT-A), the Digital Span Test (DST), the Symbol Digit Modalities Test (SDMT), and the Trail Making Test, Part B (TMT-B), to assess general cognition and other cognitive domains. The HCs also completed the MMSE, MoCA, AVLT, BNT, AFT, TMT-A, TMT-B, DST, and SDMT.
MRI data acquisition
All participants were scanned using a GE Discovery MR750 3.0 T MRI scanner with an eight-channel head coil. Foam pads and ear plugs were used to reduce head motion and noise. DKI and high-resolution 3D T1-weighted structural MRI images were acquired. DKI was acquired using a spin echo-echo planar imaging sequence: TE = 93.7 ms; TR = 5000 ms; FA = 90°; matrix = 128 × 128; slice thickness = 4 mm; field of view = 256 × 256 mm2; and number of slices = 34 (axial). DKI images were acquired from 30 gradient directions (b = 1000 and 2000 s/mm2), and included three acquisitions without diffusion weighting (b = 0). An additional b = 0 acquisition with reverse phase-encode polarity was acquired for distortion correction. The 3D T1-weighted MRI was acquired using a fast spoiled gradient-recalled sequence: repetition time (TR) = 7.336 ms; echo time (TE) = 3.036 ms; flip angle (FA) = 11°; inversion time = 450 ms; field of view (FOV) = 260 × 260 mm2; matrix = 256 × 256; number of slices = 196 (sagittal); and slice thickness = 1.2 mm.
Diffusion image preprocessing
DKI data preprocessing was conducted using the Diffusion Kurtosis Estimator (v.2.6, https://www.nitrc.org/projects/dke/) and MRtrix3 (v.3.0_RC3, www. mrtrix.org), FSL (v.6.0, http://www.fmrib.ox.ac.uk/fsl). DKI data were first preprocessed via de-noising to remove the Gibbs ringing artifact. Then, top-up and eddy were conducted to correct susceptibility induced by eddy currents, distortions, and movements in diffusion Data 1. Next, the skulls were first stripped from the DKI data for each participant. Finally, radial kurtosis (RK), axial kurtosis (AK), and the mean kurtosis (MK) of all the subjects were calculated with the Diffusion Kurtosis Estimator.
GM-based spatial statistics (GBSS) and Tract-based spatial statistics (TBSS) were implemented using the FSL to alleviate partial volume effects.
GBSS We used a GM analog of TBSS (namely, GBSS) to analyze GM. First, the Brain Extraction Tool was used to remove non-brain voxels from each participant’s 3D T1-weighted image. Second, each skull-stripped 3D T1-weighted image was affine- and non-linearly aligned to an MNI152 standard space at a 1-mm resolution, using the FMRIB linear and non-linear registration tools. Next, we corrected field bias and then used the FMRIB automated segmentation tool to obtain GM, WM, and CSF segmentations. To minimize interference from WM and CSF voxels, the generated GM images were used to create a median GM skeleton with a threshold of 0.2. Next, affine b0 maps of each subject were aligned to their 3D T1-weighted images (epi-reg). The aligned maps of each subject were projected onto the median GM skeleton map after affine- and non-linearly aligning all maps into an MNI152 brain common space at a 1-mm resolution.
TBSS We used the skeleton projection step of TBSS to analyze WM. First, we used the FMRIB non-linear registration tool to align all FA images to the standard Montreal Neurological Institute (MNI) space. Next, all FA images in the standard space were averaged, and the mean FA skeleton, as a representation of the centers of all tracts common to the groups, was created with a threshold of 0.2 to exclude peripheral tracts and GM and include major WM pathways. Finally, the FA images of each participant were projected onto the mean FA skeleton, and the MK images were projected onto the mean FA skeleton. The JHU atlas with 48 labels was used to extract MK values.
T1-weighted image preprocessing
Voxel-based morphometry (VBM) analysis was used to assess the volume difference between the ET and HC groups. We used the CAT12 Toolbox (Computational Anatomy Toolbox; http://dbm.neuro.uni-jena.de/cat12/) running in SPM12 (Statistical Parametric Mapping) software (www.fil.ion.ucl. ac.uk/spm) to conduct VBM. The preprocessing steps were [15] (a) All images were corrected for noise, inhomogeneities, and intensity, and then divided into WM, GM, and CSF components; (b) The divided images were normalized into a standard MNI space with the Diffeomorphic Anatomic Registration Through Exponentiated Lie algebra algorithm; (c) Spatial normalized images were adjusted to ensure the relative GMV; and (d) Weighted overall image quality algorithms, Mahalanobis distance, and mean correlations were used to quantify image quality [subjects who fell below the quality threshold (2 SDs) were removed]; (e) Subsequently, the total intracranial volume (TIV) was estimated before being smoothed; and (f) Finally, images were smoothed using a 6 mm full-width-half-maximum isotropic Gaussian kernel.
