Abstract
This review (with 340 refs) focuses on methods for specific and sensitive detection of metabolites for diagnostic purposes, with particular emphasis on electrochemical nanomaterial-based sensors. It also covers novel candidate metabolites as potential biomarkers for diseases such as neurodegenerative diseases, autism spectrum disorder and hepatitis. Following an introduction into the field of metabolic biomarkers, a first major section classifies electrochemical biosensors according to the bioreceptor type (enzymatic, immuno, apta and peptide based sensors). A next section covers applications of nanomaterials in electrochemical biosensing (with subsections on the classification of nanomaterials, electrochemical approaches for signal generation and amplification using nanomaterials, and on nanomaterials as tags). A next large sections treats candidate metabolic biomarkers for diagnosis of diseases (in the context with metabolomics), with subsections on biomarkers for neurodegenerative diseases, autism spectrum disorder and hepatitis. The Conclusion addresses current challenges and future perspectives.

This review focuses on the recent developments in electrochemical biosensors based on the use of nanomaterials for the detection of metabolic biomarkers. It covers the critical metabolites for some diseases such as neurodegenerative diseases, autism spectrum disorder and hepatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “biomarker” was first used in 1989 as a measurable indicator of some biological states or conditions [1]. These traceable substances can be utilized for physiology-related assessments such as disease risk, psychiatric disorders, environmental exposure, disease diagnosis, metabolic processes, substance abuse, pregnancy, cell line development, etc. Biomarkers reflect the entire spectrum of diseases from the earliest manifestations to the terminal stages and are one of the driving forces of pharmaceutical research and drug development. They have been approved by the U.S. Food and Drug Administration (FDA) regulation for use as surrogate endpoints in the treatment development process [2]. Biomarkers are often cheaper and easier to measure than true endpoints. Furthermore, they can also be measured more quickly and earlier. Considering these advantages, biomarkers have tremendous potential to affect the success rate of clinical trials, identify new drug targets and develop diagnostic assays for early detection of diseases [3, 4].
The metabolic biomarkers known as an important class of biomarkers indicate changes in metabolic processes [5]. The role of metabolism in diseases is emerging as an area of etiopathological interest. Defining the differential alterations in metabolic pathways for a variety of disorders has become an increasingly accessible option for investigators and clinicians. The exponential growth of metabolomic analyses and their relevant scientific literature within the clinical biochemistry over the last decade provides evidence of the utility of such approaches in distinguishing between health and disease and in defining potentially targetable disease mechanisms. The deregulated levels of metabolites are observed in abnormalities or disorders such as neurodegenerative diseases [6, 7] and autism spectrum disorder (ASD) [8, 9]. In addition, altered metabolite levels can represent chronic infectious diseases such as hepatitis [10]. Therefore, a fast and reliable detection of these biomarkers can help medical professionals to differentiate between diseases showing similar symptoms.
Nowadays there is a growing interest in the development of devices for the specific and sensitive quantification of biomarkers. Electrochemical biosensors can play a key role in the development of low cost, portable and rapid sensing techniques for this purpose. They provide reliable electrical signals resulting from specific immunoreactions. During the last years, different biosensing platforms have been described for the detection of biomarkers. The high interest raised by this issue has been highlighted in the recent reviews devoted to the use of functionalized nanomaterials with biological receptors for low-level detection of metabolic biomarkers. The signal enhancement associated with the use of nanomaterials is an effective path for ultrasensitive biosensing of biomarkers [11].
With remarkable achievements in nanotechnology, the nanomaterials have attracted significant attention in the electrochemical biosensors not only for electrical signal amplification, but also for immobilizing biological probes on the electrode surface [12]. The inherent advantages of electrochemical biosensors are associated with unique chemical and physical properties of nanomaterials, such as outstanding electronic and catalytic characteristics, high surface to volume ratio and simple modification of their surfaces. They have active surfaces that can easily be modified for immobilization of numerous biomolecules [13]. In addition, the large specific surface area and high surface free energy of nanomaterials will lead to the preparation of a variety of surface-immobilized biomolecules with improved stability. It is important to note that direct adsorption onto bulk materials may result in biomolecule denaturation and loss of bioactivity, while nanosized materials not only show a strong tendency to adsorb biomolecules but also retain their bioactivity [14]. These features combined with the functioning of biomolecules contribute to the improvement of biosensor performance in terms of sensitivity and specificity.
This review provides an updated overview of selected examples during the period 2005–2018 involving electrochemical biosensing approaches and nanomaterial-assisted signal enhancement strategies, which have been applied for the determination of a wide range of metabolic biomarkers. The aim of this effort is to provide the reader with a concise view of advances in the field of electrochemical nanobiosensors for determination of metabolic biomarkers.
Classification of electrochemical bioassays according to the bioreceptor type
Compared to non-bioreceptor sensors, the receptor-based biosensors are of particular interest, due to the high specific and strong interaction of biological probes with their target molecules, which results in a more efficient and reversible attachment of the analyte and/or ligand.
The sensitivity and selectivity of these methods essentially depend on the properties of the biorecognition elements to be used for analyte binding [15]. Electrochemical biosensors, an important subclass of biosensors, combine the high specificity of the bioreceptor with the high sensitivity of electrochemical transducers. In general, the biosensors can be classified into five major classes, according to the bioreceptors used. The enzymes, antibodies and aptamers are the main classes of bioreceptors that are mostly used in biosensing applications (Fig. 1). Although antibodies and oligonucleotides have been widely employed, enzymes are by far the most commonly used biosensing elements in biosensors.
Electrochemical enzyme-based biosensors
The field of biosensors has grown enormously since the first demonstration of the glucose enzyme electrode concept by Clark and Lyons in 1962 [16]. The entire field of biosensors can trace its origin to this original enzyme electrode. From 1962 until now, considerable efforts have been made on the creation and evolution of new enzymatic biosensors as an exciting area of biochemical research, reflecting a growing emphasis on this technology.
Depending on the assay type, two fundamental classes of enzymatic biosensors can be distinguished. In the first group, the enzyme detects the presence of a substrate, or co-substrate/co-factor. A typical example is a glucose biosensor. The second group is based on the detection of inhibitors in the presence of a substrate. The most common example of this approach is the detection of organophosphate compounds used as pesticides or warfare nerve agents. The major advantage of these approaches is the high sensitivity and specificity of catalytically active enzymes towards their target molecules.
