Abstract
A hybrid material was prepared from graphene oxide and melamine sponge, and modified with phenylboronic acid to obtain a sorbent for the enrichment of the nucleosides cytidine, uridine, inosine, guanosine and adenosine. The loading capacity typically is around 27.8 mg g−1 which is comparable to other sorbents, and highly selectivity for cis-diols is observed even if the concentration of potential interferents is 1500-fold higher. The sorbent was placed in an injector, and the process was operated semiautomatically by using a peristaltic pump. The sorbent is stable and can be re-used six times without decrease in efficiency. It was applied to the selective extraction of the cis-diols (cytidine, uridine, inosine, guanosine, adenosine) from HepG2 cells. It presents good linear between 3 to 5000 μg L−1 and the limits of detection (in HPLC analysis with UV detection) are 1–4 μg L−1. Good recoveries of 85–101% were obtained with spiked HepG2 cells samples, with relative standard deviation of ≤9.9%.

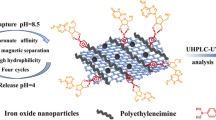
Schematic for the preparation of boronic acid modified graphene oxide/melamine sponge composite for in-syringe solid-phase extraction of nucleosides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
cis-Diols such as glycoproteins [1,2,3,4], saccharides [5, 6] and nucleosides [7,8,9] trigger numerous biological activities, so the quantitation of these molecules is very important. But due to the low abundance in cells and complexities of biological samples, the direct analysis of these compounds is very difficult. So different affinity absorbents were devoted to solve this problem. Among these, boronate affinity technology is a unique and promising method to analyze cis-diols in biological samples. The principle of this method is the reaction between boric acid and cis-diols compounds. In basic aqueous media, they can form five or six-membered cyclic esters. However, in acidic aqueous media, the cis-diols will be released into solution to realize selective enrichment and separation of cis-diols.
Although various and effective boronate affinity materials were studied in the analysis of cis-diols, searching for more absorbents with high selectivity, enrichment capacity, stability and convenient analysis process are still necessary and important. Melamine sponge is a foam-like material consisting of a formaldehyde-melamine-sodium bisulfite copolymer. It possess good ageing resistance and thermostability, and has been used as absorbent material due to its three-dimensional network structures, as well as good hydrophily, low density, fine elasticity and well capacity to reprocess. But its selectivity and enrichment capacity are not satisfied. Surface modification is an effective method including coating, grafting and growth in-situ [10,11,12]. Among these, grafting modification can only introduce few functional groups. Growth in-situ modification is time-consuming, so it is not suitable for large scale production. However, coating modification has advantages in large scale production. Yang and coworker [13] deposited lignins on the surface of melamine sponge with coating modification, then an ultra light and superhydrophobic sponge (UHS) was obtained after pyrolysis at 400 °C. This UHS sponge exhibits excellent oil/water separation performance (oil absorption capacities up to 217 times of its own weight). Liu and co-workers [14] prepared a kind of polyurethane sponge/graphite oxide/boronic acid functionalized metal-organic framework via a three-in-one strategy, this material can selectively adsorb and effectively separate luteolin from peanut shell coarse extract.
In this work, we describe a phenylboronic acid-modified hybrid sorbent with the advantages of high specific surface area from graphene oxides and good size-memory effect from melamine sponge, respectively. For realizing convenient analysis process, the synthesized absorbent was filled in an injector. Then with the help of peristaltic pump, the whole analysis process can be operated semi-automatically. This presented absorbent exhibited good selectivity, high enrichment capacity, high stability and convenient analysis process. Based on the enrichment of the material to five cis-diol nucleosides (cytidine, uridine, inosine, guanosine, and adenosine), the HPLC-UV detection method was used to detect these analytes in HepG2 cells.