Statistical analyses
Statistical analyses of demographic variables were performed using IBM SPSS Statistics for Windows, version 26.0. The Shapiro–Wilk test was used to assess the normality of the data. We used the χ2 test and independent-samples Student’s t-test, respectively, to analyze categorical variables and continuous demographic variables. The statistical significance level was set at 0.05 for all two-tailed tests. The GBSS, TBSS, and VBM analyses employed the general linear model, including independent-samples Student’s t-test, and the randomize tool was used to compare all the diffusion parameters between groups with 5000 permutations. Then, the results of the multiple comparisons were corrected by controlling for family-wise error (FWE) and applying threshold-free cluster enhancement (p < 0.05). The randomized tool was used for voxel-wise correlation analysis of each parameter with TETRAS part I, TETRAS part II, and AVLT scores. As an exploratory study, we reported the correlation analysis results with p < 0.01 (uncorrected for multiple comparisons) as statistically significant. The correlation analyses were performed with MATLAB software (version R2019a).
Results
Demographics and clinical variables
Patients with ET matched well with HCs for gender, age, and education (p > 0.05). A family history of tremor was observed in 60.3% of cases, and 13% of patients with ET were taking medication to control their tremors, including propranolol and gabapentin. Patients with ET had significantly lower AVLT scores than the HCs, including AVLT Immediate recall (p = 0.015), AVLT Short delayed recall (p = 0.003), AVLT Long delayed recall (p = 0.002), and AVLT Recognition (p = 0.035). Detailed demographic and clinical data are summarized in Table 1.
GM alterations
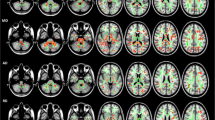
GBSS analysis showed that the MK value was significantly lower in the ET than the HC groups in temporal lobe (bilateral Temporal gyrus, bilateral Angular gyrus and bilateral Fusiform gyrus), parietal lobe (right Superior and inferior parietal gyrus, left parietal gyrus, bilateral Supra marginal gyrus, right postcentral gyrus and bilateral Cuneus), occipital lobe(bilateral occipital gyrus and left lingual gyrus) and cerebellum (bilateral Cerebellum 6,7b,8,9, left Cerebellum 4,5, Vermis 6,7, bilateral Cerebellum_Crus 1,2 and left Cerebellum_Crus 4,5,6) (Specific clusters are shown in Fig. 1 and Table 2). No significant differences in AK or RK values were found between the ET and HC groups, and no significant difference in GM volume was found between the two groups.
WM alterations
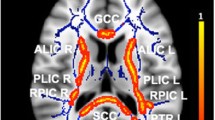
Compared with the HCs, the patients with ET showed significant alterations in WM microstructure in extensive bilateral regions. The TBSS analysis of the ET group showed that RK was significantly decreased in cerebellar peduncle, corpus callosum, corticospinal tract, medial lemniscus, cerebral peduncle, anterior, posterior limb of internal capsule, retrolenticular part of internal capsule, anterior, superior corona radiata, posterior corona radiata, posterior thalamic radiation, sagittal stratum, external capsule, cingulum, fornix (cres)/stria terminalis, superior longitudinal fasciculus and uncinate fasciculus (Specific clusters are shown in Fig. 2 and Table 2), and no significant differences were observed in MK or AK values between the HC and ET groups.
