Abstract
2,4-Difluoro-3-formyl-phenylboronic acid (DFFPBA)-modified magnetic attapulgite (ATP-Fe3O4-NH2-DFFPBA) was synthesized and employed to capture and enrich cis-diol-containing biomolecules. The resulting material exhibited a high saturation magnetization value of 20.71 emu/g, allowing the absorbent to be conveniently magnetically separated. Combining the Fe3O4 nanoparticles with the high specific surface area of attapulgite yielded a material with a high capture capacity (13.78 mg/g) for adenosine. Furthermore, ATP-Fe3O4-NH2-DFFPBA was found to possess remarkable selectivity for adenosine at a low molar ratio of adenosine/2-deoxyadenosine (1:500). The potential applications of this material were explored by using it to extract five nucleosides from urine samples, and the results demonstrate that it can decrease matrix interference and selectively enrich analytes.

Boronic-acid-functionalized magnetic attapulgite could selectively enrich the nucleosides in urine samples with the help of an external magnet
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most cis-diol biomolecules (CDB) play important roles in diverse fundamental biological processes. For example, nucleosides—building blocks of nucleic acids—regulate and modulate various physiological activities. However, the direct analysis of nucleosides from real samples is seriously hampered by their sub-stoichiometric nature due to serious matrix interference. Therefore, selectively enriching target nucleosides and eliminating any matrix effect are important tasks in clinical diagnosis. Boronic acids can covalently form five- or six-membered cyclic esters with 1,2- or 1,3-cis-diol in basic aqueous media, and these cyclic esters dissociate at acidic pH [1]. This useful characteristic makes boronic acids ideal affinity ligands for the specific capture of CDB. Many studies have been published on different types of boronate-functionalized materials, such as monoliths [2], mesoporous silica [3], and magnetic nanomaterials [4]. Among these materials, magnetic nanoparticles have received increasing attention for their quick magnetic resonance and ease of separation. However, materials with high loading capacities must still be found due to the relatively low surface areas of the magnetic particles currently used. To improve the loading capacity, porous materials with large specific surface areas (such as attapulgite, ATP) have been introduced into nucleoside enrichment schemes.
In the study reported in the present paper, we prepared 2,4-difluoro-3-formyl-phenylboronic acid (DFFPBA)-functionalized magnetic ATP via a coprecipitation technique and applied it to enrich nucleosides. DFFPBA was chosen as the ligand because of its low pK a value (6.5) and strong affinity [5] due to the presence of an electron-withdrawing group (fluorine). The resulting material exhibits a high saturation magnetization value, good selectivity, and a high adsorption capacity. Finally, ATP-Fe3O4-NH2-DFFPBA was applied to selectively enrich nucleosides from urine samples.
Experimental
Materials
Ferric chloride hexahydrate (FeCl3·6H2O) and ferrous chloride tetrahydrate (FeCl2·4H2O) were provided by Tianjin Chemicals Corporation (Tianjin, China). 2,4-Difluoro-3-formyl-phenylboronic acid (DFFPBA) was purchased from Sigma (St. Louis, MO, USA). Attapulgite (ATP) was supplied by Gansu ATP Co. Ltd. (Gansu, China). Prior to use, it was dried in a vacuum at 110 °C for 48 h. (3-Aminopropyl)trimethoxysilane (APTMS), sodium cyanoborohydride, cytidine, uridine, inosine, guanosine, adenosine, and 2′-deoxyadenosine monohydrate were obtained from Aladdin Chemistry Co., Ltd. (Shanghai, China). Ammonium hydroxide (NH4OH, 25 % ammonia), toluene, and methanol were received from Tianjin Chemical Co. (Tianjin, China). All of the chemicals mentioned above were of analytical grade. HPLC-grade acetonitrile was purchased from Dima Technology (Richmond Hill, ON, USA). Deionized (D.I.) water was prepared from a Milli-Q system (Millipore, Bedford, MA, USA).