Notwithstanding all these advantages, the enzymes have some drawbacks that have limited their widespread application. They often suffer from lack of chemical and thermal stabilities. Exposure to certain conditions, such as elevated temperatures or organic solvents, can lead to denaturation and concomitant loss of activity. There have been significant improvements in the field of enzymatic biosensors. For example, the use of new genetically engineered enzymes has allowed the improved performance characteristics of current biosensors for the detection of established analytes. In fact, the application of modern engineering techniques in enzyme biosensors has enabled the optimization of their properties [17, 18].
Electrochemical antibody-based biosensors
Antibody-based biosensors known as immunosensors have revolutionized diagnostics for the detection of a variety of biomarkers [19, 20]. The first immunosensor reported in the 1950s opened the doors to the possibility of immuno-diagnosis [21]. Since then, a widespread effort has been conducted to develop immunosensors for clinical diagnostics.
Antibodies, as a member of the biorecognition elements are categorized into two main classes of monoclonal and polyclonal antibodies. Monoclonal antibodies have a monovalent affinity, in that they specifically bind to the same epitope. In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different plasma cell lineages. They have higher overall antibody affinity against the antigen due to the recognition of multiple epitopes of an antigen. Polyclonal antibodies can be produced in large quantities in a short time, without complicated technologies and at low cost; however, they suffer from lack of specificity [22]. Thus, for such applications as biosensing and therapeutic drug development that require antibodies specific to a single epitope, the monoclonal antibodies might be more apptopriate. They offer a combination of high specificity with an excellent ability for affinity purification. However, they have long production time and high cost.
Nanobodies (Nbs) as the single-domain antibody fragments derived from heavy-chain antibodies of camelids and cartilaginous fish were discovered in camelidae in the early 1990s [23]. Like a whole antibody, they are able to bind selectively to a specific antigen. In contrast to common antibodies, they are well expressed in microorganisms and are less lipophilic and more soluble in water [24]. Due to their low molecular weight, high physico-chemical stability and ability to bind antigens inaccessible to conventional antibodies, they have broadly used in biosensing applications. Theses single-domain antibodies can be coupled more densely on biosensor surfaces. In addition to their advantage in targeting less accessible epitopes, their conformational stability also leads to higher resistance to surface regeneration conditions.
Electrochemical aptamer-based biosensors
Aptamers are artificial single-stranded nucleic acid ligands that can bind a wide range of target molecules including small molecules [25], tumor markers [11], ions [26], proteins [27], cells [28] and tissues and organisms [29] with high specificity and selectivity. Aptamers are screened through an in vitro process called SELEX and can replace antibodies in different applications. Aptamers are similar to antibodies regarding their binding affinities, but they offer a number of advantages over antibodies such as chemical and thermal stability, adaptability to various targets, ease in synthesis and storage, and versatility in labeling, immobilization, signaling and regeneration [30]. These outstanding properties make the aptamers promising diagnostic and therapeutic tools for the future biomedical and analytical applications.
Since their discovery in 1990 [31,32,33], aptamers have demonstrated important advantages in the field of biosensing, especially for the development of devices that allow the detection of disease biomarkers. The first aptasensor proposed in 1998 for the detection of thrombin was based on the use of an aptamer labeled with a fluorescent marker [34]. Since then, a considerable advancement has occurred in biosensor design as well as the use of signal amplification probes to achieve small molecules sensing. Although the potential biosensing applications are unlimited, most of the current applications are foreseen in the areas of biomarker detection, cancer clinical testing, and detection of infectious microorganisms and viruses [35].
Strategic applications of nanomaterials in electrochemical biosensing
The need for ultrasensitive bioassays and the trend towards miniaturized assays make the nanomaterials one of the hot fields in biosensor technology. Ever since the pioneering study of Mirkin and co-workers [36] on nanoparticle-based biosensors, a variety of nanostructured materials have demonstrated their appropriateness for biosensing applications. The intelligent use of nanomaterials can lead to clearly enhanced performances with increased sensitivities and lowered detection limits of several orders of magnitudes.
In this review, we will briefly describe the classification of nanomaterials and provide a brief overview of their role in the development of ultrasensitive electrochemical biosensors. Special attention is paid to the major role of nanomaterials as the signal amplifiers. Applications of nanoparticles in electrochemical signal amplification are mainly based on three mechanistic types: (1) nanomaterials that increase the loading of bioreceptors; (2) nanomaterials that act as the electrochemical signal generating probes; (3) nanomaterials that enhance the loading of electrochemically detectable species (Fig. 2).
Classification of nanomaterials
Nanomaterials are defined as materials in which at least one length dimension is below 100 nanometers. In this size regime, these materials exhibit particular and tunable optical, electrical and mechanical properties that are not present at the macro-scale. The synthesis of novel nanomaterials is a growing research area due to the potential applications for the progress of novel technologies. In the past two decades, hundreds of novel nanomaterials have been introduced. So, the preparation, characterization and classification of nanomaterials, as well as their applications, are essential.
The first classification idea of nanomaterials was given in 1995 [37] and further explained in 2000 [38]. A new classification scheme was reported in 2007 to overcome the limitations of previous classification methods [39]. In this scheme, the various types of nanostructures were discriminated based on their dimensionality. They were characterized as i) zero-dimensional (0D), ii) one-dimensional (1D), iii) two-dimensional (2D) and iv) three-dimensional (3D).
Zero-dimensional nanomaterials
Zero-dimensional nanomaterials are the elementary building blocks in the design of nanostructures, represented by nanoparticles, quantum dots and nanoclusters (Fig. 3a–c). All three dimensions of zero-dimensional nanomaterials are within 100 nm, more specifially less than 50 nm in the most cases.