Statistical map shows voxels that reveal differences in DKI parameters between ET patients and healthy controls (red or yellow). The background images are standard MNI T1 template and the RK skeleton (green). TBSS analysis shows that RK is significantly (p < 0.05, TFCE-corrected) lower in the patient group than in controls. There was no significant difference in AK and MK values between groups. The results were amplified
Correlation analysis
The correlations between the abnormal DKI parameters of the GM and WM regions and tremors and memory in patients with ET are shown in Figs. 3 and 4 and Table 3. We found that cognitive scores were positively correlated with MK values and the RK values in left Cerebelum_Crus 1,2 and anterior limb of internal capsule, posterior limb of internal capsule, retrolenticular part of internal capsule, anterior corona radiate, right posterior thalamic radiation (including the optic radiation) and left external capsule, respectively. In addition, we found tremor scores generally had a negative correlation with MK values and RK values in left Cerebelum Crus 1,2, Vermis_7, right Temporal_Mid and Genu of corpus callosum, Posterior thalamic radiation (including the optic radiation), Fornix (cres)/Stria terminalis, right Superior cerebellar peduncle, left retrolenticular part of internal capsule, right Superior corona radiata, left sagittal stratum (including the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus).
Correlation between MK values and AVLT are shown. MK positively correlates with AVLT immediate (a) and short decayed recall (b) scores and negatively correlates with TETRAS ADL (c) and EXEC (d) (GBSS analysis, p < 0.01, uncorrected). Among them, the correlation results between MK values and AVLT were amplified
Correlation between RK values and clinical scores are shown. The background images are the standard MNI T1 template and the RK skeleton (green). RK positively (yellow) correlates with AVLT recognition score (a) and negatively (blue) correlates with TETRAS ADL (b) and EXEC (c) scores (TBSS analysis, p < 0.01,uncorrected). The correlation results were amplified
Discussion
This study evaluated GM and WM abnormalities in patients with ET using advanced diffusion imaging technology and data analysis methods and investigated the correlations between brain microstructural abnormalities and motor and cognitive symptoms. Building on DKI, we found, first, that the ET group (compared to the HC group) had microstructural abnormalities in several brain regions, as demonstrated by MK indices, and that microstructural changes in the cerebellum and temporal gyrus were associated with tremor and cognitive scores. Second, we did not find volume differences between the ET and HC groups, indicating that ordinary structural MRI did not detect structural changes between the groups, whereas DKI was more sensitive to detecting microstructure changes. Third, we found, in addition to GM, that widespread microstructural damage affected most of the WM bundles. Moreover, these changes were found to be correlated with clinical scores.
Gray matter changes
This study compared the differences in GM structure between the patients with ET and the HCs using the GBSS. We observed lower MK values in the cerebellum, occipital lobe, temporal lobe, and parietal lobe of patients with ET. MK is the most commonly used dispersion kurtosis parameter in clinical research. MK represents the overall microstructure complexity of brain tissue. Disintegration of axons and loss of cell bodies lead to a loss of microstructural complexity and an increase in extracellular free diffusion space, which are eventually manifested as a decrease in MK [10, 16]. Therefore, the decrease in MK values represents changes in GM microstructure, which may be due to neurodegeneration, including changes in the structure of the axons of Purkinje cells, and decreased dendrite spines and cell densities [17]. However, these changes do not necessarily cause observable volumetric differences on structural imaging. No significant structural changes were observed in the T1 phase of this study. Although previous studies have found that patients with ET have volume loss and cortical thickness changes of cerebrum and cerebral cortex, the samples of these studies are relatively small and the results are not consistent. Therefore, we believe that volumetric changes may not be sensitive enough compared to microstructural changes based on diffusion imaging, or that microstructural changes occur earlier than gray matter atrophy. These results also suggest that DKI is a sensitive means to detect ET damage and a potential imaging marker to identify ET.
ET is thought to be associated with cognitive deficits in multiple domains, such as executive functions, attention, memory, language, and visuospatial functions, all of which may eventually lead to dementia [18]. The interaction between cerebellum and cortical regions is essential to many aspects of cognition [19, 20]. Our findings showed the microstructure changes of the left cerebellum Crus 1 and2 were correlated with the impairment of memory. The Crus 1 and 2 regions occupy a large proportion of the posterior cerebellar hemisphere, which has been considered to be the cognitive cerebellum [21, 22]. There is evidence that the cerebellar Crus 1 and 2 are related to vocabulary acquisition and verbal retrieval, which interacts with cerebral executive control circuitry [19, 23]. Different from previous studies, the Crus 1 and 2 appear to play an important role in memory in this study. In addition, we assessed the effect of microstructure changes on motor symptoms in patients with ET. We found microstructural changes in the Vermis 7 and Crus 1 and 2 were correlated with tremor scores. Prior anatomical studies have indicated that the vermis may play a role in motion control and regulation [24, 25]. A f-MRI study of patients with Parkinson’s disease found the functional connectivity between Crus 1 and 2 and the STN was associated with the severity of motor problems [26]. Our findings confirmed that the vermis and Crus 1 and 2 may play a role in motion control and regulation. In addition, the correlation between the right middle temporal gyrus and tremor scores found in our study suggests that the temporal lobe may be involved in tremor formation, which needs further exploration.