Preparation of ATP-Fe3O4-NH2-DFFPBA composites
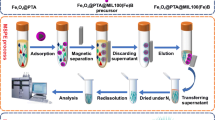
The preparation scheme for magnetic ATP modified with DFFPBA is shown in Scheme S1. First, ATP-Fe3O4 was prepared via a simple coprecipitation method according to a procedure reported previously [6]. Then ATP-Fe3O4-NH2 was prepared by mixing 1.0 g ATP-Fe3O4 with 0.8 mL APTMS in 50 mL anhydrous toluene, and the mixture was refluxed for 8 h under a nitrogen atmosphere. The resultant was cleaned by magnetic separation, washed with ethanol, and then dried in a vacuum. Finally, the ligand DFFPBA was bound to ATP-Fe3O4-NH2 composite nanoparticles through a Schiff-base reaction. Typically, 1.6 g ATP-Fe3O4-NH2 was suspended in 80 mL anhydrous methanol containing 0.3 g DFFPBA by ultrasound, and the reaction was allowed to continue for 12 h at 25 °C. During the course of the reaction, 100 mg sodium cyanoborohydride were added to the mixture every 4 h until a total of 300 mg had been added. Finally, the resulting ATP-Fe3O4-NH2-DFFPBA nanoparticles were washed with ethanol and water.
Preparation of standard solutions and biological samples
The stock solution of nucleosides (cytidine, uridine, inosine, guanosine, adenosine) at a concentration of 0.5 mg/mL was prepared by dissolving the commercial standards in ultrapure water, and the resulting solution was stored at 4 °C. Early-morning urine samples were collected from six healthy adults in the authors’ work group and stored at −20 °C. Before the analysis, the samples were thawed at room temperature and centrifuged to eliminate precipitates for 5 min at 14,797×g. The supernatant was collected and adjusted to pH 8.5 using 50 mM phosphate buffer. Spiked urine samples were obtained by adding different concentrations (from 0.1 to 5 μg/mL) of the stock solution, and were subsequently diluted 10 times with 50 mM (pH 8.5) phosphate buffer.
SPE procedure
The nucleoside sample was prepared by diluting the stock solution (0.5 mg/mL) with 50 mM phosphate buffer (pH 8.5) to 1.0 μg/mL. ATP-Fe3O4-NH2-DFFPBA (10 mg) was dispersed in 2 mL of the above solution to carry out the extraction for 9 min, and then separated by a magnet. The supernatant fluid was decanted. Subsequently, 0.5 mL of formic acid (20 mmol/L, pH 3.0) were used to elute the analytes for 5 min. Then the eluate was evaporated at 50 °C and the residue was reconstituted with 200 μL of ammonium formate/acetonitrile (v/v, 3:97) for HPLC analysis.
Results and discussion
Characterization of the materials
The resulting materials ATP-Fe3O4, ATP-Fe3O4-NH2, and ATP-Fe3O4-NH2-DFFPBA were characterized by FT-IR spectra (see Fig. S1 of the “Electronic supplementary material,” ESM), X-ray photoelectron spectrometry (XPS; see Fig. S2 of the ESM), TEM (see Fig. S3 of the ESM), and a vibrating sample magnetometer (see Fig. S4 of the ESM), respectively. As shown in Fig. S1 of the ESM, the presence of the DFFPBA component was confirmed by the occurrence of peaks at 1384 cm−1 corresponding to the stretching vibration of B–O. The amount of boronic acid ligand loaded onto the surface of the ATP-Fe3O4-NH2 was quantified by XPS (Fig. S2 of the ESM). This showed the presence of Si and O (from ATP), C, N, and Fe (from the modification steps), and B (from the final product), which proved that DFFPBA was successfully bound to ATP-Fe3O4-NH2. According to Fig. S3 of the ESM, it is clear that the materials largely maintained their original morphologies after they had been modified with amino groups and boronic acid groups. Furthermore, the surface area of ATP-Fe3O4-NH2-DFFPBA was 89.5 m2/g based on the Brunauer–Emmett–Teller (BET) method. It was also observed that ATP-Fe3O4, ATP-Fe3O4-NH2, and ATP-Fe3O4-NH2-DFFPBA exhibit superparamagnetic behavior and that their saturation magnetization values are 35.48, 23.14, and 20.71 emu/g at 25 °C, respectively (Fig. S4 of the ESM). The decrease in saturation magnetization from ATP-Fe3O4 to ATP-Fe3O4-NH2 to ATP-Fe3O4-NH2-DFFPBA can be attributed to surface chemical modification. Nevertheless, the relatively high saturation magnetization of ATP-Fe3O4-NH2-DFFPBA is still sufficient to allow particles to be separated from solution with an external magnet, which is very useful in applications of these particles.
