Abstract
A magnetite@graphene oxide nanocomposite was first coated with polyethylenimine and then modified with phytic acid and titanium(IV) ions. The high loading with Ti(IV) and the good hydrophilicity of PEI and PA result in a material that can be applied to the efficient extraction of highly polar nucleobases, nucleosides and nucleotides. The physicochemical properties of the composite were investigated by scanning electron microscopy, transmission electron microscopy, energy dispersive X-ray spectroscopy, Fourier transform infrared spectroscopy, water contact angle measurements, thermogravimetric analysis, and vibrating sample magnetometry. A series of parameters that affect extraction and elution under the conditions of immobilized metal affinity chromatography (IMAC) and hydrophilic interaction liquid chromatography (HILIC) were examined. The analytes were eluted from the nanocomposites using 10 mM trisodium phosphate as the elution solution in the IMAC mode, and 50% methanol-water as elution solution in the HILIC mode. Figures of merit include (a) an intra-day precision of 0.1–1.0% in the IMAC mode; (b) an intra-day precision of 0.4%–0.8% in the HILIC mode; (c) detection limits between 1.8–2.8 ng mL−1 in the IMAC mode; and (d) detection limits of 4.0–10.5 ng mL−1 in the HILIC mode. The method was applied to the extraction of the nucleotides cytidine-5′-monophosphate (CMP), uridine-5′-monophosphate (UMP), guanosine-5′-monophosphate (GMP), and adenosine-5′-monophosphate (AMP), and the nucleobases and nucleosides hypoxanthine, adenosine, cytosine, inosine and cytidine from Cordyceps sinensis, Lentinus edodes and plasma samples.

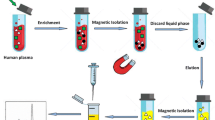
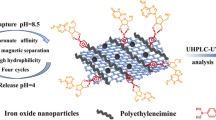
Schematic presentation of the workflow for the extraction of nucleobases, nucleosides and nucleotides using phytic acid-Ti(IV) functionalized magnetite@graphene oxide nanocomposites under two distinct modes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nucleobases-based compounds (viz. nucleobases, nucleosides and nucleotides) are ubiquitous in cells and play essential roles in various biological functions [1]. Apart from in field of biology [2,3,4], nucleobases-based compounds such as monophosphate nucleotides are nutrients of special importance during periods of rapid growth or after injury, and are indispensable supplementary nutrients in neonatal feeding [5]. Nucleobases, nucleosides and nucleotides also exist in some medicinal herbs [i.e. Ganoderma lucidum, Cordyceps sinensis (C. sinensis)] as important bioactive compounds related to multiple pharmacological activities [6,7,8]. To date, several analytical methods, including reverse phase-high performance liquid chromatography (RP-HPLC) [9], ultra-high performance liquid chromatography [10], ion-pairing RP-HPLC [11], hydrophilic interaction liquid chromatography (HILIC) [12], capillary electrophoresis or capillary electro-chromatography [13, 14], have been developed for the quantification of nucleobases-based compounds and their analogs in natural plants, foods and biological fluids. However, sometimes these analytical methods still show limitations to analyze all nucleobases-based components directly when they are in low abundance. Therefore, effective and selective pre-treatment approach for these compounds from complex samples before chromatographic analysis is necessary.
The number of established extraction methods of nucleobase compounds are very limited. The most conventional strategy is solvent-based extraction, and aqueous solution was always employed since the highly polar characteristic of the compounds [9,10,11, 15]. Solid phase extraction (SPE) methods, mainly commercial materials, such as OASIS WAX and Oasis MCX cation exchange cartridges [16, 17], are also applied occasionally for the efficient extraction of polar nucleobases-based compounds. However as the nature of needing several steps for column activation and equilibrium procedure, the whole extraction process of commercial sorbents is a little time-consuming. On the other hand, considering the cis-diol structure in the cyclic ribose on nucleosides, new SPE materials based on the boronate affinity are considered as a selective extraction technique for them [18,19,20]. Similarly, the metal-based affinity strategy, mainly including immobilized metal ion affinity chromatography (IMAC) and metal oxide affinity chromatography, are employed for enrichment of cis-diol nucleosides [21,22,23,24]. While except for the affinity with cis-diol structure, the metal-based affinity materials present the metal electrostatic interaction and/or chelation effect on phosphate-containing compounds. However this characteristic was mainly employed for phosphoproteins and phosphopeptides analysis [25,26,27,28], and never applied for nucleotides. In reality, boronate modified sorbents and metal-based affinity materials have some drawbacks. Firstly, because the external shell of the boronate modified sorbents is an organic species, the secondary hydrophobic interactions and aromatic interactions between sorbents and interferes may suppress selectivity for cis-diol compounds. Secondly, chemical cleavage of the bonded boric acid groups may be induced by intense conditions such as copper salts, silver salts, or hot water. The most troublesome problem is, both boronate modified sorbents and metal-based affinity materials cannot be used to extract compounds that had neither a cis-diol nor a phosphate structure (i.e. nucleobases and deoxynucleosides related compounds). Therefore, it will be highly attractive to prepare a kind of SPE material with adsorption capacity for nucleobases, nucleosides and nucleotides simultaneously.