Typical SEM and TEM images of different kinds of nanostructures based on their dimensionality; a Aunanoparticles [40], b Au nanoclusters [40], c carbon dots [41], d carbon nanotubes [42], e Au nanorods [43], f Au nanowires [44], g CdSe nanobelts [45], h graphene oxide nanoribbons [46], i ceramic nanofibers made of anatase and rutile [47], j Ag nanoplates [48], k graphene oxide nanosheets [49], l copper nanowalls [50], m silica nanodisks [51], n carbon nanocoils [52], o graphene nanoballs with copper cores [53], and p Pd nanoflowers [54]; Reprinted by permission of ACS Publishers
One-dimensional nanomaterials
Nanostructures like nanotubes, nanorods, nanowires, nanobelts, nanoribbons and nanofibers with two dimensions in the nanoscale regime and the third dimension in the microscale are known as one-dimensional nanomaterials (Fig. 3d–i).
Two-dimensional nanomaterials
Two-dimensional nanomaterials are the thinnest materials with only one dimension whithin 100 nm whereas the other two dimensions are those of bulk materials. The common representations of two-dimensional nanomaterials are nanoplates, nanosheets nanowalls and nanodisks (Fig. 3j–m). These nanomaterials generally possess very high specific area and good aspect ratio.
Three-dimensional nanomaterials
Three-dimensional nanomaterials such as nanocoils, nanoballs and nanoflowers are nanophase materials consisting of equiaxed nanometer-sized grains (Fig. 3n–p). These nanomaterials have attracted intensive research interests because they have higher surface areas and supply enough absorption sites for all involved molecules in a small space.
Electrochemical signal amplification approaches using nanomaterials
The electrochemical signal amplification based on nanomaterials for obtaining lower and lower detection limit has recently attracted considerable attention due to the need for ultrasensitive bioassays. Especially, most nanomaterials are biocompatible, which permit them to act in direct contact with the biological environment. In order to achieve a good performance for biosensing, three approaches including biosensing nanoplatforms, signal generating nanoprobes and nanocarriers for signal probes in label-free sandwich detection strategies have been introduced.
Nanomaterials as the platforms of sensing elements
Nanotechnology offers unique opportunities for creating highly sensitive biosensing platforms. Electrode nanostructuration with nanoscale materials is currently a regular operation for preparing electrochemical scaffolds with improved conductivity and enhanced ability for immobilization of biomolecules. In fact, the stabilization of functionalized nanomaterials on the electrode surface increases the effective surface area and promotes the accumulation of bioreceptors. They act as signal amplification platforms for bioreceptors, owing to the existence of reactive groups on their surface, the fast electron transfer kinetics (particularly beneficial for electrochemical biosensors) and the high surface to volume ratio. It is widely believed that the large surface area to volume ratio is directly related to the high sensitivity of nanomaterials based biosensors. In addition, the ion centers and high electrical conductivity of some nanostructures can also have a major role in accelerating the electron transfer process.
A wide variety of electroconductive or semi-electroconductive nanomaterials with small sizes and appropriate surface modifications have been actively utilized as the signal amplifiying nanoplarforms for fabrication of electrochemical receptor based biosensors. In particular, nanomaterials such as noble metal nanoparticles (Au, Pt) [27, 55], carbon based nanostructures [56, 57], magnetic nanoparticles (MNPs) [19, 58], quantum dots (QDs) [59, 60], metal oxide nanoparticles [61, 62] and polymer nanomaterials [63, 64] have been attracted a lot of attention in this field.
Within the group of carbon nanostructures, graphene and its derivatives are considered as the rapidly “rising star” carbon nanomaterials due to tailorable chemical functionalities originated from the pristine sp2 hybridization, superior mechanical strength, good chemical and thermal stabilities, low density, excellent thermal conductivities and low toxicity [65]. Graphene oxide (GO) well-known for its distinct physiochemical properties and a high quantity of oxygen-containing functional groups is electrically semiconducting and has low electronic conductivity. In some cases, it is necessary to regain graphene's desirable characteristics such as electrical conductivity or catalytic activity. Reduced graphene oxide (rGO) with higher electrical conductivity than GO still contains some oxygen related functional groups which can bind to the biological probes [66]. Graphene quantum dots (GQDs), as the newest member of graphene family, are graphene sheets with lateral size smaller than 100 nm in single, double and multiple layers, and diameters spanning the range 3–20 nm mainly [25, 67]. They combine the advantages of graphene with the quantum confinement of carbon dots for electrochemical biosensing applications.
Nanomaterials as the signal generating probes
Growing demand for developing ultrasensitive electrochemical bioassays has led to the design of numerous signal amplification strategies based on nanoparticle electroactive labels. The importance of the use of nanomaterials as the signal tags for amplification of electrochemical responses has been reflected in the number of reviews published on this topic [68]. Nanoparticles consist of thousands of atoms, which in principle can be oxidized or reduced electrochemically. Consequently, when all bioreceptor molecules are labeled with nanoparticles, the loading of electroactive species on the electrode surface significantly increases, which leads to enhanced sensitivity of the biosensor. Nanoparticles of Au [69, 70] and Ag [71, 72] have been used for this purpose. AuNPs can be electrochemically oxidized in HCl to produce electroactive AuCl4-, which is then reduced to give a detectable signal. Conjugated metal sulfide nanoparticles, such as CdS [73], PbS [74] and ZnS [75], have also been extensively used as labels in the development of ultrasensitive electrochemical affinity bioassays. These metal sulfides are easily dissolved in HNO3 medium to obtain Cd2+, Pb2+ and Zn2+, which can then be detected with high sensitivity using stripping voltammetry.
Nanomaterials as the carriers for signal tags in label-free sandwich detection strategies
Along with the use of nanomaterials for the construction of nanostructured electrode surfaces, their utilization as carriers of signal tags for electrochemical signal amplification is another less widespread but equally relevant application. Nanomaterials, especially carbon nanostructures, have been demonstrated to be excellent carriers for signal probes with their good conductivity and biocompatibility in label-free electrochemical sandwich-type biosensors. Nanoscale materials have a greater surface area, which increases the amount of the accumulation of redox probes, which can be led to high sensitivity. Typically, graphene and its derivatives are the promising supports for immobilization of redox tags such as thionine or methylene blue [76]. The graphene nanosheets offer a large surface area for immobilization of electroactive compounds as the electrochemical signal probes. In addition, the high electrical conductance is a distinctive character for graphene, which leads to increased current density and high sensitivity. Our group developed a sandwich-type electrochemical aptasensor for detection of MUC 1 in breast cancer patients [77]. In this work, rGO-N'1,N'3 dihydroxymalonimidamide nanosheets with large surface area, excellent electroconductivity and good adsorption capacity were employed, not only as an ideal carrier to immobilize numerous secondary aptamers, but also as a suitable sorbent for the accumulation of electroactive thionine. Another example of these nanocarries is core-shell nanocomposites. Valipour and Roushani [78] reported an electrochemical sandwich-type immunoassay for the core antigen of hepatitis C based on a nafion coated TiO2 nanocomposite as the carrier of Celestine Blue tags.