Optimization of the desorption and extraction conditions
The effects of different desorption time and desorption solvents on the extraction efficiency were investigated (see Fig. S5 in the ESM). Formic acid, acetic acid, and phosphate buffer (pH 3.0) were tested as desorption solvents. The extraction time was set at 30 min to ensure that the analytes were absorbed as completely as possible. The highest response was obtained with formic acid, so this was selected as the desorption solvent. All of the analytes were desorbed efficiently from the materials within 5 min. The extraction time profiles for the five nucleosides showed that the peak area increased from 3 to 9 min and then remained stable with increasing extraction time. Therefore, 9 min was chosen as the extraction time in subsequent experiments. Control over the capture and release of the analytes is achieved by adjusting the pH. We investigated the effect of varying the binding pH in the range 7.0–9.0, since the pK a of DFFPBA is 6.5. The results indicate that the extraction efficiency increases as the pH increases from neutral to weakly alkaline before reaching an equilibrium value at pH 8.5.
Binding capacity
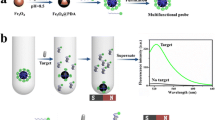
The cis-diol binding capacity of ATP-Fe3O4-NH2-DFFPBA was measured using adenosine as the test analyte. The material (10 mg) was incubated in 2 mL of adenosine solution containing different concentrations of adenosine (from 0.005 to 0.6 mg/mL) for 9 min. The suspension was then directly measured by HPLC. The saturation absorption capacity of the nanoparticles was as high as 13.78 mg/g, higher than that of a boronic-acid-functionalized material reported previously (see Table S2 of the ESM). To evaluate the specificity of ATP-Fe3O4-NH2-DFFPBA for nucleosides, adenosine was selected as the target while 2-deoxyadenosine was used as a comparison. Adenosine and 2-deoxyadenosine have similar structures, but adenosine is a cis-1,2-diol compound while 2-deoxyadenosine is not. As shown in Fig. 1, ATP-Fe3O4-NH2-DFFPBA selectively captured adenosine rather than 2-deoxyadenosine, even when the molar ratio of interferent to target was increased from 1:1 to 500:1. These results demonstrate that the material has high interaction affinity and selectivity for CDB.
Furthermore, in order to evaluate the linearity of the measurements, 2-mL urine samples spiked with nucleosides at levels ranging from 0.1 to 5 μg/mL were extracted by 10 mg ATP-Fe3O4-NH2-DFFPBA. After elution, the eluate was allowed to evaporate under an N2 atmosphere and the residue was dissolved in 200 μL of ammonium formate/acetonitrile (v/v, 3:97) for LC-MS analysis. As shown in Table S3 of the ESM, the square of the correlation coefficient ranged from 0.990 to 0.998. LODs (S/N = 3) in the range 2–41 ng/mL and LOQs (S/N = 10) in the range 8–137 ng/mL were obtained, which mean that this technique could be used to analyze nucleosides in urine samples. Compared with the other nucleosides, uridine has a higher LOQ (137 ng/mL) in urine owing to the matrix effect, while a lower LOQ (11 ng/mL) was obtained in an artificial water sample (data not shown). In addition, the accuracy of the method was examined at three levels: 0.2, 1.0, and 3.0 μg/mL. The results in Table 1 show that good intraday (RSD 1.1-9.9 %) and interday (RSD 1.3-9.9 %) precisions were obtained for the analysis of nucleosides. Recoveries varied from 86.9 % to 111.3 %, with RSDs in the range 1.1–9.7 %.