White matter changes
As studies have shown, identifying the WM areas associated with motor and non-motor signs of ET may help explain the different pharmacotherapy responses in ET and help develop reliable therapeutic interventions [27]. At present, the role of WM alterations in the pathogenesis of ET remains controversial. In particular, the first diffusion study did not report WM abnormalities in ET patients [28]. Herein, we analyzed the DKI images of ET and HC group using TBSS to compare differences in WM structure between the two groups. The ET group exhibited lower RK values in several WM bundles. Our findings indicate widespread microstructural changes in the WM of patients with in ET and are partially consistent with the findings of previous DTI studies [29, 30]. Variations in RK values in WM areas are thought to reflect microstructural changes, including myelin abnormalities, neuronal damage, microtubule breakdown, and/or axonal loss [31]. Axonal demyelination can affect nerve conduction velocity and even block the conduction of action potentials [32].
With respect to the clinical relevance of WM abnormalities, we found a significant correlation between microstructural damage in WM and TETRAS and AVLT scores. We found that decreased RK values the in right superior cerebellar peduncle were positively correlated with tremor scores. The altered RK values in the cerebellar peduncles support the hypothesis that cerebellar connections are pathophysiologically related to ET [33]. The superior cerebellar peduncle is mainly composed of efferent fibers whose outputs are a part of the cerebellar-thalamo- cortical pathway, which are involved in the origin of ET [34]. Damage to the cerebellar peduncle may lead to structural disconnection and clinical symptoms. The positive correlation between the RK values of the right superior cerebellar peduncle and TETRAS scores in our study may reflect adaptive compensatory reorganization of nerve circuitry, thereby allowing patients with ET to remain at the same level of motor performance as healthy controls. The decreased RK values in other tracts were negatively correlated with the tremor scores. WM abnormalities in our study were also correlated with memory. The decreased RK values of these fiber bundles indicate that the integrity of their cell membranes and myelin sheaths is impaired, which also contributes to the development of motor and cognitive disorders.
WM is the connection between encephalic regions, and it is responsible for information exchange and communication between GM regions. WM abnormalities are thought to be the result of Wallerian degeneration secondary to the neuronal loss of GM [3, 35, 36]. This reminds us that we cannot analyze the microstructure changes in WM and GM separately. In addition, there is evidence that the correlation between GM metabolic changes and atrophy and clinical abnormalities is stronger than changes in the WM bundles [10]. Our study helps to fill the gap in knowledge about GM microstructure and its clinical relevance, which may be critical for understanding the pathogenic mechanism of ET and assist in early diagnosis and disease monitoring.
The study is not without its limitations. First, the diagnosis of ET was based on the judgment of experienced neurologists rather than a pathological diagnosis. Second, our study used a cross-sectional design, which cannot determine causal relationships. Longitudinal studies with larger samples are needed to explore causal relationships between DKI indices and clinical features of ET. Third, as an exploratory study, our correlation results were not corrected for multiple comparisons and may have false positives, so the results should be interpreted carefully. Finally, DKI has some inherent limitations, including its sensitivity to imaging artifacts and thermal noise, although the denoised, de-Gibbs ringing artifact, top-up and eddy were conducted to solve these issues.
Conclusion
Compared with MRI T1, the microstructural changes of DKI sequences in patients with ET were more extensive, suggesting that DKI is more sensitive to early brain microstructural changes. These results support the neurodegenerative hypothesis of ET and suggest that neurodegeneration may play a role in cognitive and motor disorders. Biomarkers sensitive to microstructural changes are expected to provide information that complements morphological evidence that can improve the early diagnosis and disease assessment of ET.