Experimental
Materials and reagents
Graphene oxide (GO) was purchased from Shanghai Simbatt Energy Technology Co., Ltd. (Shanghai, China, http://www.simbatt.com). Thionyl chloride, 3-aminophenylboronic acid were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China, http://macklin.company.lookchem.cn). Melamine sponges (MMS, 10 × 10 × 1 cm) was obtained from Feng Tai Nano Materials Co., Ltd. (Zhengzhou, China, http://foamtech.cn.tonbao.com). Gold nanoparticles (AuNPs, 5 nm, 277 μM) was purchased from BBI Solutions (Beijing, China, https://www.bbisolutions.com/cn). Uridine, cytidine, inosine, adenosine, and 2′-deoxyadenosine monohydrate were supplied by Aladdin Chemistry Co., Ltd. (Shanghai, China, http://www.aladdin-e.com). 2′-deoxycytidine, spongouridine, 2′-deoxyuridinewere provided by J&K Scientific (Beijing, China, http://www.jkchemical.com/index.aspx). Vidarabine was commercial available from Shanghai Future Industrial Limited By Share Ltd. (Shanghai, China, http://jianglai.company.lookchem.cn/). Cytarabine was supplied by TCI Development Co., Ltd. (Shanghai, China, http://tcisha.company.lookchem.cn/). The dichloromethane (CH2Cl2) and trimethylamine were analytical grade and used after evaporation to remove water. Acetonitrile (HPLC grade) was provided by Dima Technology (Richmond Hill, ON, USA, http://lasallescientific.com/dikma-technologies/). The water used in all experiments was purified with a Milli-Q Advantage A10 (Millipore, Bedford, MA, USA). Other reagents used were analytical grade.
Experimental
Preparation of MMS-GO-PBA
The commercial available Melamine sponge (MMS, 3 × 3 × 1 cm) were immersed in acetone for 6 h to remove impurities and then dried at 50 °C. After that, they were immersed in 10 mL different concentrations of GO suspension (1, 2, 4, 6, 8 g L−1). They were continuous extruded the absorbed solution and then dried at 60 °C under vacuum overnight. The products were denoted as MMS-GO.
The acyl chlorination of MMS-GO (named MMS-GO-Cl) and 3-aminophenylboronic acid modified on MMS-GO-Cl (named MMS-GO-PBA) were realized according to the modified methods [15] and details were put in the Electronic Supplementary Material (ESM). The preparation and analysis process was illustrated in Fig. 1.
Extraction procedure
Before use, MMS-GO-PBA was punched into symmetrical cylindric plugs (1.0 cm in height and 1.2 cm in diameter). The sponge-based syringe was prepared by stuffing a piece of cylindric MMS-GO-PBA into the barrel of a 5 mL plastic syringe, and then sealed with a plunger. The syringe containing MMS-GO-PBA was fixed on the syringe pump. During extraction, 2.0 mL of sample solution containing 25 mM ammonium chloride-ammonia buffer (pH 10) was aspirated into the syringe by backward movement of the syringe pump plunger at a flow rate of 1.0 mL min−1. Then the solution in syringe was extruded very fast by pushing syringe plunger. Elution was carried out by loading 0.6 mL of trifluoroacetic acid (TFA)/methanol (5/95, v/v) into the syringe at a flow rate of 0.6 mL min−1. The desorption step was repeated twice. The eluates were combined into a 2.0 mL centrifuge tube and were concentrated to dryness under a nitrogen stream at 50 °C. The residue was dissolved in 100 μL of initial mobile phase and 20 μL were injected into the HPLC system for analysis.
Results and discussion
Characterization
To prove the successful synthesis of materials, the variation of elemental composition was measured by elemental analysis and ICP-OES. The results are illustrated in Table S1 (see in ESM). Compare MMS-GO with MMS-GO-Cl, the successful acylation of carboxyl acid in GO can be concluded from the decrease of H content. The achievement of modification of 3-aminophenylboronic acid can be demonstrated by the increase of B content.
The morphology of porous MMS and MMS-GO (4 g L−1) was observed by scanning electron microscopy (see in Fig. S1 in the ESM). As can be seen from Fig. S1a, the untreated MMS has a three-dimensional honeycomb-web-like structure with pore sizes in the range of 50–100 μm. After modification with GO, the MMS maintains an intact 3D porous structure, indicating that the porous structure of MMS was not destroyed during the dip-coating process (Fig. S1b). The magnified images (Fig. S1c, d) show a wrinkled surface morphology of the GO coating on MMS. The deposition is associated with the hydrogen interaction between the NH groups in MMS and large amount of OH, COOH groups in GO, which promoted the GO coating on MMS [16].
Nitrogen sorption measurements were performed to characterize the pore parameters of the materials. (Fig. S2) Both MMS-GO and MMS-GO-PBA show type-IV isotherm. A summary of Brunauer-Emmett-Teller (BET) specific surface area and the average pore size of the materials are given in Table S2. MMS-GO has a BET surface area of 50 m2 g−1 with a pore volume of 0.42 cm3 g−1, while MMS-GO-PBA has a 44 m2 g−1 BET surface area with a pore volume of 0.39 cm3 g−1. MMS-GO-PBA possesses decreased surface area and pore volume comparing with MMS-GO. These results proved that PBA was successfully grafted on the acyl chlorination of MMS-GO.