Real sample analysis
The practical applicability of the developed method was tested by determining five nucleosides in urine samples from healthy volunteers under optimal conditions. As shown in Table 2, most of the nucleosides could be detected. In addition, recovery experiments were carried out by spiking analytes at 0.4 μg/mL, and the recoveries for the five target nucleosides ranged from 85.2 % to 115.0 %. Few of the nucleosides were detected in samples analyzed directly by LC-MS due to the complex urine–matrix interference (Fig. 2). However, after extraction, most of the nucleosides were clearly observed. It is worth noting that the extraction ratio of adenosine was lower in urine samples than in others—even lower than its original concentration in urine. A possible reason for this is the matrix effect in urine. Some of the compounds in urine appear to affect the adsorption of adenosine, because the extraction ratio of adenosine from the artificial water sample with an adenosine concentration that was 10 times higher than those of the other studied nucleosides was normal. Thus, the higher concentration of adenosine in urine [7] was not the reason for the low extraction ratio. These results therefore demonstrate that the magnetic nanoparticles can reduce the matrix effect and selectively enrich CDB in complex real samples.
a–e LC-MS chromatograms for the extraction of endogenous nucleosides from a urine sample. a Cytidine, b uridine, c inosine, d guanosine, and e adenosine. For all the chromatograms, (i) shows the direct analysis of standards (1 μg/mL), (ii) corresponds to pre-enrichment, and (iii) represents post-enrichment with ATP-Fe3O4-NH2-DFFPBA
Conclusion
In summary, we successfully synthesized DFFPBA-modified magnetic ATP using a facile method. High surface area and saturation magnetization mean that the resulting material is easily separated and has a high adsorption capacity. It is significant that this absorbent is able to capture adenosine even in the presence of 500-fold interference in the artificial sample. In addition, the successful application of the absorbent to the selective extraction of nucleosides from urine samples demonstrated that the proposed method provides an alternative method of achieving affinity enrichment of target CDB in biological samples.
References
James TD, Sandanayake KRAS, Shinka S (1996) Saccharide sensing with molecular receptors based on boronic acid. Angew Chem Int Ed Engl 35:1910–1922
Chen M, Lu Y, Ma Q, Guo L, Feng YQ (2009) Boronate affinity monolith for highly selective enrichment of glycopeptides and glycoproteins. Analyst 134:2158–21643
Xu Y, Wu Z, Zhang L, Lu H, Yang P, Webley PA, Zhao D (2009) Highly specific enrichment of glycopeptides using boronic acid-functionalized mesoporous silica. Anal Chem 81:503–508
Li H, Shan Y, Qiao L, Dou A, Shi X, Xu G (2013) Facile synthesis of boronate-decorated polyethyleneimine-grafted hybrid magnetic nanoparticles for the highly selective enrichment of modified nucleosides and ribosylated metabolites. Anal Chem 85:11585–11592
Li QJ, Lü CC, Liu Z (2013) Preparation and characterization of fluorophenylboronic acid functionalized monolithic columns for high affinity capture of cis-diol containing compounds. J Chromatogr A 1305:123–130
Liu YS, Liu P, Su ZX, Li FS, Wen FS (2008) Attapulgite-Fe3O4 magnetic nanoparticles via co-precipitation technique. Appl Surf Sci 255:2020–2025
Rodriguez-Gonzalo E, Hernandez-Prieto R, Garcia-Gomez D, Carabias-Martinez R (2013) Capillary electrophoresis–mass spectrometry for direct determination of urinary modified nucleosides. Evaluation of synthetic urine as a surrogate matrix for quantitative analysis. J Chromatogr B 942:21–30
Acknowledgments
The authors thank the support provided by the National Science Foundation of China (nos. 21375052 and J1103307).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 407 kb)
Rights and permissions
About this article
Cite this article
Cheng, T., Li, H., Ma, Y. et al. Synthesis of boronic-acid-functionalized magnetic attapulgite for selective enrichment of nucleosides. Anal Bioanal Chem 407, 3525–3529 (2015). https://doi.org/10.1007/s00216-015-8550-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8550-4