HILIC is a promising solution for highly polar and hydrophilic compounds, displayed outstanding performance in the separation of polar molecules. Various hydrophilic-based materials for sample pretreatment have received much attention to academic researchers [29, 30]. It is mainly focused on the enrichment of glycan, glycopeptide and glycoprotein, and as far as we know have not yet been applied to enrichment of hydrophilic nucleobases-based compounds in complicated matrix.
In order to achieve simultaneous pretreatment of nucleobases, nucleosides and nucleotides in complex samples, the phytic acid-Ti4+ modified magnetite@ graphene oxide nanocomposites was synthesized with polyethylenimine (PEI) as a kind of post functionalization candidate (magGO@PEI@PA@Ti4+). The material possesses metal coordinated and hydrophilic dual functions. It can extract nucleobases-based compounds with two distinct mechanisms, i.e. IMAC for nucleotides and HILIC for nucleobases and nucleosides. The uniqueness of the present approach is mainly in the utility of the characteristics of all involved monomers on the sorbent to maximize the adsorption capacity for target compounds. Specifically, 1) the feature of Fe3O4 ensures the easily and directly isolation of target analytes; 2) the high density of amino groups on the PEI makes it a modifiable and hydrophilic intermediate; 3) GO provides ultrahigh specific surface area for increasing compound binding sites; 4) as a natural compound composed of six phosphate groups, PA possesses excellent hydrophilic and metal ion coordination ability; and 5) immobilization of Ti4+ on the surface of the magGO@PEI@PA@Ti4+ composite makes the material bind with phosphate-containing compounds. The physicochemical properties of synthesized materials were thoroughly characterized and some important parameters involved in extraction/elution process were carefully optimized. Finally, the synthesized sorbent was successfully applied for the extraction of nucleobases, nucleosides and nucleotides in medicinal mushroom C. sinensis, natural foods Lentinus edodes (Berk.) sing (L. edodes) and biological plasma samples.
Experimental
Reagents and samples
Iron(III) chloride hexahydrate (FeCl3·6H2O), polyethyleneimine (PEI, 50%, relative molecular mass (Mr) = 70,000), sodium hydroxide (NaOH), sulfuric acid (H2SO4), hydrochloric acid (HCl), acetic acid (AC), 30% hydrogen peroxide, ammonium hydroxide (NH3·H2O), potassium permanganate (KMnO4), ammonium acetate (NH4AC), disodium hydrogen phosphate (Na2HPO4), and potassium dihydrogen phosphate (KH2PO4), sodium acetate, sodium citrate, ethanol, ethylene glycol, were all of analytical grade (>97%) and from Chron Chemicals (Chengdu Chron Chemicals Co., Ltd., China, http://www.chronchem.com/en/). Titanium sulfate [Ti(SO4)2] was purchased from DiBai Biotechnology Co., Ltd. (Shanghai, China, http://www.chemxyz.com/index.php). Graphite powder (2000 mesh) and phytic acid (PA) were from Aladdin Chemical Technology Co., Ltd. (Shanghai, China, https://www.aladdin-e.com). Water used for all the experiments was purified by a water purification system (ATSelem 1820A, Antesheng Environmental Protection Equipment Co., Ltd., Chongqing, China, http://www.atshb.com/). All the solvents used in the HPLC analysis such as methanol (MeOH) and acetonitrile (ACN) were of HPLC-grade and purchased from Adamas-beta (Adamas Reagent Co., Ltd., Shanghai, China, http://www.adamas-beta.com/).
The reference compounds, namely, cytidine-5′-monophosphate (CMP), uridine-5′-monophosphate (UMP), adenosine-5′-monophosphate (AMP), guanosine-5′-monophosphate (GMP) (>98%, determined by HPLC) were purchased from Adamas-beta, hypoxanthine, adenosine, cytosine, inosine and cytidine were all purchased from Sigma (St Louis, MO, USA, www.sigmaaldrich.com), their chemical structures were shown in Fig. S1. Natural C. sinensis was from Langxian, Tibet province and was compared with the features described in the Chinese Pharmacopoeia (2015 edition). The dried L. edodes were purchased from the local supermarket in June 2018. Samples were deposited at the Pharmaceutical Engineering Laboratory in the School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, China.