Optimization of the extraction and desorption condition
In order to achieve the best extraction efficiency of the MMS-GO-PBA for selected nucleosides, the physical and chemical parameters involved during the extraction process were studied (see in Fig. S3 in the ESM). During preparation of adsorbent, different amounts of GO were deposited onto the surface of MMS, and the extraction efficiencies were investigated. As can be seen from Fig. S3a, the adsorption efficiencies increase when the concentrations of GO increased from 1 to 4 mg mL−1. So, the concentration of 4 mg mL−1 GO was selected for adsorbent preparation. The flow rate of the extraction was studied in the range of 0.5–4 mL min−1. As shown in Fig. S3b, the analyte extraction was improved by decreasing the extraction flow rate from 4 to 0.5 mL min−1. Further decrease of the extraction flow rate to 1 mL min−1 did not led to further improvement. Consequently, the flow rate of 1 mL min−1 was employed for further experiment.
The influence of both desorption volume and its flow rate were investigated, to ensure effective elution of the analytes from the sorbent. Four different volumes (0.4, 0.5, 0.6 and 0.7 mL) were studied and the results are illustrated in Fig. S3c. The best extraction yield was obtained for 0.6 mL. Fig. S3d shows that the analytes responses enhanced as desorption flow rate was decreased and remained constant at flow rate of 0.6 mL min−1. As shown in Fig. S3e, the desorption efficiency reaches 93% with twice elution and each time only needs 1 min. Therefore, the whole extraction and desorption procedure can be finished within 4 min.
Evaluation of adsorption isotherms
Langmuir and Freundlich isotherms were used to describe the adsorption results of adsorbents. The binding isotherms were presented in Fig. S4 (see in the ESM). The model constants and the linear regression coefficients were listed in Table S3 (see in the ESM). It showed that the adsorption of adenosine correlated better with the Langmuir model than the Freundlich model, indicating surface homogeneity of MMS-GO-PBA and monolayer adsorption. The maximum adsorption capacity calculated from Langmuir model was 27.8 mg g−1. In Table 1, a comparison of enrichment capacities toward nucleosides of our work and other reported materials are listed. Although, the enrichment capacity is not the highest, the equilibrium time is shorter than those of most materials.
Selectivity evaluation
Selectivity is a major parameter for absorbent materials. Accordingly, the specific selectivity of our material was evaluated by extracting cytidine, uridine and adenosine from complex samples containing some non-cis-diol compounds as interferences, including 2′-deoxycytidine, cytarabine, deoxyuridine, spongouridine, deoxyadenosine and vidarabine. Among them, the concentration of interferences were 1, 500, 1000 and 1500 times higher than that of analytes, respectively. As shown in Fig. 2, our material exhibited very good selectivity toward cis-diol compounds, even the concentration of interferences were 1500 times higher than the cis-diols. Therefore, material presented very satisfying specific selectivity toward cis-diols.
Chromatograms of mixture containing non-cis-diols and 1,2-cis-diols before (i) and after (ii) enrichment by MMS-GO-PBA with molar ratios of 1:1 (a); 500:1 (b); 1000:1 (c); 1500:1 (d). Peaks: 1, cytidine; 2, cytarabine; 3, 2′-deoxycytidine; 4, uridine; 5, spongouridine; 6, 2′-deoxyuridine; 7, vidarabine; 8, adenosine; 9, 2′-deoxyadenosine
Method validation
With the optimized conditions, the calibration curves of these five nucleosides were established with their aqueous solutions, respectively. The results were summarized in Table S4 (see in the ESM). Obviously, the linear relationship between peak area (Y) and the concentration of nucleosides (X) were obtained with correlation coefficients of R2 ≥ 0.995. The limits of detection (LODs) were in the range of 1–4 μg L−1, which were calculated based on the 3σ/m criterion (where σ is the standard deviation of the blank and m is the slope of the calibration plot). In table S5, the LODs in this work are comparable with them except Li’s work, which have the lowest LOD. In Li’s work, extra ionic liquid was used during enrichment, and the material was powder. In the present work, bulk material was used, which is easy to recover from the solution. The higher sensitivity can be achieved when using mass spectrum (MS) as the detector. Recoveries at spiking levels of 100, 500 and 2000 μg·L−1 are in the range of 85.8–101% and the relative standard deviation (RSD) are less than 9.9% for all analytes (Table S6).
