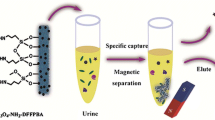
Abstract
Boronate affinity materials have been widely used for selective capture of cis-diols such as nucleosides. Adsorbents with features of low binding pH and high adsorption capacity are highly desired. However, most reported materials only possess one of the two features. We have synthesized a 1,3,5-triazine-containing binary boronic acid by reacting cyanuric chloride with 3-aminophenylboronic acid, and the product was then grafted onto attapulgite (a fibrous aluminum-magnesium silicate). The resulting functionalized attapulgite exhibit low binding pH (5.0) and display high adsorption capacity (19.5 ± 1.1 mg⋅g−1 for adenosine). The material exhibits high selectivity for cis-diols even in the presence of a 1000-fold excess of interferences. It was applied to the selective extraction of nucleosides from human urine. Typical features of the method include (a) limits of detection in the range from 4 to 17 ng⋅mL−1, (b) limits of quantification between 13 and 57 ng⋅mL−1, (c) relative standard deviations of ≤9.1 %, and (d) recoveries of nucleosides from spiked human urine between 85.0 and 112.9 %. In our perception, the material and method offer a promising strategy for selective capture of cis-diols in the areas of proteomics, metabolomics and glycomics.

We have prepared 1,3,5-triazine-containing binary boronic acid-functionalized attapulgite. The material exhibited low binding pH (5.0) and high adsorption capacity (19.5 ± 1.1 mg⋅g‾1 for adenosine).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The level of modified nucleosides excreted into urine is directly related to the degradation degree of ribonucleic acids (RNAs) in the organism. When RNAs are degraded, normal nucleosides are reutilized to synthesize nucleic acids or undergo degradation. However, modified nucleosides are directly excreted into urine due to the lack of specific phosphorylases [1]. Transfer RNAs (tRNAs) exhibit a very different modification pattern in tumor cells [2], and irregular tRNA metabolism leads to a higher level of excreted modified nucleosides [3]. Therefore, modified nucleosides in urine have been studied as cancer biomarkers [4].
Nucleosides belong to cis-diols. Various analytical methods have been developed for the selective capture of cis-diols, including hydrazide chemistry [5], boronic acid chemistry [6–8], hydrophilic interaction liquid chromatography [9] and lectin-based method [10]. Among the above strategies, boronate affinity materials (BAMs) based on boronic acid chemistry have been widely used [11–15]. The principle is that boronic acids can covalently form five- or six membered cyclic esters with 1,2- or 1,3- cis-diols under basic conditions and these cyclic esters dissociate at acidic pH values. Binding pH and adsorption capacity are two important properties of BAMs. BAMs with the two features of low binding pH and high adsorption capacity are highly appreciated. The basic binding conditions result in inconvenience of operation and the degradation of labile molecules. Four strategies have been used to reduce binding pH: introducing an electron-withdrawing group into the phenyl ring [16], utilizing intramolecular B–N [17] or B–O coordination [18], and setting up a molecular team [19]. There are two methods to improve adsorption capacity. One is increasing the amount of binding sites on a given material by improving the graft efficiency of boronate ligand [20, 21]. The other is utilizing supporting materials with large specific surface area [22, 23]. However, most reported BAMs only possess one of the two features.
In the present work, a binary boronic acid denoted as DBA was prepared by reacting cyanuric chloride with 3-aminophenylboronic acid, and then it was grafted onto attapulgite (a fibrous aluminum-magnesium silicate). There existed a strong electron-deficient 1,3,5-triazine ring in DBA, making the functionalized attapulgite to bind cis-diols at lower pH values. Moreover, since attapulgite possessed large specific surface area and DBA exhibited two binding sites, the adsorbent displayed high adsorption capacity. Therefore, the prepared material possessed the two features of low binding pH value and high adsorption capacity. Finally it was successfully applied to selective extraction of nucleosides from human urine.