Apparatus and instruments
A field-emission scanning electron microscope (FESEM) (Quanta 650, FEI, Hillsboro, OR, https://www.fei.com/) and an energy dispersive X-ray spectroscopy (EDX) were used to study surface morphology at 20.0 kV and elemental content of the nanocomposite using Cu Kα radiation. Transmission electron microscope (TEM) image was taken using a JEM 2100 (JEOL Ltd. Tokyo, Japan, https://www.jeol.co.jp/en/) transmission electron microscope working at 200 kV. Fourier transform infrared (FTIR) spectra was collected between 4000 and 400 cm−1 on a Bruker Tensor 27 FTIR Spectrometer (Bruker, USA, https://www.bruker.com/) in KBr media. The water contact angle was recorded using an OCA40 optical contact angle measurement instrument (Dataphysics, Germany, https://www.dataphysics-instruments.com/). Thermal gravimetric analysis (TGA) was carried out on Mettler TGA/DSC1/1600LF (Mettler-Toledo AG, Analytical, Switzerland, https://www.mt.com/cn/zh/home.html) from 25 °C to 800 °C at a heating rate of 10 °C min−1 in N2 gas flow. The magnetic property of the material was measured by a vibrating sample magnetometer (VSM) model AGFM/VSM 3886 (Kashan, Iran) at room temperature (about 25 °C) in a magnetic field strength of 2 T.
Preparation of phytic acid and Ti(IV) (PA-Ti4+) modified magnetite@GO nanocomposites
The preparation process of PA-Ti4+ modified magnetite@GO composites included three main steps: 1) preparation of GO and Fe3O4 magnetic nanoparticles (MNPs) separately; 2) functionalization of Fe3O4 MNPs with PEI and deposited it on the surface of GO; 3) grafting of the magnetic graphene oxide polyethyleneimine composite (magGO@PEI) with PA and Ti4+, the scheme is shown in Fig. 1. GO was prepared by Hummers method with some modifications, Fe3O4 MNPs were prepared by a solvothermal reaction method. The specific preparation procedure of GO, Fe3O4, and Fe3O4@PEI were described in Electronic Supplementary Material (ESM). PA and Ti4+ were immobilized on magGO@PEI through electrostatic interaction. magGO@PEI was prepared according to the previous reports with minor modification [31] and the details were described in ESM. Next, the above magGO@PEI composite was dispersed in 100 mL PA (7 mg mL−1) and stirred for 6 h at room temperature (about 25 °C) to form magGO@PEI@PA. Finally, the magGO@PEI@PA was incubated with 100 mM Ti(SO4)2 solution (100 mL) for 2 h to immobilize Ti4+. The product magGO@PEI@PA@Ti4+ composite was rinsed with water for three times and dried at 45 °C under vacuum for overnight.
Magnetic solid-phase extraction (MSPE) procedure
In the investigations of extraction conditions for nucleobases, nucleotides and nucleosides, the extraction rate was selected as the evaluation index to compare the influence of different extraction conditions on the result. The extraction rate is defined as (C0-C)/C × 100%, where C0 and C are the initial and equilibrium concentrations of each compound, respectively.
Firstly, 2.5 mg magGO@PEI@PA@Ti4+ was added to 1 mL sample solution in a glass vial. The mixture was oscillated on a temperature-controlled air bath shaker (SHZ-82, Jintan Zhengrong Experimental Instrument Factory, Jiangsu, China, http://www.zenroelab.com/). Then, the magGO@PEI@PA@Ti4+ composites were isolated from the solution phase by employing a magnet under the bottom of the vial, and the supernatant was decanted and subjected to HPLC analysis to determine C for calculating extraction rate. After the solution being totally removed, the same volume of desorption solvent was added, and the mixture was oscillated for another period of time. The resulting desorption solution was subjected to HPLC analysis.
Preparation of sample solutions
The solutions of four nucleotides were separately prepared in distilled water at a concentration of about 500 μg mL−1. The five nucleobases and nucleosides were prepared in acetate buffer at a concentration of about 300 μg mL−1, respectively. All of the solutions were kept in the dark under 4 °C. The stock solution was prepared by mixing the same volume of the analyte solution, and a final concentration of about 10 μg mL−1 for each analyte was used in the process of MSPE parameters optimization.