Candidate metabolic biomarkers for diagnosis of diseases
Metabolomics, a postgenomic approach used to rapidly identify global metabolic changes in biological systems, has been increasingly applied to diagnosis of diseases, measurement of the response to treatment, discovery of biomarkers and identification of perturbed pathways. In this review, we attempted to explore the electrochemical biosensing strategies for the potential biomarkers related to some diseases and for validation of these biomarkers as predictors to diagnose the neurodegenerative diseases, autism spectrum disorder diseases and hepatitis.
Neurodegenerative diseases
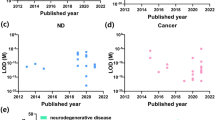
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and Amyotrophic Lateral Sclerosis (ALS) are incurable and debilitating conditions that result in progressive degeneration and death of nerve cells. Currently, the human and animal studies are developing new and compelling ideas about the early diagnosis of these disorders, with the goal of slowing or stopping their progression. These efforts reveal the presence of abnormal metabolites in many degenerative diseases that interfere with normal cellular functions. The ultrasensitive assessment of these functional biomarkers for neurodegenerative diseases is important in diagnosis, management and treatment of these disorders. Considering the studies performed in the previous decade, 101 metabolites have been identified as putative biomarkers for AD, PD and ALS. Notably, alanine, choline, creatinine, creatine and uric acid are the shared metabolite signatures among these three diseases [6, 7]. Thus, a variety of electrochemical biosensors based on enzymatic reacrions have been developed for the selective and sensitive determination of these metabolic biomarkers. In the most of these cases, the measurement is based on the amperometric detection of hydrogen peroxide (H2O2), as a side product of the choline oxidase catalysed reaction.
Alanine
D-amino acid oxidase (DAAO) as a peroxisomal enzyme has been used in several biosensors for the determination of the D-amino acids such D-alanine in biological fluids. As seen in Table 1, with combination of excellent electron transfer ability of CNTs and high bioactivity of DAAO, various signal amplification biosensors for assessment of D-alanine have been designed [79,80,81,82,83]. The high sensitivity and selectivity of these catalytic biosensors indicate the good conductivity of designed nanoplatforms and successful maintenance of DAAO bioactivity.
Choline
Choline is a precursor of the neurotransmitter acetylcholine, one of the crucial brain chemicals involved in memory. Individuals suffer from various nerve disorders such as PD and AD due to the lack of acetylcholine [84]. The quantitative determination of choline is important in clinical analysis, especially in the early diagnosis of brain disorders. Among different methods available for choline detection, the electrochemical biosensors based on choline oxidase (ChO) and nanomaterials present advantages such as simplicity, reliability, high sensitivity and selectivity. A broad range of the electrochemical biosensors for determination of choline using the electrocatalytic properties of conductive nanomaterials and enzymatic activity of ChO has been listed in Table 2 [85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111].
Creatinine and creatine
Creatinine is the end product of creatine catabolism in mammals [112]. It is found together with creatine in muscle tissue and in blood. Determination of these metabolic biomarkers in biological fluids is essential for detection of neurodegenerative diseases. For this purpose, the commercial enzymes containing creatininase (CA), creatinase (CI) or sarcosine oxidase (SO) immobilized on the nanomaterial modified electrodes have been extensively used as the biosensing interfaces. Table 3 represents the recent developments in design and fabrication of electrochemical biosensors for the determination of creatine [113] and creatinine [114,115,116,117,118,119,120].
Uric acid
Uric Acid (UA), considered as a waste of cellular metabolism, has now received increasing attention because it was found to directly participate in the pathogenesis of many human diseases including neurological disorders. UA protects neurons in neurodegenerative disorders via antioxidative effects. Fast and accurate determination of UA in human physiological fluids has been recognized as a vital clinical test in the diagnosis of patients suffering from numerous metabolic disorders. Thus, continuous monitoring of UA levels in the blood is of paramount importance. The important challenge in the electrochemical detection of UA is the co-existence of many interfering compounds in biological systems such as dopamine (DA) and ascorbic acid (AA). To overcome this limitition, non-enzymatic sensing strategies have been designed using the materials with high electrocatalytic activity to oxidation of UA [121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136]. These nanocomposites electrocatalytically oxidize UA, DA and AA at different potentials, so that the simultaneous determination of the analytes by these sensors will be in rich. Although these sensors can effectively achieve UA detection, there is a scope for improvement in the receptor based biosensors. The binding of UA to the active site of uricase enzyme is a very specific and strong interaction. Thus, considerable efforts have been focused on the development of new enzyme-based biosensors. Table 4 presents a list of the electrochemical enzymatic biosensors for evaluation of UA in biological fluids [137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175].
Autism Spectrum Disorder
The pattern of behavioral symptoms now described as Autism Spectrum Disorder (ASD) was first recognized in 1943 [176]. ASD is a complex neurodevelopmental condition that occurs within the first 3 years of life, which is marked by social skills and communication deficits along with stereotyped repetitive behavior [177]. The prevalence of autism has been increased by more than tenfold in the last decade. This growing prevalence has stimulated intense research into the identification of biochemical markers related to autism would be advantageous for earlier clinical diagnosis and intervention.
The etiology of this developmental disorder is poorly understood, and no biomarker has definitely been identified. However, many investigators are addressing the concept of autism as a general metabolic disorder. Some studies suggest that oxidative stress-induced mechanisms and reduced antioxidant defense, mitochondrial dysfunction and impaired energy metabolism (nicotinamide adenine dinucleotide (NAD), adenosine triphosphate (ATP), pyruvate) and altered tryptophan metabolism are major causes of ASD [178,179,180]. They have shown a disturbance in energy metabolism in the brains of autistic children with a marked increase in the size especially in areas related to social cognitive processes. In addition, tryptophan is a precursor of important compounds, such as serotonin, quinolinic acid and kynurenic acid, which are involved in neurodevelopment and synaptogenesis. The decreased tryptophan metabolism may alter brain development, neuroimmune activity and mitochondrial function [181].