Experimental
Materials and chemicals
Attapulgite (ATTA) was provided by Jiangsu Jiuchuan Nano-material Technology Co. Ltd. (Jiangsu, China, http://www.chinaclay.net), and it was activated by 6 mol L−1 hydrochloric acid before use. Cyanuric chloride was purchased from J&K Chemical Co. Ltd. (Beijing, China, http://www.jkchemical.com/index.aspx). 3-Aminophenylboronic acid (APBA) monohydrate was purchased from Energy Chemical (Shanghai, China, http://www.energy-chemical.com.cn). 3-Aminopropyltrimethoxysilane (APTMS), N,N-diisopropylethylamine (DIPEA), cytidine, uridine, guanosine, adenosine and deoxyadenosine were purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China, http://www.aladdin-e.com). All other reagents were of analytical grade (Tianjin, China, http://www.rionlon.com/yyhx.html). Acetonitrile (ACN) of HPLC grade was from Dikma Technology (VA, USA, http://www.dikma.com.cn). Purified water was provided by a Milli-Q system (Millipore, Bedford, MA, USA, http://www.millipore.bioon.com.cn) and used throughout the experiments. The standard solution of 0.1 mg mL−1 for each analyte was prepared in water and stored at 4 °C in the dark. With these standard solutions, sample solutions were spiked to the desired concentrations for experiments.
Preparation of boronate affinity adsorbent
Synthesis of 3 , 3 ′ - ( 6 - chloro - 1 , 3 , 5-triazine - 2 , 4 - diyl ) bis ( azanediyl ) bis ( 3 , 1 - phenylene ) diboronic acid ( DBA )
DBA was synthesized according to the method described in literature [24]. A solution of cyanuric chloride (4.5 mmol, 0.84 g) in 20 mL acetic acid and a solution of APBA monohydrate (9.0 mmol, 1.39 g) with sodium acetate (11.25 mmol, 0.92 g) in 10 mL acetic acid-water (v/v, 1:1) were combined at room temperature. Moderate cooling was applied to the resulting solution to maintain a temperature below 25 °C for 3 h. Precipitates were separated by filtration, and washed sequentially with acetic acid and water. The solid was then dried under vacuum at 80 °C overnight. IR (KBr, Fig. S1 of ESM): 696, 795, 852 (C–Cl), 1003, 1363 (B–O), 1536, 1599, 1637, 3365 cm−1. 1H NMR (400 MHz, DMSO-d6, Fig. S2 of ESM): δ 7.27 (t, 2H), 7.50 (d, 2H), 7.79 (s, 4H), 7.86 (s, 4H), 9.96 (s, 2H). HRMS (ESI, m/z) Calcd. for [C15H14B2ClN5O4 + H+]: 386.0999, found: 386.0990.
Preparation of amine - functionalized attapulgite ( ATTA - NH 2 )
ATTA-NH2 was synthesized in a manner similar to that described in literature [25]. ATTA (1.0 g) and APTMS (2.0 mL) were dispersed in toluene (30 mL) with ultrasonic agitation for 30 min. The mixture was then refluxed for 8 h under nitrogen atmosphere. After cooling to room temperature, the precipitates were separated by filtration and washed with ethanol. The solid was then dried under vacuum at 60 °C overnight.
Preparation of binary boronic acid - functionalized attapulgite ( ATTA - NH 2 -DBA )
ATTA-NH2-DBA was synthesized in a manner similar to that described in literature [26]. DBA (1.0 g) and DIPEA (435 μL) were dissolved in N,N-dimethylformamide (DMF, 40 mL). ATTA-NH2 (1.24 g) was dispersed in the above solution with ultrasonic agitation for 30 min. The mixture was then stirred at 90 °C for 8 h under nitrogen atmosphere. After cooling to room temperature, the precipitates were separated, and washed sequentially with DMF and ethanol. The solid was then dried under vacuum at 60 °C overnight.
The synthetic route is illustrated in Fig. 1.
Dispersive solid-phase extraction procedure
For each extraction, ATTA-NH2-DBA (50 mg) was dispersed in 2.0 mL of loading sample to carry out ultrasonic agitation for 3 min, and then it was collected by centrifugation for 5 min at 14,797 g. Subsequently, the adsorbent was rinsed sequentially with 0.5 mL water and 0.5 mL ACN-H2O (v/v, 1:1) with ultrasonic agitation for 3 min each time. Analytes were then eluted from the adsorbent with 3 × 0.5 mL of 100 mM formic acid with ultrasonic agitation for 3 min each time. The eluate was concentrated under a gentle stream of N2 with a Termovap sample concentrator (HP5016SY, Shanghai, China). The residue was dissolved in water (200 μL) for chromatographic analysis.