C. sinensis and L. edodes material were oven-dried at 35 °C for 48 h. All dried materials were pulverized and griddled through 50 mesh sieves before extraction. A total of 0.5 g C. sinensis and 1.0 g L. edodes fine powder were accurately transferred to a glass-stoppered conical flask, respectively. Then 10 mL boiling ultrapure water (95–100 °C) was added and ultrasonic extracted under 75 °C for 30 min. The obtained solution was centrifuged at 5000×g for 10 min; the upper solution was collected and used for MSPE.
Rabbit plasma was also used for MSPE and its preparation procedure was provided in ESM.
HPLC analysis
HPLC analysis was performed on an Agilent 1260 Series liquid chromatography system(Agilent Technologies, USA, www.agilent.com), which was equipped with a vacuum degasser, a quaternary pump, an auto-sampler, and a diode array detector (DAD) and was controlled with the Agilent Chem Station software. The details of HPLC analysis were described in ESM.
Results and discussion
Characterization of magnetic nanomaterials
The morphology and configuration of the magnetic particles were presented in both SEM and TEM images. As shown in Fig. 2a, some spherical shape, even size, and rough surface particles are presented after gradually modifying Fe3O4 with PEI, PA and Ti4+. Moreover, it is also clearly observed that the Fe3O4 particles are spread on some lamellar structure (area in the blue circle), confirming the successful synthesis of magnetic graphene nanocomposite. Furthermore, TEM was employed to further evaluate the size and surface morphology of the magGO@PEI@PA@Ti4+ particle. As shown in Fig. 2b, c, the magGO@PEI@PA@Ti4+ possesses uniformly spherical shape with typical core-shell structure and a mean diameter of ~340 nm, and the further modification results in the formation of a layer (~28 nm) on the surface of the Fe3O4 nanoparticles. Similarly, the lamellar GO structure can also be observed in the TEM image (area in the red circle). In addition, the composition of the composites was analyzed by EDX, and the results are illustrated in Fig. 2d, e. As compared with magGO@PEI, new P and Ti elements are appeared attributed to the abundant P and Ti existed in PA (C6H18O24P6) and Ti(SO4)2.
FTIR were used to characterize the functional groups, and the spectra are presented in Fig. 3a. The characteristic band of Fe3O4 appeared at 585 cm−1 (Fe-O vibration), which is observed in all synthesized composites. The strong adsorption peak in the region of 3500–3200 cm−1 is assigned to O-H stretching vibration for hydroxyl and carboxyl arising from GO in Fig. 3a (curve b, c). Most importantly, bands at 1125 and 880 cm−1 in Fig. 3a (curve c), which confirm the successful decoration of PA on magnetic materials, are separately assigned to the P=O and P-O-H stretching vibration in phosphateester group. As one of the significant characteristics, the water-compatibility of the composites was evaluated. One drop of water was deposited on the surface of magGO@PEI@PA@Ti4+ slice, where it rapidly spread over the contact area (Fig. 3b). The water contact angle approaches 10 ̊ within 1 s, which indicates the hydrophilicity of the surface of the material.
Characterization of synthesized materials. a FTIR spectra of Fe3O4@PEI (curve a), magGO@PEI (curve b), magGO@PEI@PA@Ti4+ (curve c); b The profiles of a water drop on the films of magGO@PEI@PA@Ti4+; c TGA curves of Fe3O4 (curve a) and magGO@PEI@PA@Ti4+ (curve b); d VSM curves and photo of magnetic separation (inset) of Fe3O4 and magGO@PEI@PA@Ti4+
Thermogravimetric analysis was used to compare the thermal behavior of Fe3O4, magGO@PEI and magGO@PEI@PA@Ti4+. As illustrated in Fig. 3c, the mass decrease of Fe3O4 is evidently less than that of magGO@PEI, proves the stability of the magnetic core. The curve of magGO@PEI shows the weight loss of 17.45%, while the total loss of magGO@PEI@PA@Ti4+ after decorating is 17.83% and the value is continuing falling. This demonstrates that new substances had been coated on the basis of magGO@PEI. Specifically, the TGA curve of magGO@PEI@PA@Ti4+ indicates a 5.58% weight loss between 25 and 180 °C, which is related to the evaporation of surface-adsorbed water and water in the crystal structure. The same weight loss is also observed for Fe3O4 and magGO@PEI. An increasing weight loss down to 400 °C is observed for magGO@PEI@PA@Ti4+ compared with magGO@PEI, which can be attributed to the decomposition of adsorbed groups (PA and Ti4+) on the surface.