These findings provided initial support for the possibility that the evaluation strategies of biomarkers may be effective for a broad range of individuals with ASD. Thus, a variety of biosensors have been developed for blood analysis of these children. Table 5 provides renewed insight related to electrochemical biosensors for ultrasensitive detection of ASD metabolic biomarkers [61, 182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233].
It should be noted that most of these electrochemical biosensors have been fabricated based on the formation of aptamer-target complexes. The DNA aptamer for adenosine is one of the most studied since the initial report in 1995 by Huizenga and Szostak [234]. It has a similar affinity to a few adenosine derivatives including adenosine monophosphate (AMP), cyclic adenosine monophosphate (cAMP) and ATP, but it cannot bind other nucleosides such as guanosine [235].
Tryptophan aptamers with different dissociation constants have been extensively studied for the affinity based bioassays [236]. The investigation of specificity showed that these aptamers strongly bind to tryptophan and possess almost no binding to other amino acids, so that they can be used as the efficient biorecognition tools for biosensing applications.
Hepatitis
Hepatitis refers to an inflammatory condition of the liver. It may be caused by viruses, drugs or alcohol, although the most common cause is viruses. Viral hepatitis can be caused by five hepatitis viruses including A, B, C, D and E. During a five-year period, 10–20% of chronic hepatitis leads to cirrhosis [237]. Accordingly, the major clinical risk factor for hepatocellular carcinoma (HCC) development is liver cirrhosis as 70–90% of HCCs develop in a cirrhotic liver [238]. These data clearly indicate the critical importance of early diagnosis of hepatitis. Therefore, the reliable and noninvasive diagnostic methods to predict and assess liver hepatitis are needed. The blood testing including enzyme immunoassay, polymerase chain reaction (PCR) assay, recombinant immunoblot assay, biomarker assay and quantification of hepatitis RNA in serum are the most common methods for detection of hepatitis. Among these, the analysis of metabolic biomarkers is a powerful strategy to advance the diagnosis, treatment and prevention of hepatitis.
Liver is a major metabolic organ and its infection is expected to result in measurable changes in such metabolite levels as tryptophan, phenylalanine, histidine, tyrosine, ethanol, lactic acid, L-proline and fumaric acid [239]. Especially, the concentration of amino acids is very often found altered in liver diseases [240]. The high blood level of lactate also plays an important role in liver dysfunction [241]. Both acute and chronic hepatic diseases can result in lactate accumulation and lactic acidosis. In addition, several studuies reported the relationship between blood alcohol concentration and hepatic enzymes in these patients [242]. Hepatic enzymes can be used as a predictor of hepatic injury.
A variety of electrochemical biosensing methods have been developed to detect metabolic biomarkers for the fast diagnosis of hepatitis. Most of these biosensors have been designed based on the amperometric determination of H2O2, a by-product of the enzyme reactions. They reveal good performances such as increased sensitivity, excellent selectivity and good repeatability, when compared with non-enzymatic biosensors [243, 244]. Table 6 summarizes a list of reported electrochemical biosensors based on the biorecognition elements for ultrasensitive detection of hepatitis metabolic biomarkers [228, 230,231,232,233,234, 245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340]. The analytical characteristics of these biosensors confirm that they can serve as potential devices for the detection of metabolism dysregulations in patients with hepatitis.
Conclusion
With increasing acute and chronic diseasese, sensitive and reliable diagnostic tools will improve treatment outcomes. However, the conventional diagnostics used for such purposes are extremely powerful; most of these are limited by time and cost-consuming protocols and require higher volume of test sample. In this review, we have presented a snapshot of the recent developments in the field of electrochemical biosensors and an overview of signal amplification strategies based on the nanomaterials. However, researchers have developed many signal enhancement strategies in the fabrication of electrochemical biosensors such as nuclease amplification, rolling circle amplification, catalyzed hairpin assembly amplification and etc. The nanomaterial-assisted signal amplification is more efficient because that they are not only employed as ideal carriers to immobilize various bioreceptors but also act as the sorbents for the accumulation of redox indicators. One of the fascinating aspects of nanomaterials is that the chemical properties of their surface can be manipulated by surface modification and functionalization. Furthermore, some of these nanomaterials exhibit unique electrical conductivity with high performance for electrochemical biosensors. The combination of high affinity and specificity of bioreceptors and unique properties of nanomaterias provides promising opportunities for various biosensing applications. Despite considerable progresses in this area, there are definitely challenges that must be addressed. (I) Most of electrochemical biosensors have been constructed for in vitro detection of metabolic biomarkers; therefore, the design of biosensors for in vivo measurements should be a priority in the future. The real-time measurement of metabolic biomarkers requires the development of in vivo biosensors, which necessitates the synthesis of non-toxic nanomaterials, improved stability of enzymatic immobilization and enhanced resistance to substance interference. (II) The fabrication of multiplexed nanoscale biosensors for simultaneous detection of different analytes has still remained a major challenge at the nanotechnology frontier. Development of biosensors that allow for simultaneous detection of multiple metabolic biomarkers of a disease can achieve higher detection sensitivity while reducing false positives. For this purpose, integration of analytical technologies on a single platform is recommended. (III) The design of microfluidics platforms for electrochemical biosensors is required to overcome the limitations of conventional bioassays through the development of biosensors that can provide continuous, in situ and rapid measurement of targets. The developments in microfluidics should be directed towards fabrication of biomimetic human organoid models that simulate both the biology and the physiological microenvironment of the human system, termed organs-on-chips. Microfluidic organs-on-a-chip platforms allow the continual monitoring of (multiple) secreted biomolecules in a noninvasive manner while consuming only small volumes of media. (IV) Despite all significant achievements in the design and development of highly sensitive and selective electrochemical biosensors, there is a need to address the issue of transition from development stages towards commercialization of biosensors for point-of-care diagnostics of diseases. By driving the development of suitable bench-top technology through product development, other practical aspects including portability, costs and fabrication techniques can also be examined. These biosensors will then be well poised for speedy translation into point-of-care diagnostics. Finally, it is expected that the nanomaterials will have a great practical foundation in design of novel biosensors for point-of-care clinical diagnostics. Taking into account the continuous progress in the development of novel nanomaterials, the application of new electrochemical sensing scaffolds based on multifunctional nanomaterials is expected to be widely developed in the future.