Selectivity evaluation
An aqueous solution containing adenosine (cis-diol, 1 μg mL−1) and deoxyadenosine (non-cis-diol, 1 μg mL−1) was used to investigate the selectivity of ATTA-NH2-DBA. This solution (2.0 mL) was incubated with ATTA-NH2-DBA (20 mg) for 3 min. Then the supernatant was collected by centrifugation for 5 min at 14,797 g. The initial solution and the supernatant were injected into the chromatographic system. The process was repeated with 10-fold, 100-fold and 1000-fold excesses of the interfering analog to determine the ability of ATTA-NH2-DBA to capture cis-diols from complex samples.
Adsorption capacity evaluation
ATTA-NH2-DBA (20 mg) was incubated with aqueous solutions (2.0 mL) of adenosine for 3 min. The concentrations of adenosine solutions ranged from 0 to 800 μg mL−1. Supernatants were collected by centrifugation for 5 min at 14,797 g and then injected for HPLC analysis. The equilibrium adsorption amount (q e , mg g−1) was calculated according to the following formula:
where c 0 and c e (μg mL−1) are the concentration of adenosine solution before and after adsorption, respectively; V (mL) is the volume of the adenosine solution; and m (mg) is the mass of ATTA-NH2-DBA.
Urine sample preparation
Urine samples were collected from healthy volunteers and stored at −20 °C. After thawing, urine sample was diluted with water at a ratio of 1:9 (v/v), and then mixed with a vortex mixer. The obtained mixture was centrifuged for 5 min at 14,797 g, and the supernatant was used for the extraction procedure. Spiked sample was prepared by addition of nucleoside standards in urine.
Results and discussion
Choice of materials
Cyanuric chloride was chosen to synthesize boronate ligand due to its following features. (i) It is a trivalent molecule and its three chlorine atoms are easily displaced by various nucleophiles in a controlled manner. Therefore, it reacts with two boronic acid molecules to obtain a binary boronic acid. One remaining chlorine atom in the binary boronic acid can react with the nucleophilic groups on supporting materials, thus the binary boronic acid can be grafted onto supporting materials. There exist two binding sites on the binary boronic acid, thus adsorption capacity of the prepared adsorbent is improved. (ii) There exhibits a strong electron-deficient 1,3,5-triazine ring in the binary boronic acid, making the resulting material to bind cis-diols at lower pH values. APBA was selected to react with cyanuric chloride, because the amino group in APBA is nucleophilic and can react with the chlorine atoms in cyanuric chloride. We chose ATTA as the supporting material. Our previous work [23] has demonstrated that ATTA-based boronate affinity adsorbent exhibited high adsorption capacity due to the large specific surface area provided by ATTA.
Characterization
FT-IR spectra of ATTA, ATTA-NH2 and ATTA-NH2-DBA are shown in Fig. 2. C–H absorbance band at 2931 cm−1 in the FT-IR spectra of ATTA-NH2 suggested that APTMS was successfully grafted onto ATTA. Moreover, XPS confirmed the presence of C, N, O, Si and B elements in ATTA-NH2-DBA (Fig. 3). The existence of B element suggested that ATTA-NH2-DBA was synthesized successfully. Besides, ICP was used to characterize ATTA-NH2-DBA, and the result showed that the mass percent of B was 0.3528 % in ATTA-NH2-DBA. This further confirmed the successful synthesis of ATTA-NH2-DBA.
TEM images were obtained to investigate the morphology of the prepared materials. ATTA was shown to have a fibrous morphology that was unchanged after modification (Fig. S3 of ESM). Additionally, nitrogen adsorption-desorption measurement at 77 K showed that the BET surface area of ATTA-NH2-DBA was 114.7 m2 g−1.
Optimization of method
The following parameters were optimized: sample pH value and ionic strength, the equilibrium time of adsorption and desorption, and the number of elutions. The results are given in the ESM (Fig. S4 and S5). The binding pH of ATTA-NH2-DBA was 5.0 (Fig. S4). The low binding pH was attributed to the strong electron-withdrawing 1,3,5-triazine ring in DBA. As the pH of urine varies from 4.5 to 8.0 [27], the pH of urine was above 5.0 after ten-fold dilution with water. Therefore, nucleosides were directly extracted from ten-fold diluted urine sample without pH adjustment. Other optimized experimental conditions were as follows: (a) NaCl concentration in sample: 0 mM, (b) adsorption time: 3 min, and (c) desorption condition: 3 × 0.5 mL of 100 mM formic acid with ultrasonic agitation for 3 min each time.