The magnetic properties of Fe3O4 and magGO@PEI@PA@Ti4+ were measured by VSM in the field ranges from −20,000 to +20,000 Oe at room temperature (about 25 °C). As shown in Fig. 3d, the magnetization curves of Fe3O4 and magGO@PEI@PA@Ti4+ do not show hysteresis, indicating that these two materials exhibit superparamagnetic behavior. The magnetization saturation values of Fe3O4 and magGO@PEI@PA@Ti4+ are 55.6 emu g−1 and 45.9 emu g−1, respectively. Because the surface of Fe3O4 is grafted, the magnetization saturation value of magGO@PEI@PA@Ti4+ is declined slightly. Also, the magGO@PEI@PA@Ti4+ nanocomposites can be dispersed uniformly in water via sonication to form a homogeneous solution. Under an external magnetic field, the nanocomposites are quickly aggregated in 5 s (NdFeB magnet, size 8 × 4 mm, coercive force 876 kA m−1, remanent 1.17 T, intrinsic coercivity 20 kA m−1, maximum energy product 36 kJ s−1, density 7.5 g cm3–1, operating temperature 150 °C). Therefore, owing to the very good superparamagnetism, magGO@PEI@PA@Ti4+ can be rapidly and easily separated from the solution, which can facilitate the SPE process.
Extraction and desorption of nucleotides in the immobilized metal affinity chromatography (IMAC) mode
In order to achieve the maximal extraction efficiency of the magGO@PEI@PA@Ti4+ for four nucleotides, the following parameters in MSPE protocol were optimized: extraction time, kind of acids and ionic strength of extraction solution, as well as type and concentration of eluent. All parameters were examined in three independent experiments (n = 3), the results and respective data are given in the ESM and Fig. S2. After being systemically optimized, the following experimental conditions were found to give the best results: (a) an extraction time of 15 min; (b) deionized water as loading buffer; (c) no ion was added in sample; (d) 10 mM Na3PO4 as elution solution for 10 min desorption.
Extraction and desorption of nucleobases and nucleosides in the hydrophilic interaction liquid chromatography (HILIC) mode
D.V. McCalley investigated the effect of various conditions on the selectivity of the separation of a set of neutral, acidic and basic solutes in HILIC mode, providing a practical guide in the selection of suitable conditions for establishing a separation. The magnitude of the effect of changes produced from the correlation of retention factor was estimated as: stationary phase > mobile phase pH > organic solvent concentration > buffer concentration > column temperature [32]. Accordingly, a systemic optimization of conditions for the extraction of nucleobases and nucleosides were performed, and the elution effects of various solvent systems were also compared. Each experiment was repeated for three times (n = 3). The results are shown in ESM and Fig. S3. Finally the following optimum conditions were adapted: (a) pH of extraction solution was 7.5; (b) 99% ACN was added into the loading buffer; (c) the ion strength was controlled at 2.5 mM with NH4AC buffer; (d) extraction under 35 °C; (e) an extraction time of 20 min; (f) desorption with 1 mL 50% MeOH for 20 min under 25 °C.
Analytical performance
The MSPE-HPLC method was systematically validated by determining the linearity range, limits of detection (LOD) (S/N = 3), limits of quantification (LOQ) (S/N = 10), inter- and intra-day precision under two different mode, respectively. The determination was performed through extracting and analyzing reference solutions spiked with different concentrations of analytes under the optimized extraction and desorption conditions. As it can be seen in Table S2 and Table S3, good linearity are obtained for all nine compounds in the range of 0.098–6.25 μg mL−1 and with a relatively high coefficients (R2 ≥ 0.9990). The LOD for four nucleotides are between 1.8 and 2.8 ng mL−1, while LOQ are between 8.1 and 13.1 ng mL−1; The LOD for five nucleobases and nucleosides are between 4.0 and 10.5 ng mL−1, and LOQ are between 6.1 and 32.8 ng mL−1. For precision test, the 6.25 μg mL−1 mixed references solutions were analyzed for six times within one day (n = 6) and the inter-day reproducibility was examined in duplicates for consecutive three days (n = 6). Intra-day RSD and inter-day RSD values are 0.1%–1.0% and 1.6%–3.4% for nucleotides, 0.4%–0.8% and 2.3%–4.3% for nucleobases and nucleosides, respectively. The satisfactory precisions illustrate the achievement of good reproducibility using the MSPE-HPLC method under both IMAC and HILIC modes.