References
Vasan RS (2006) Biomarkers of cardiovascular disease. Circulation 113:2335–2362
Strimbu K, Tavel JA (2010) What are Biomarkers? Curr Opin HIV AIDS 5:463–466
Bradley E (2012) Incorporating biomarkers into clinical trial designs: points to consider. Nature Biotechnol 30:596–599
Lee JM, Han JJ, Altwerger G, Kohn EC (2011) Proteomics and biomarkers in clinical trials for drug development. J Protoemics 74:2632–2641
Baumgartner C, Osl M, Netzer M, Baumgartner D (2011) Bioinformatic-driven search for metabolic biomarkers in disease. J Clin Bioinforma 1:1–10
Havelund JF, Heegaard NHH, Færgeman NJK, Gramsbergen JB (2017) Biomarker research in Parkinson’s disease using metabolite profiling. Metabolites 7:42
Kori M, Aydın B, Unal S, Arga KY, Kazan D (2016) Metabolic biomarkers and neurodegeneration: A pathway enrichment analysis of Alzheimer’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis. OMICS: J Integrative Biol 20:645–661
Emond P, Mavel S, Aidoud N, Nadal-Desbarats L, Montigny F, Bonnet-Brilhault F, Barthélémy C, Marten M, Sarda P, Laumonnier F, Vourc'h P, Blasco H, Andres CR (2013) GC-MS-based urine metabolic profiling of autism spectrum disorders. Anal Bioanal Chem 405:5291–5300
Esparham AE, Smith T, Belmont JM, Haden M, Wagner LE, Evans RG, Drisko JA (2015) Nutritional and metabolic biomarkers in autism spectrum disorders: An exploratory study. Integr Med 14:1–14
Schoeman JC, Hou J, Harms AC, Vreeken RJ, Berger R, Hankemeier T, Boonstra A (2016) Metabolic characterization of the natural progression of chronic hepatitis B. Genome Med 8:64
Farzin L, Shamsipur M (2018) Recent advances in design of electrochemical affinity biosensors for low level detection of cancer protein biomarkers using nanomaterial-assisted signal enhancement strategies. J Pharmaceut Biomed Anal 147:185–210
Zhu C, Yang G, Li H, Du D, Lin Y (2015) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem 87:230–249
Chopra N, Gavalas VG, Bachas LG, Hinds BJ, Bachas LG (2007) Functional one dimensional nanomaterials: applications in nanoscale biosensors. Anal Lett 40:2067–2096
Abu-Salah KM, Ansari AA, Alrokayan SA (2010) DNA-based applications in nanobiotechnology. J Biomed Biotechnol 2010:1–15
Hock B, Seifert M, Kramer A (2002) Engineering receptors and antibodies for biosensors. Biosens Bioelectron 17:239–249
Clark LC, Lyons C (1962) Electrode system for continuous monitoring in cardiovascular surgery. Ann NY Acad Sci 102:29–45
Campas M, Prieto-Simon B, Marty JL (2009) A review of the use of genetically engineered enzymes in electrochemical biosensors. Semin Cell Dev Biol 20:3–9
Gurung N, Ray S, Bose S, Raj V (2013) A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res Int 2013:329121
Emami M, Shamsipur M, Saber R, Irajirad R (2014) An electrochemical immunosensor for detection of a breast cancer biomarker based on antiHER2-iron oxide nanoparticle bioconjugates. Analyst 139:2858–2866
Eissa S, Chinnappan R, Zourob M (2017) Label-free impedimetric immunosensors for liver cancer stem cells. Procedia Technol 27:287–289
Donahue AC, Albitar M (2010) Antibodies in biosensing. In: Zourob M (ed) Recognition receptors in biosensors. Springer, New York, pp 221–248
Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F (2005) Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J 46:258–268
Siontorou CG (2013) Nanobodies as novel agents for disease diagnosis and therapy. Int J Nanomedicine 8:4215–4227
Harmsen MM, De Haard HJ (2007) Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol 77:13–22
Shahdost-Fard F, Roushani M (2017) Designing an ultra-sensitive aptasensor based on an AgNPs/thiol-GQD nanocomposite for TNT detection at femtomolar levels using the electrochemical oxidation of Rutin as a redox probe. Biosens Bioelectron 87:724–731
Farzin L, Shamsipur M, Sheibani S (2017) A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 174:619–627
Shamsipur M, Farzin L, Tabrizi MA (2016) Ultrasensitive aptamer-based on-off assay for lysozyme using a glassy carbon electrode modified with gold nanoparticles and electrochemically reduced graphene oxide. Microchim Acta 183:2733–2743
Farzin L, Shamsipur M, Samandari L, Sheibani S (2018) Signaling probe displacement electrochemical aptasensor for malignant cell surface nucleolin as a breast cancer biomarker based on gold nanoparticle decorated hydroxyapatite nanorods and silver nanoparticle labels. Microchim Acta 185:154
Liu X, Marrakchi M, Xu D, Dong H, Andreescu S (2016) Biosensors based on modularly designed synthetic peptides for recognition, detection and live/dead differentiation of pathogenic bacteria. Biosens Bioelectron 80:9–16
Yüce M, Ullah N, Budak H (2015) Trends in aptamer selection methods and applications. Analyst 140:5379–5399
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Robertson DL, Joyce GF (1990) Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 344:467–468
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Potyrailo RA, Conrad RC, Ellington AD, Hieftje GM (1998) Adapting selected nucleic acid ligands (aptamers) to biosensors. Anal Chem 70:3419–3425
Hong P, Li W, Li J (2012) Applications of aptasensors in clinical diagnostics. Sensors 12:1181–1193
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA (1997) Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277:1078–1081