Selectivity evaluation
The cis-diol, adenosine, and non-cis-diol, deoxyadenosine, were used as analytes to evaluate the selectivity of ATTA-NH2-DBA. As shown in Fig. 4, only adenosine was bound by ATTA-NH2-DBA. Moreover, the signal intensity of adenosine after adsorption was stable when the amount of deoxyadenosine increased. The above all suggested that ATTA-NH2-DBA had high selectivity for cis-diols.
Adsorption capacity evaluation
The data of adsorption experiments was analyzed by the Langmuir and Freundlich adsorption isotherm models (Fig. S6 of ESM) as expressed in Eqs. (2) and (3), respectively.
where q e and q m (mg g−1) are the equilibrium adsorption amount and the maximum adsorption amount, respectively; c e (μg mL−1) is the equilibrium concentration of adenosine in solution after adsorption; b (mL μg−1) is the adsorption equilibrium constant; k (mL μg−1) and n are Freundlich isotherm constant related to adsorption capacity and adsorption intensity, respectively.
Parameters from the fitting of Langmuir and Freundlich adsorption isotherm models are presented in Table S1 of ESM. It showed that Langmuir model was more appropriate to describe the isothermal adsorption behavior of adenosine on ATTA-NH2-DBA. The adsorption capacity of ATTA-NH2-DBA calculated from Langmuir model was 19.48 ± 1.13 mg g−1. The high adsorption capacity of ATTA-NH2-DBA was contributed to two factors. (a) One was the large specific surface area provided by ATTA. We prepared 2,4-difluoro-3-formyl-phenylboronic acid-modified magnetic ATTA in our previous work [23]. It has been demonstrated that the large specific surface area provided by ATTA made the adsorbent to exhibit high adsorption capacity (13.78 mg g−1 for adenosine). (b) The other was the binary boronic acid DBA. Compared with 2,4-difluoro-3-formyl-phenylboronic acid-modified magnetic ATTA, ATTA-NH2-DBA possessed higher adsorption capacity. This suggested that the prepared binary boronic acid improved adsorption capacity.
Binding pH and adsorption capacity are two important properties of BAMs. BAMs are expected to have the two features of low binding pH and high adsorption capacity. ATTA-NH2-DBA possessed the two features. Table 1 lists a comparison between ATTA-NH2-DBA and reported BAMs. Most reported adsorbents only had one of the two features: (a) low binding pH but with low adsorption capacity [16–19, 29], (b) high adsorption capacity but with high binding pH [20, 30]. Although the material in literature [31] possessed the two features, its binding pH was higher and adsorption capacity was lower compared with ATTA-NH2-DBA.
Methodological investigation
Under the optimal conditions, the features of the method were investigated. As shown in Table S2 of ESM, the limits of detection (LODs) and limits of quantification (LOQs) were in the range of 4–17 ng mL−1 and 13–57 ng mL−1, respectively. In addition, the intra- and inter-day relative standard deviations (RSDs) were ≤7.7 % and ≤9.1 %, respectively (Table S3 of ESM). The recoveries were measured by analyzing the spiked human urine at three different concentrations ranging from 0.1 to 2.0 μg mL−1. The result showed that the recoveries were in the range of 85.0–112.9 % (Table S3 of ESM). Figure 5 shows the chromatograms of nucleosides standard and spiked urine sample. The chromatogram of spiked urine sample after extraction exhibited the peaks of non-target compounds. It was because there existed a variety of nucleosides in urine and those non-target nucleosides were also adsorbed by the adsorbent [21].
A comparison between this method and reported methods is presented in Table 2. It was observed that this method had relatively low LODs and consumed less adsorbent. Moreover, our method did not need the procedure of pH adjustment, avoiding inconvenience of operation and the degradation of labile molecules.
Conclusion
A 1,3,5-triazine-containing binary boronic acid (denoted as DBA) was synthesized. It was then grafted onto ATTA, generating ATTA-NH2-DBA. The applicable pH range of ATTA-NH2-DBA (≥ 5.0) covered the pH ranges of frequently used biosamples, such as blood, tear and saliva. This enabled ATTA-NH2-DBA to extract analytes from these biosamples without pH adjustment. Moreover, ATTA-NH2-DBA possessed high adsorption capacity and can capture more targeted molecules. Thus we expect the application of this material in the areas of proteomics, metabolomic and glycomics. However, ATTA-NH2-DBA had the limitation of separation. Centrifugation was applied to separate ATTA-NH2-DBA from sample solutions, which was inconvenient and time-consuming. We will further try to settle this issue and improve the method.