Data availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Hopfner F, Ahlf A, Lorenz D, Klebe S, Zeuner KE, Kuhlenbäumer G et al (2016) Early- and late-onset essential tremor patients represent clinically distinct subgroups. Mov Disord 31:1560–1566. https://doi.org/10.1002/mds.26708
Ågren R, Awad A, Blomstedt P, Fytagoridis A (2021) Voxel-based morphometry of cerebellar lobules in essential tremor. Front Aging Neurosci 13:667854. https://doi.org/10.3389/fnagi.2021.667854
Juttukonda MR, Franco G, Englot DJ, Lin YC, Petersen KJ, Trujillo P et al (2019) White matter differences between essential tremor and Parkinson disease. Neurology 92:e30–e39. https://doi.org/10.1212/WNL.0000000000006694
Louis ED, Faust PL (2020) Essential tremor pathology: neurodegeneration and reorganization of neuronal connections. Nat Rev Neurol 16:69–83. https://doi.org/10.1038/s41582-019-0302-1
Benito-León J, Serrano JI, Louis ED, Holobar A, Romero JP, Povalej-Bržan P et al (2018) Essential tremor severity and anatomical changes in brain areas controlling movement sequencing. Ann Clin Transl Neurol 6:83–97. https://doi.org/10.1002/acn3.681
Serrano JI, Romero JP, Castillo MDD, Rocon E, Louis ED, Benito-León J (2017) A data mining approach using cortical thickness for diagnosis and characterization of essential tremor. Sci Rep 7:2190. https://doi.org/10.1038/s41598-017-02122-3
Han Q, Hou Y, Shang H (2018) A voxel-sise meta-analysis of gray matter abnormalities in essential tremor. Front Neurol 9:495. https://doi.org/10.3389/fneur.2018.00495
Sarica A, Quattrone A, Crasà M, Nisticò R, Vaccaro MG, Bianco MG et al (2022) Cerebellar voxel-based morphometry in essential tremor. J Neurol 269:6029–6035. https://doi.org/10.1007/s00415-022-11291-9
Bhalsing KS, Upadhyay N, Kumar KJ, Saini J, Yadav R, Gupta AK et al (2014) Association between cortical volume loss and cognitive impairments in essential tremor. Eur J Neurol 21:874–883. https://doi.org/10.1111/ene.12399
Gong NJ, Chan CC, Leung LM, Wong CS, Dibb R, Liu C (2017) Differential microstructural and morphological abnormalities in mild cognitive impairment and Alzheimer’s disease: evidence from cortical and deep gray matter. Hum Brain Mapp 38:2495–2508. https://doi.org/10.1002/hbm.23535
Khairnar A, Ruda-Kucerova J, Szabó N, Drazanova E, Arab A, Hutter-Paier B et al (2017) Early and progressive microstructural brain changes in mice overexpressing human α-Synuclein detected by diffusion kurtosis imaging. Brain Behav Immun 61:197–208. https://doi.org/10.1016/j.bbi.2016.11.027
Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX (2009) Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturationstudy. Neuroimage 45:386–392. https://doi.org/10.1016/j.neuroimage.2008.12.018
Bai X, Zhou C, Guo T, Guan X, Wu J, Liu X et al (2021) Progressive microstructural alterations in subcortical nuclei in Parkinson’s disease: a diffusion magnetic resonance imaging study. Parkinsonism Relat Disord 88:82–89. https://doi.org/10.1016/j.parkreldis.2021.06.003
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED et al (2018) Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 33:75–87. https://doi.org/10.1002/mds.27121
Louis ED (2019) The roles of age and aging in essential tremor: an epidemiological perspective. Neuroepidemiology 52:111–118. https://doi.org/10.1159/000492831
Bester M, Jensen JH, Babb JS, Tabesh A, Miles L, Herbert J et al (2015) Non-Gaussian diffusion MRI of gray matter is associated with cognitive impairment in multiple sclerosis. Mult Scler 21:935–944. https://doi.org/10.1177/1352458514556295
Louis ED (2016) Essential tremor: a common disorder of Purkinje neurons? Neuroscientist 22:108–118. https://doi.org/10.1177/1073858415590351
Sengul Y, Temur HO, Corakcı Z, Sengul HS, Dowd H, Ustun I et al (2022) Brain microstructural changes and cognitive function in non-demented essential tremor patients: a diffusion tensor imaging study. Int J Neurosci 132:154–164. https://doi.org/10.1080/00207454.2020.1803859
Sokolov AA, Miall RC, Ivry RB (2017) The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 21:313–332. https://doi.org/10.1016/j.tics.2017.02.005
Cerasa A, Quattrone A (2016) Linking essential tremor to the cerebellum- neuroimaging evidence. Cerebellum 15:263–275. https://doi.org/10.1007/s12311-015-0739-8