Gleiter H (2000) Nanostructured materials: basic concepts and microstructure. Acta Mater 48:1–29
Skorokhod V, Ragulya A, Uvarova I (2001) Physico-chemical kinetics in nanostructured systems. Academperiodica, Kyiv, pp 180–192
Pokropivny VV, Skorokhod VV (2007) Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater Sci Eng C 27:990–993
Zheng K, Setyawati MI, Leong DT, Xie J (2017) Antimicrobial gold nanoclusters. ACS Nano 11(7):6904–6910
Ding H, Wei JS, Zhong N, Gao Q, Xiong HM (2017) Highly efficient red-emitting carbon dots with gram-scale yield for bioimaging. Langmuir 33:12635–12642
Miyako E, Sugino T, Okazaki T, Bianco A, Yudasaka M, Iijima S (2013) Self-assembled carbon nanotube honeycomb networks using a butterfly wing template as a multifunctional nanobiohybrid. ACS Nano 7:8736–8742
Park K, Drummy LF, Wadams RC, Koerner H, Nepal D, Fabris L, Vaia RA (2013) Growth mechanism of gold nanorods. Chem. Mater. 25:555–563
Nouh ESAE, Baquero EA, Lacroix LM, Delpech F, Poteau R, Viau G (2017) Surface-engineering of ultrathin gold nanowires: Tailored self-assembly and enhanced stability. Langmuir 33:5456–5463
Zhang L, Jia Y, Wang S, Li Z, Ji C, Wei J, Zhu H, Wang K, Wu D, Shi E, Fang Y, Cao A (2010) Carbon nanotube and CdSe nanobelt schottky junction solar cells. Nano Lett 10:3583–3589
Higginbotham AL, Kosynkin DV, Sinitskii A, Sun Z, Tour JM (2010) Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 4:2059–2069
Xue J, Xie J, Liu W, Xia Y (2017) Electrospun nanofibers: New concepts, materials, and applications. Acc Chem Res 50:1976–1987
Liu Z, Zhou H, Lim YS, Song JH, Piao L, Kim SH (2012) Synthesis of silver nanoplates by two-dimensional oriented attachment. Langmuir 28:9244–9249
Geng H, Yao B, Zhou J, Liu K, Bai G, Li W, Song Y, Shi G, Doi M, Wang J (2017) Size fractionation of graphene oxide nanosheets via controlled directional freezing. J Am Chem Soc 139:12517–12523
Jiang C, Zhang W, Liu Y, Qian Y (2006) Self-assembled copper nanowalls into microstructures with different shapes: A facile aqueous approach. Crystal Growth & Design 6:2603–2606
Banerjee S, Datta A (2010) Photoluminescent silica nanotubes and nanodisks prepared by the reverse micelle sol-gel method. Langmuir 26:1172–1176
Chen X, Zhang S, Dikin DA, Ding W, Ruoff RS (2003) Mechanics of a carbon nanocoil. Nano Letters 3:1299–1304
Lee S, Hong J, Koo JH, Lee H, Lee S, Choi T, Jung H, Koo B, Park J, Kim H, Kim YW, Lee T (2013) Synthesis of few-layered graphene nanoballs with copper cores using solid carbon source. ACS Appl Mater Interfaces 5:2432–2437
Ma N, Liu X, Yang Z, Tai G, Yin Y, Liu S, Li H, Guo P, Zhao XS (2018) Carrageenan assisted synthesis of palladium nanoflowers and their electrocatalytic activity toward ethanol. ACS Sustainable Chem Eng 6:1133–1140
Roushani M, Shahdost-fard F (2016) An aptasensor for voltammetric and impedimetric determination of cocaine based on a glassy carbon electrode modified with platinum nanoparticles and using rutin as a redox probe. Microchimica Acta 183:185–193
Wang Z, Dai Z (2015) Carbon nanomaterial-based electrochemical biosensors: an overview. Nanoscale 7:6420–6431
Yáñez-Sedeño P, Campuzano S, Pingarrón JM (2017) Carbon nanostructures for tagging in electrochemical biosensing: A review. J Carbon Res 3:3
Shamsipur M, Emami M, Farzin L, Saber R (2018) A sandwich-type electrochemical immunosensor based on in situ silver deposition for determination of serum level of HER2 in breast cancer patients. Biosens Bioelectron 103:54–61
Shahdost-Fard F, Roushani M (2016) An impedimetric aptasensor based on water soluble cadmium telluride (CdTe) quantum dots (QDs) for detection of Ibuprofen. J Electroanal Chem 763:18–24
Shamsipur M, Farzin L, Tabrizi MA, Shanehsaz M (2016) CdTe amplification nanoplatforms capped with thioglycolic acid for electrochemical aptasensing of ultra-traces of ATP. Mater Sci Eng C 69:1354–1360
Haghighi N, Hallaj R, Salimi A (2017) Immobilization of glucose oxidase onto a novel platform based on modified TiO2 and graphene oxide, direct electrochemistry, catalytic and photocatalytic activity. Mater Sci Eng C 73:417–424
Shahdost-fard F, Roushani M (2017) The use of a signal amplification strategy for the fabrication of a TNT impedimetric nanoaptasensor based on electrodeposited NiONPs immobilized onto a GCE surface. Sens Actuators B-Chem 246:848–853
Hui N, Sun X, Niu S, Luo X (2017) PEGylated polyaniline nanofibers: antifouling and conducting biomaterial for electrochemical DNA sensing. ACS Appl Mater Interfaces 9:2914–2923
Bangar MA, Shirale DJ, Chen W, Myung NV, Mulchandani A (2009) Single conducting polymer nanowire chemiresistive label-free immunosensor for cancer biomarker. Anal Chem 81:2168–2175
Liu B, Xie J, Ma H, Zhang X, Pan Y, Lv J, Ge H, Ren N, Su H, Xie X, Huang L, Huang W (2017) From graphite to graphene oxide and graphene oxide quantum dots. Small 13:1601001
Oh YJ, Yoo JJ, Kim YII, Yoon JK, Yoon HN, Kim JH, Park SB (2014) Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim Acta 116:118–128
Valipour A, Roushani M (2017) Using silver nanoparticle and thiol graphene quantum dots nanocomposite as a substratum to load antibody for detection of hepatitis C virus core antigen: Electrochemical oxidation of riboflavin was used as redox probe. Biosens Bioelectron 89:946–951