References
Bjork GR, Ericson JU, Gustafsson CED, Hagervall TG, Jonsson YH, Wikstrom PM (1987) Transfer-RNA modification. Annu Rev Biochem 56:263–287
Fink K, Adams WS, Davis FW, Nakatani M (1963) The identification of 2-dimethylamino-6-hydroxypurine and its ribonucleoside in urine of normal and leukemic subjects. Cancer Res 23:1824–1829
Nakano K, Nakao T, Schram KH, Hammargren WM, Mcclure TD, Katz M, Petersen E (1993) Urinary-excretion of modified nucleosides as biological marker of RNA turnover in patients with cancer and AIDS. Clin Chim Acta 218:169–183
Seidel A, Seidel P, Manuwald O, Herbarth O (2015) Modified nucleosides as biomarkers for early cancer diagnose in exposed populations. Environ Toxicol 30:956–967
Chen R, Jiang XN, Sun DG, Han GH, Wang FJ, Ye ML, Wang LM, Zou HF (2009) Glycoproteomics analysis of human liver tissue by combination of multiple enzyme digestion and hydrazide chemistry. J Proteome Res 8:651–661
Liu YS, Yu J (2016) Oriented immobilization of proteins on solid supports for use in biosensors and biochips: a review. Microchim Acta 183:1–19
Mader HS, Wolfbeis OS (2008) Boronic acid based probes for microdetermination of saccharides and glycosylated biomolecules. Microchim Acta 162:1–34
Trupp S, Schweitzer A, Mohr GJ (2006) Fluororeactands for the detection of saccharides based on hemicyanine dyes with a boronic acid receptor. Microchim Acta 153:127–131
Fang CL, Xiong ZC, Qin HQ, Huang G, Liu J (2014) One-pot synthesis of magnetic colloidal nanocrystal clusters coated with chitosan for selective enrichment of glycopeptides. Anal Chim Acta 841:99–105
Bertok T, Katrlik J, Gemeiner P, Tkac J (2013) Electrochemical lectin based biosensors as a label-free tool in glycomics. Microchim Acta 180:1–13
Hasegawa U, Nishida T, van der Vilies AJ (2015) Dual stimuli-responsive phenylboronic acid-containing framboidal nanoparticles one-step aqueous dispersion polymerization. Macromolecules 48:4388–4393
Tan L, Chen KC, Huang C, Peng RF, Luo XY, Yang R, Cheng YF, Tang YW (2015) A fluorescent turn-on detection scheme for α-fetoprotein using Quantum dots placed in a boronate-modified molecularly imprinted polymer with high affinity for glycoproteins. Microchim Acta 182:2615–2622
Wang ST, Chen D, Ding J, Yuan BF, Feng YQ (2013) Borated titania, a new option for the selective enrichment of cis-diol biomolecules. Chem Eur J 19:606–611
Wang HQ, Feng W, Jia Q (2015) A graphene oxide functionalized with 3-aminophenylboronic acid for the selective enrichment of nucleosides, and their separation by capillary electrophoresis. Microchim Acta 182:185–192
He XM, Zhu GT, Zhu YY, Chen X, Zhang Z, Wang ST, Yuan BF, Feng YQ (2014) Facile preparation of biocompatible sulfhydryl cotton fiber-based sorbents by “thiol-ene” click chemistry for biological analysis. ACS Appl Mater Interfaces 6:17857–17864
Liu YC, Ren LB, Liu Z (2011) A unique boronic acid functionalized monolithic capillary for specific capture, separation and immobilization of cis-diol biomolecules. Chem Commun 47:5067–5069
Li HY, Liu YC, Liu J, Liu Z (2011) A wulff-type boronate for boronate affinity capture of cis-diol compounds at medium acidic pH condition. Chem Commun 47:8169–8171
Li HY, Wang HY, Liu YC, Liu Z (2012) A benzoboroxole-functionalized monolithic column for the selective enrichment and separation of cis-diol containing biomolecules. Chem Commun 48:4115–4117
Ren LB, Liu Z, Liu YC, Dou P, Chen HY (2009) Ring-opening polymerization with synergistic co-monomers: access to a boronate-functionalized polymeric monolith for the specific capture of cis-diol-containing biomolecules under neutral conditions. Angew Chem Int Ed 48:6704–6707