Morimoto C, Uematsu A, Nakatani H, Takano Y, Iwashiro N, Abe O et al (2021) Volumetric differences in gray and white matter of cerebellar Crus I/II across the different clinical stages of schizophrenia. Psychiatry Clin Neurosci 75:256–264. https://doi.org/10.1111/pcn.13277
Guell X, Gabrieli JDE, Schmahmann JD (2018) Embodied cognition and the cerebellum: perspectives from the dysmetria of thought and the universal cerebellar transform theories. Cortex 100:140–148. https://doi.org/10.1016/j.cortex.2017.07.005
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V et al (2009) Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594. https://doi.org/10.1523/JNEUROSCI.1868-09.2009
Coffman KA, Dum RP, Strick PL (2011) Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci U S A 108:16068–16073. https://doi.org/10.1073/pnas.1107904108
Bostan AC, Dum RP, Strick PL (2013) Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17:241–254. https://doi.org/10.1016/j.tics.2013.03.003
Kawabata K, Watanabe H, Bagarinao E, Ohdake R, Hara K, Ogura A et al (2020) Cerebello-basal ganglia connectivity fingerprints related to motor/cognitive performance in Parkinson’s disease. Parkinsonism Relat Disord 80:21–27. https://doi.org/10.1016/j.parkreldis.2020.09.005
Nestrasil I, Svatkova A, Rudser KD, Chityala R, Wakumoto A, Mueller BA et al (2018) White matter measures correlate with essential tremor severity—a pilot diffusion tensor imaging study. Brain Behav 8:e01039. https://doi.org/10.1002/brb3.1039
Martinelli P, Rizzo G, Manners D, Tonon C, Pizza F, Testa C et al (2007) Diffusion-weighted imaging study of patients with essential tremor. Mov Disord 2:1182–1185. https://doi.org/10.1002/mds.21287
Benito-León J, Mato-Abad V, Louis ED, Hernández-Tamames JA, Álvarez-Linera J, Bermejo-Pareja F et al (2017) White matter microstructural changes are related to cognitive dysfunction in essential tremor. Sci Rep 7:2978. https://doi.org/10.1038/s41598-017-02596-1
Pietracupa S, Bologna M, Bharti K, Pasqua G, Tommasin S, Elifani F et al (2019) White matter rather than gray matter damage characterizes essential tremor. Eur Radiol 29:6634–6642. https://doi.org/10.1007/s00330-019-06267-9
Bhalsing KS, Kumar KJ, Saini J, Yadav R, Gupta AK, Pal PK (2015) White matter correlates of cognitive impairment in essential tremor. AJNR Am J Neuroradiol 36:448–453. https://doi.org/10.3174/ajnr.A4138
Becktepe JS, Busse J, Jensen-Kondering U, Toedt I, Wolff S, Zeuner KE et al (2021) White matter hyperintensities are associated with severity of essential tremor in the elderly. Front Neurol 12:694286. https://doi.org/10.3389/fneur.2021.694286
Nicoletti G, Manners D, Novellino F, Condino F, Malucelli E, Barbiroli B et al (2010) Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology 74:988–994. https://doi.org/10.1212/WNL.0b013e3181d5a460
Hallett M (2014) Tremor: pathophysiology. Parkinsonism Relat Disord 20(Suppl 1):S118-122. https://doi.org/10.1016/S1353-8020(13)70029-4
Padovani A, Borroni B, Brambati SM, Agosti C, Broli M, Alonso R et al (2006) Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 77:457–463. https://doi.org/10.1136/jnnp.2005.075713
Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G et al (2002) White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry 72:742–746. https://doi.org/10.1136/jnnp.72.6.742
Acknowledgements
We thank all the patients and healthy controls who participated in the study, which was supported by the Major Health Science and Technology Program of Zhejiang Province (Grant No. WKJ-ZJ-2208) and the Medical and Health Research Project of Zhejiang Province (No. 2021RC065).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical approval
This study has been approved by the Institutional Review Board and has therefore been approved performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, J., Zhou, C., Pu, J. et al. Brain microstructural changes in essential tremor patients and correlations with clinical characteristics: a diffusion kurtosis imaging study. J Neurol 270, 2106–2116 (2023). https://doi.org/10.1007/s00415-023-11557-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11557-w