To illustrate the potential application of this method in the analysis of real complicate samples, the hybrid material was used to extract 1.0 × 105 HepG2 cells. It can be seen from Fig. 3 that, there exist obvious matrix interferents with untreated cells. However, after extraction with the synthesized materials, major interfering matrix were eliminated and the peaks of five nucleosides are easy to be observed. These results further proved the selectivity and potential application in the analysis of complex samples.
Furthermore, to study the influence of AuNPs on the metabolism of cells. The presented method was used to analyze the nucleosides in HepG2 cells which were incubated with different concentrations of AuNPs (0, 5, 100 μM) for 6 h and 24 h, respectively. As shown in Fig. 4, the content of nucleosides became higher with the increase of amounts of AuNPs. With the same concentration of AuNPs, the content of nucleosides were also higher with the increase of incubated time. It means that AuNPs can affect the metabolism of nucleosides in HepG2 cells. According to previous research, the toxicity of AuNPs was caused by oxidative stress, and further caused cells cycle changes. So AuNPs may also have effect on the metabolism of small molecules in HepG2 cells [25].
Concentration levels (μg L−1) of five nucleosides determined in the 1.0 × 105 HepG2 cells by the standard addition method. a Blank HepG2 cells, b HepG2 cells incubated with 5 μM AuNPs, c HepG2 cells incubated with 100 μM AuNPs. Data are presented as the average ± standard deviation (n = 3, 0.01 < *p ≤ 0.05,**p ≤ 0.01, *p and **p stand for significant difference between 0 μM and 5 μM in b, *p and **p stand for significant difference between 5 μM and 100 μM in c)
These results also support the proposal that nanoparticles can induce cytotoxicity.
Reusability
Reusability can decrease the cost of analysis obviously, so we survey the reuse ability of our absorbent by comparing the change of enrichment capacities. From Fig. S4, this presented material showed good stability and reproduced performance, because it can keep 93.1% of the maximum enrichment capacity after 6 recyclings. We think the good elasticity and mechanical capacity of the material make the major contribution to this performance. It means the modification with GO do not change the mechanical capacity of MMS and surface. GO is also very stable during multiple suction filtrations and presses.
Conclusion
In conclusion, an easily accessed and well-performed boron affinity absorbent based on MMS was synthesized and its enrichment of nucleosides was studied. The deposition of GO on the surface of MMS can increase the specific surface area and enlarge the grafting amount of affinity groups. The absorbent can be filled in solid phase extraction column, then the enrichment can be realized semi-automatically with the help of injection pump. Owing to the excellent stability of this material, it can be reused for 6 times without obvious decrease of performance. The method also have good selectivity and enrichment capacity owing to the specific reaction of boric acid group. At last, this absorbent was applied in practical samples to analyze nucleosides from HepG2 cells. It is still need to improve the adsorption capacity of these kind of materials for the analysis in the more complicated biological samples.
References
Ren L, Liu Y, Dong M, Liu Z (2009) Synthesis of hydrophilic boronate affinity monolithic capillary for specific capture of glycoproteins by capillary liquid chromatography. J Chromatogr A 1216:8421–8425
Yang J, He X, Chen L, ZhangY (2017) Thiol-yne click synthesis of boronic acid functionalized silica nanoparticle-graphene oxide composites for highly selective enrichment of glycoproteins. J Chromatogr A 1513:118–125
Wu Q, Jiang B, Weng Y, Liu J, Li S, Hu Y, Yang K, Liang Z, Zhang L, Zhang Y (2018) 3-Carboxybenzoboroxole functionalized polyethylenimine modified magnetic graphene oxide nanocomposites for human plasma glycoproteins enrichment under physiological condition. Anal Chem 90:2671–2677
Su J, He X, Chen L, Zhang Y (2018) A combination of "thiol-ene" click chemistry and surface initiated atom transfer radical polymerization: fabrication of boronic acid functionalized magnetic graphene oxide composite for enrichment of glycoproteins. Talanta 180:54–60
Okutucu B, Önal S (2011) Molecularly imprinted polymers for separation of various sugars from humanurine. Talanta 87:74–79