Comparison of the method with previous reports
The extraction performance of magGO@PEI@PA@Ti4+ based MSPE-HPLC analysis is compared with other previous reports involving the pretreatment of nucleobase-based compounds (Table 1). The merits of the present sorbent can be concluded as following aspects: a) relatively better specificity than traditional solvent extraction methods; b) the simpler and shorter extraction process compared with commercial sorbents as their nature of needing several steps for column activation and equilibrium procedures; c) saves solvent and sorbents compared with traditional liquid-liquid extractions and commercial SPE sorbents; d) proposes a new material employing MSPE method for those characteristic nucleobase-based compounds including nucleobases, nucleosides and nucleotides which have rarely been explored with SPE sorbents before; e) to extract nucleobases-based compounds from the same sample by two distinct mechanisms, IMAC for nucleotides while HILIC for nucleobases and nucleosides, and achieves good results with relatively low LOD and LOQ values. In addition, the synthesized materials have a wide range of application potential. Considering the mechanism of the magGO@PEI@PA@Ti4+, some other species can also be extracted employing this material. Compounds contained amino or hydroxyl group (i.e. catecholamines, histidine-tagged proteins etc.) can coordinate with the metal ions on the surface of the adsorbent under IMAC mode, while some highly polar drugs (metformin, aminoglycoside antibiotic) which cannot be well retained on the common reverse phase SPE column can also be pretreated by the magGO@PEI@PA@Ti4+ under HILIC mode.
Application to real samples
The practicality of the magGO@PEI@PA@Ti4+ based MSPE method was investigated and applied to determine nucleobases, nucleosides and nucleotides in real samples, including medicinal mushroom C. sinensis, edible mushroom L. edodes and animal plasma. Three concentrations (low, medium and high) of reference compounds were spiked in sample, and the accuracy of the present method was validated by calculating the recovery. The relative recovery (RR%) was acquired from the equation below:
wherein, Cfound, Creal and Cadded were the concentration of the analyte after addition of a known amount of reference compound into the real sample, the concentration of analyte in the real sample and the concentration of a known amount of the reference spiked into the real sample, respectively. All measurements were replicated three times (n = 3), Fig. 4 presents representative chromatograms of determining nucleotides, nucleobases and nucleosides in the different samples after pretreatment by magGO@PEI@PA@Ti4+ under IMAC and HILIC mode, respectively. The mean values of the results are summarized in Tables 2 and 3.
Typical chromatograms for blank (black line, lower) and spiked (red line, upper) samples. a Sample after MSPE under IMAC mode: C. sinensis (a), L. edodes (b) and rabbit plasma (c); b Sample after MSPE under HILIC mode: C. sinensis (d), L. edodes (e) and rabbit plasma (f). All chromatograms were present as samples spiked with medium level concentration of reference compounds, the specific concentrations were consistent with Tables 2 and 3. (1) CMP; (2) UMP; (3) GMP; (4) AMP; (5) Hypoxanthine; (6) Adenosine; (7) Cytosine; (8) Inosine; (9) Cytidine
The recoveries of CMP, GMP, AMP, cytosine, inosine and cytidine are all in the range of 71.6%–106.3% with RSD between 0.4%–12.6%. The complex natural and biological matrix have some substantial interference on the determination of these six important ingredients in the real samples, but the values are comparable with some reported methods toward nucleosides [18, 21]. The recovery of the other three compounds, UMP, hypoxanthine, and adenosine, are not quite satisfactory with a RR value between 50.7%–86.3%. The possible reason for this result might be due to the relatively low extraction rate of the material for these three compounds: (a) the dissociation of UMP under the extraction condition causing some interference for the chelation of the compound with the metal; (b) the insufficient hydrophilicity of adenosine and hypoxanthine; and (c) the strong matrix interference in complicated natural products and biological matrix.
Conclusions
A metal coordinated and hydrophilic dually functional sorbent based on PA-Ti4+ modified magnetite@GO nanocomposites was prepared. Because of the high loading amount of Ti4+ and hydrophilicity of PEI and PA, large surface area and good magnetic property contributed from GO and Fe3O4, the magGO@PEI@PA@Ti4+ nanocomposites were employed to extract typical nucleotides under IMAC mode and nucleobases and nucleosides under HILIC mode. The satisfactory precision and low detection limits facilitate its implementation in the determination of some nucleobases, nucleosides and nucleotides in complicated spiked C. sinensis, L. edodes and animal plasma samples. This is the first attempt to extract these three kinds of highly polar compounds from a complex matrix with one SPE material under two distinct mechanisms. Although the enriched nucleobase-based compounds from the complex samples are very limited due to the limitations of the extraction efficiency of the material, the synthesis and application of the combined properties of IMAC- and HILIC- material would be of potential value in the SPE field to pretreat and determine more kinds of polar nucleobase-based compounds from real matrices. Further efforts are required for the development of the enrichment materials owing high capacity under two different modes, and the strategy of combination of different metal ions or hydrophilic modifying materials can be taken into consideration for enhancing enrichment efficiency in the future.