Wang W, He MF, Wang CZ, Wei YM (2015) Enhanced binding capacity of boronate affinity adsorbent via surface modification of silica by combination of atom transfer radical polymerization and chain-end functionalization for high-efficiency enrichment of cis-diol molecules. Anal Chim Acta 886:66–74
Li H, Shan YH, Qiao LZ, Dou A, Shi XZ, Xu GW (2013) Facile synthesis of boronate-decorated polyethyleneimine-grafted hybrid magnetic nanoparticles for the highly selective enrichment of modified nucleosides and ribosylated metabolites. Anal Chem 85:11585–11592
Zhu XY, Gu JL, Zhu JY, Li YS, Zhao LM, Shi JL (2015) Metal-organic frameworks with boronic acid suspended and their implication for cis-diol moieties binding. Adv Funct Mater 25:3847–3854
Chen T, Li HH, Ma Y, Liu XY, Zhang HX (2015) Synthesis of boronic-acid-functionalized magnetic attapulgite for selective enrichment of nucleosides. Anal Bioanal Chem 407:3525–3529
Kolmakov KA (2008) An efficient, “green” approach to aryl amination of cyanuric chloride using acetic acid as solvent. J Heterocycl Chem 45:533–539
Chen XM, Qin F, Liu YQ, Huang XD, Zou HF (2004) Synthesis of chiral stationary phases with radical polymerization reaction of cellulose phenylcarbamate derivatives and vinylized silica gel. J Chromatogr A 1034:109–116
Lubian E, Baldini F, Giannetti A, Trono C, Carofiglio T (2010) Solid-supported Zn(II) porphyrin tweezers as optical sensors for diamines. Chem Commun 46:3678–3680
Thongboonkerd V, Mungdee S, Chiangjong W (2009) Should urine pH be adjusted prior to gel-based proteome analysis? J Proteome Res 8:3206–3211
Dou P, Liang L, He JG, Liu Z, Chen HY (2009) Boronate functionalized magnetic nanoparticles and off-line hyphenation with capillary electrophoresis for specific extraction and analysis of biomolecules containing cis-diols. J Chromatogr A 1216:7558–7563
Li DJ, Li QJ, Wang SS, Ye J, Nie HY, Liu Z (2014) Pyridinylboronic acid-functionalized organic-silica hybrid monolithic capillary for the selective enrichment and separation of cis-diol-containing biomolecules at acidic pH. J Chromatogr A 1339:103–109
Du J, He MF, Wang XM, Fan H, Wei YM (2015) Facile preparation of boronic acid functionalized magnetic nanoparticles with a high capacity and their use in the enrichment of cis-diol-containing compounds from plasma. Biomed Chromatogr 29:312–320
Li DJ, Li Y, Li XL, Bie ZJ, Pan XH, Zhang Q, Liu Z (2015) A high boronate avidity monolithic capillary for the selective enrichment of trace glycoproteins. J Chromatogr A 1384:88–96
He HB, Sun YR, Li B, Yu QW, Wang TL, Feng YQ (2013) Boronate affinity solid-phase extraction based on functionalized magnesia-zirconia composite for enrichment of nucleosides in human urine. Anal Method 5:1435–1441
Wang CZ, Xu HH, Wei YM (2016) The preparation of high-capacity boronate affinity adsorbents by surface initiated reversible addition fragmentation chain transfer polymerization for the enrichment of ribonucleosides in serum. Anal Chim Acta 902:115–122
Chen ML, Wei SS, Yuan BF, Feng YQ (2012) Preparation of methacrylate-based monolith for capillary hydrophilic interaction chromatography and its application in determination of nucleosides in urine. J Chromatogr A 1228:183–192
Szymanska E, Markuszewski MJ, Bodzioch K, Kaliszan R (2007) Development and validation of urinary nucleosides and creatinine assay by capillary electrophoresis with solid phase extraction. J Pharmaceut Biomed 44:1118–1126
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21375052, 21575055 and J1103307).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 767 kb)
Rights and permissions
About this article
Cite this article
Li, H., Zhu, S., Cheng, T. et al. Binary boronic acid-functionalized attapulgite with high adsorption capacity for selective capture of nucleosides at acidic pH values. Microchim Acta 183, 1779–1786 (2016). https://doi.org/10.1007/s00604-016-1808-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1808-5