Huang W, Yang X, Zhao S, Zhang M, Hu X, Wang J, Zhao H (2013) Fast and selective recognizes polysaccharide by surfacemolecularly imprinted film coated onto aldehyde-modified magnetic nanoparticles. Analyst 138:6653–6661
Wang C, Xu H, Wei Y (2016) The preparation of high-capacity boronate affinity adsorbents by surface initiated reversible addition fragmentation chain transfer polymerization for the enrichment of ribonucleosides in serum. Anal Chim Acta 902:115–122
Li H, Zhang X, Zhang L, Wang X, Kong F, Fan D, Li L, Wang W (2016) Preparation of a boronate affinity silica stationary phase with enhanced binding properties towards cis-diol compounds. J Chromatogr A 1473:90–98
Li H, Zhu S, Cheng T, Wang S, Zhu B, Liu X, Zhang H (2016) Binary boronic acid-functionalized attapulgite with high adsorption capacity for selective capture of nucleosides at acidic pH values. Microchim Acta 183:1779–1786
Pham VH, Dickerson JH (2014) Superhydrophobic silanized melamine sponges as high efficiency oil absorbent materials. ACS Appl Mater Interfaces 6:14181–14188
Lei Z, Deng Y, Wang C (2016) Ambient-temperature fabrication of melamine-based sponges coated with hydrophobic lignin shells by surface dip adsorbing for oil/water separation. RSC Adv 6:106928–106934
Liu Q, Meng K, Ding K, Wang Y (2015) A superhydrophobic sponge with hierarchical structure as an efficient and recyclable oil absorbent. Chem Plus Chem 80:1435–1439
Yang Y, Yi H, Wang C (2015) Oil absorbents based on melamine/lignin by a dip adsorbing method. ACS Sustain Chem Eng 3:3012–3018
Liu S, Pan J, Ma Y, Qiu F, Niu X, Zhang T, Yang L (2016) Three-in-one strategy for selective adsorption and effective separation of cis-diol containing luteolin from peanut shell coarse extract using PU/GO/BA-MOF composite. Chem Eng J 306:655–666
Zhou M, Xie J, Yan S, Jiang X, Ye T, Wu W (2014) Graphene@poly(phenylboronic acid)s microgels with selectively glucose-responsive volume phase transition behavior at a physiological pH. Macromolecules 47:6055–6066
Liu T, Zhao G, Zhang W, Chi H, Hou C, Sun Y (2015) The preparation of superhydrophobic graphene/melamine composite sponge applied in treatment of oil pollution. J Porous Mater 22:1573–1580
Ma J, Wang C, Wei Y (2016) Polyethyleneimine-facilitated high-capacity boronate affinity membrane and its application for the adsorption and enrichment of cis-diol-containing molecules. RSC Adv 6:43648–43655
Wang ST, Chen D, Ding J, Yuan BF, Feng YQ (2013) Borated titania, a new option for the selective enrichment of cis-diol biomolecules. Chem-Eur J 19:606–612
Yang F, Mao J, He XW, Chen LX, Zhang YK (2013) Preparation of a boronate-functionalized affinity hybrid monolith for specific capture of glycoproteins. Anal Bioanal Chem 405:5321–5331
Cheng T, Li H, Ma Y, Liu X, Zhang H (2015) Synthesis of boronic-acid-functionalized magnetic attapulgite for selective enrichment of nucleosides. Anal Bioanal Chem 407:3525–3529
Li D, Li Y, Li X, Bie Z, Pan X, Zhang Q, Liu Z (2015) A high boronate avidity monolithic capillary for the selective enrichment of trace glycoproteins. J Chromatogr A 1384:88–96
Ding J, Mao LJ, Guo N, Yu L, Feng YQ (2016) Determination of endogenous brassinosteroids using sequential magnetic solid phase extraction followed by in situ derivatization/desorption method coupled with liquid chromatography-tandem mass spectrometry. J Chromatogr A 1446:103–113
Gao L, Wang C, Wei Y (2016) Enhanced binding capacity of boronate affinity fibrous material for effective enrichment of nucleosides in urine samples. RSC Adv 6:28470–28476
Li H, Cheng T, Wang S, Zhu X, Zhang H (2016) High-efficiency extraction of nucleosides based on the combination of self-assembly ionic liquid layer and boronic acid-functionalized attapulgite. Talanta 153:71–78
Yang Y, Nan J, Hou J, Yu B, Zhao T, Xu S, Lv S, Zhang H (2014) Cytotoxicity of gold nanoclusters in human liver cancer cells. Int J Nanomedicine 9:5441–5448
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21375052 and 21575055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1590 kb)
Rights and permissions
About this article
Cite this article
Cheng, T., Jiao, Z., Liu, X. et al. Selective, fast and semi-automatic enrichment of nucleosides by using a phenylboronic acid modified hybrid material composed of graphene oxide and melamine sponge. Microchim Acta 185, 348 (2018). https://doi.org/10.1007/s00604-018-2878-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2878-3