References
Watt DL, Buckland RJ, Lujan SA, Kunkel TA, Chabes A (2016) Genome-wide analysis of the specificity and mechanisms of replication infidelity driven by imbalanced dNTP pools. Nucleic Acids Res 44(4):1669–1680
Conti JB, Belardinelli L, Utterback DB, Curtis AB (1995) Endogenous adenosine is an antiarrhythmic agent. Circulation 91(6):1761–1767
Frickenschmidt A, Frohlich H, Bullinger D, Zell A, Laufer S, Gleiter CH, Liebich H, Kammerer B (2008) Metabonomics in cancer diagnosis: mass spectrometry-based profiling of urinary nucleosides from breast cancer patients. Biomarkers 13(4):435–449
Struck W, Waszczuk-Jankowska M, Kaliszan R, Markuszewski MJ (2011) The state-of-the-art determination of urinary nucleosides using chromatographic techniques "hyphenated" with advanced bioinformatic methods. Anal Bioanal Chem 401(7):2039–2050
Carver JD (2003) Advances in nutritional modifications of infant formulas. Am J Clin Nutr 77(6):1550s–1554s
Anfossi G, Russo I, Massucco P, Mattiello L, Cavalot F, Balbo A, Trovati M (2002) Adenosine increases human platelet levels of cGMP through nitric oxide - Possible role in its antiaggregating effect. Thromb Res 105(1):71–78
Li SP, Su ZR, Dong TTX, Tsim KWK (2002) The fruiting body and its caterpillar host of Cordyceps sinensis show close resemblance in main constituents and anti-oxidation activity. Phytomedicine 9(4):319–324
Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J (2008) Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (review). Int J Oncol 32(3):527–535
Gao JL, Leung KSY, Wang YT, Lai CM, Li SP, Hu LE, Lu GH, Jiang ZH, Yu ZL (2007) Qualitative and quantitative analyses of nucleosides and nucleobases in Ganoderma spp. by HPLC-DAD-MS. J Pharmaceut Biomed 44(3):807–811
Studzinska S, Buszewski B (2012) A new way to fast and high resolution determination of modified nucleosides. J Chromatogr B 887:93–101
Vinas P, Campillo N, Melgarejo GF, Vasallo MI, Lopez-Garcia I, Hernandez-Cordoba M (2010) Ion-pair high-performance liquid chromatography with diode array detection coupled to dual electrospray atmospheric pressure chemical ionization time-of-flight mass spectrometry for the determination of nucleotides in baby foods. J Chromatogr A 1217(32):5197–5203
Zhou GS, Pang HG, Tang YP, Yao X, Ding YH, Zhu SQ, Guo S, Qian DW, Shen J, Qian YF, Su SL, Zhang L, Jin C, Qin Y, Duan JA (2014) Hydrophilic interaction ultra-performance liquid chromatography coupled with triple-quadrupole tandem mass spectrometry (HILIC-UPLC-TQ-MS/MS) in multiple-reaction monitoring (MRM) for the determination of nucleobases and nucleosides in ginkgo seeds. Food Chem 150:260–266
Szymanska E, Markuszewski MJ, Heyden YV, Kaliszan R (2009) Efficient recovery of electrophoretic profiles of nucleoside metabolites from urine samples by multivariate curve resolution. Electrophoresis 30(20):3573–3581
Chen XJ, Yang FQ, Wang YT, Li SP (2010) CE and CEC of nucleosides and nucleotides in food materials. Electrophoresis 31(13):2092–2105
Hayama T, Kiyokawa E, Yoshida H, Imakyure O, Yamaguchi M, Nohta H (2015) Selective extraction of nucleotides with Fluorous biphasic system utilizing Perfluoroalkylamine as an ion-pair reagent. Chromatography 36(1):13–18. https://doi.org/10.15583/jpchrom.2015.001
Cohen S, Megherbi M, Jordheim LP, Lefebvre I, Perigaud C, Dumontet C, Guitton J (2009) Simultaneous analysis of eight nucleoside triphosphates in cell lines by liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B 877(30):3831–3840
Jeng LB, Lo WY, Hsu WY, Lin WD, Lin CT, Lai CC, Tsai FJ (2009) Analysis of urinary nucleosides as helper tumor markers in hepatocellular carcinoma diagnosis. Rapid Commun Mass Sp 23(11):1543–1549
Wang HQ, Feng W, Jia Q (2015) A graphene oxide functionalized with 3-aminophenylboronic acid for the selective enrichment of nucleosides, and their separation by capillary electrophoresis. Microchim Acta 182(1–2):185–192
Ma J, Wang CZ, Wei YM (2016) Polyethyleneimine-facilitated high-capacity boronate affinity membrane and its application for the adsorption and enrichment of cis-diol-containing molecules. RSC Adv 6(49):43648–43655
Cheng T, Zhang Y, Liu XY, Zhang XY, Zhang HX (2017) A filter paper coated with phenylboronic acid-modified mesoporous silica for enrichment of intracellular nucleosides prior to their quantitation by HPLC. Microchim Acta 184(10):4007–4013
Wan LZ, Zhu HJ, Guan YF, Huang GM (2017) Nanocoating cellulose paper based microextraction combined with nanospray mass spectrometry for rapid and facile quantitation of ribonucleosides in human urine. Talanta 169:209–215
Xu J, Zhang Z, He XM, Wang RQ, Hussain D, Feng YQ (2018) Immobilization of zirconium-glycerolate nanowires on magnetic nanoparticles for extraction of urinary ribonucleosides. Microchim Acta 185(1):43
Xu YY, Yang Y, Xue AF, Chen H, Li SQ (2018) In situ precipitation of hydrous titanium dioxide for dispersive micro solid-phase extraction of nucleosides and their separation. New J Chem 42(7):4909–4914
Pan YN, Guo XM, Li SS, Liu XY, Zhang HX (2018) A boronate-decorated porous carbon material derived from a zinc-based metal-organic framework for enrichment of cis-diol-containing nucleosides. New J Chem 42(3):2288–2294
Tan SY, Wang JD, Han Q, Liang QL, Ding MY (2018) A porous graphene sorbent coated with titanium(IV)-functionalized polydopamine for selective lab-in-syringe extraction of phosphoproteins and phosphopeptides. Microchim Acta 185(7):316
Wang MM, Chen S, Zhang DD, Yu YL, Wang JH (2018) Immobilization of a Ce(IV)-substituted polyoxometalate on ethylenediamine-functionalized graphene oxide for selective extraction of phosphoproteins. Microchim Acta 185(12):553
Lin HZ, Chen HM, Shao X, Deng CH (2018) A capillary column packed with azirconium(IV)-basedorganic framework for enrichment of endogenous phosphopeptides. Microchim Acta 185(12):562
Jiang JB, Sun XN, She XJ, Li JJ, Li Y, Deng CH, Duan GL (2018) Magnetic microspheres modified with Ti(IV) and Nb(V) for enrichment of phosphopeptides. Microchim Acta 185(6):309
Zhang Q, Yang FQ, Ge LY, Hu YJ, Xia ZN (2017) Recent applications of hydrophilic interaction liquid chromatography in pharmaceutical analysis. J Sep Sci 40(1):49–80
Tang F, Yu QW, Yuan BF, Feng YQ (2017) Hydrophilic materials in sample pretreatment. Trac-Trend Anal Chem 86:172–184
Li N, Chen J, Shi YP (2019) Magnetic polyethyleneimine functionalized reduced graphene oxide as a novel magnetic sorbent for the separation of polar non-steroidal anti-inflammatory drugs in waters. Talanta 191:526–534. https://doi.org/10.1016/j.talanta.2018.09.006
McCalley DV (2017) Understanding and manipulating the separation in hydrophilic interaction liquid chromatography. J Chromatogr A 1523:49–71
Fan H, Li SP, Xiang JJ, Lai CM, Yang FQ, Gao JL, Wang YT (2006) Qualitative and quantitative determination of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography-electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS). Anal Chim Acta 567(2):218–228
Struck W, Siluk D, Yumba-Mpanga A, Markuszewski M, Kaliszan R, Markuszewski MJ (2013) Liquid chromatography tandem mass spectrometry study of urinary nucleosides as potential cancer markers. J Chromatogr A 1283:122–131
Fan H, Chen PH, Wang CZ, Wei YM (2016) Zirconium-doped magnetic microspheres for the selective enrichment of cis-diol-containing ribonucleosides. J Chromatogr A 1448:20–31
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21275169), project No. 2018CDXYHG0028 supported by the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 528 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhou, DD., Li, F. et al. Extraction of nucleobases, nucleosides and nucleotides by employing a magnetized graphene oxide functionalized with hydrophilic phytic acid and titanium(IV) ions. Microchim Acta 186, 187 (2019). https://doi.org/10.1007/s00604-019-3308-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3308-x