Ding L, Bond AM, Zhai J, Zhang J (2013) Utilization of nanoparticle labels for signal amplification in ultrasensitive electrochemical affinity biosensors: A review. Anal Chim Acta 797:1–12
Omidfar K, Zarei H, Gholizadeh F, Larijani B (2012) A high-sensitivity electrochemical immunosensor based on mobile crystalline material-41-polyvinyl alcohol nanocomposite and colloidal gold nanoparticles. Anal Biochem 421:649–656
Xu Q, Yan F, Lei J, Leng C, Ju H (2012) Disposable electrochemical immunosensor by using carbon sphere/gold nanoparticle composites as labels for signal amplification. Chem Eur J 18:4994–4998
Ting BP, Zhang J, Gao Z, Ying JY (2009) A DNA biosensor based on the detection of doxorubicin-conjugated Ag nanoparticle labels using solid-state voltammetry. Biosens Bioelectron 25:282–287
Zhang J, Ting BP, Jana NR, Gao Z, Ying JY (2009) Ultrasensitive electrochemical DNA biosensors based on the detection of a highly characteristic solid-state process. Small 5:1414–1417
Ding C, Zhang Q, Zhang S (2009) An electrochemical immunoassay for protein based on bio bar code method. Biosens Bioelectron 24:2434–2440
Zhu N, Zhang A, Wang Q, He P, Fang Y (2004) Lead sulfide nanoparticle as oligonucleotides labels for electrochemical stripping detection of DNA hybridization. Electroanalysis 16:577–582
Wang J, Liu G, Merkoci A (2003) Electrochemical coding technology for simultaneous detection of multiple DNA targets. J Am Chem Soc 125:3214–3215
Şinoforoğlu M, Gür B, Arık M, Onganer Y, Meral K (2013) Graphene oxide sheets as a template for dye assembly: graphene oxide sheets induce H-aggregates of pyronin (Y) dye. RSC Adv 3:11832–11838
Farzin L, Sadjadi S, Shamsipur M, Chabok A, Sheibani S (2017) A sandwich-type electrochemical aptasensor for determination of MUC 1 tumor marker based on PSMA-capped PFBT dots platform and high conductive rGO-N'1,N'3-dihydroxymalonimidamide/thionine nanocomposite as a signal tag. J Electroanal Chem 807:108–118
Valipour A, Roushani M (2017) TiO2 nanoparticles doped with Celestine Blue as a label in a sandwich immunoassay for the hepatitis C virus core antigen using a screen printed electrode. Microchim Acta 184:2015–2022
Shoja Y, Rafati AA, Ghodsi J (2017) Enzymatic biosensor based on entrapment of D-amino acid oxidase ongold nanofilm/MWCNTs nanocomposite modified glassy carbonelectrode by sol-gel network: Analytical applications for D-alanine inhuman serum. Enzyme Microb Technol 100:20–27
Wang Q, Lin X, Xu J, Xuan C, Zhu S, Fu Y (2016) Highly-sensitive and selective electrochemiluminescence biosensor for the specific detection of D-alanine. J Electrochem Soc 163:B373–B378
Xia Q, Huang Y, Lin X, Zhu S, Fu Y (2016) Highly sensitive D-alanine electrochemical biosensor based on functionalized multi-walled carbon nanotubes and D-amino acid oxidase. Biochem Eng J 113:1–6
Lata S, Batra B, Kumar P, Pundir CS (2013) Construction of an amperometric D-amino acid biosensor based on D-amino acid oxidase/carboxylated mutliwalled carbon nanotube/copper nanoparticles/polyalinine modified gold electrode. Anal Biochem 437:1–9
Wcislo M, Compagnone D, Trojanowicz M (2007) Enantioselective screen-printed amperometric biosensor for the determination of D-amino acids. Bioelectrochemistry 71:91–98
Li Y, Huang H, Shi F, Li Y, Su X (2013) Optical choline sensor based on a water-soluble fluorescent conjugated polymer and an enzyme-coupled assay. Microchimica Acta 180:1135–1140
Magar HS, Ghica ME, Abbas MN, Brett CMA (2017) A novel sensitive amperometric choline biosensor based on multiwalled carbon nanotubes and gold nanoparticles. Talanta 167:462–469
Yu G, Zhao Q, Wu W, Wei X, Lu Q (2016) A facile and practical biosensor for choline based on manganese dioxide nanoparticles synthesized in-situ at the surface of electrode by one-step electrodeposition. Talanta 146:707–713
Mazurenko I, Tananaiko O, Biloivan O, Zhybak M, Pelyak I, Zaitsev V, Etienne M, Walcarius A (2015) Amperometric biosensor for choline based on gold screen-printed electrode modified with electrochemically- deposited silica biocomposite. Electroanalysis 27:1685–1692
Rahman MM, Asiri AM (2015) Selective choline biosensors based on choline oxidase co-immobilized with self-assembled monolayers onto micro-chips at low potential. Anal Methods 7:9426–9434
Zhang L, Chen J, Wang Y, Yu L, Wang J, Peng H, Zhu J (2014) Improved enzyme immobilization for enhanced bioelectrocatalytic activity of choline sensor and acetylcholine sensor. Sens Actuators B-Chem 193:904–910
Özdemir M, Arslan H (2014) Choline-sensing carbon paste electrode containing polyaniline (pani)-silicon dioxide composite-modified choline oxidase. Artif Cells Nanomed Biotechnol 42:27–31
Keihan AH, Sajjadi S, Sheibani N, Moosavi-Movahedi AK (2014) A highly sensitive choline biosensor based on bamboo-like multiwall carbon nanotubes/ionic liquid/Prussian blue nanocomposite. Sens Actuators B-Chem 204:694–703
Wu X, Chai Y, Yuan R, Liang W, Yuan D (2014) A novel electrochemiluminescence choline biosensor based onbiofunctional AMs-ChO biocomposite. Sens Actuators B-Chem 204:429–436
Chen HC, Tsai RY, Chen YH, Lee RS, Hua MY (2013) A colloidal suspension of nanostructured poly(N-butyl benzimidazole)-graphene sheets with high oxidase yield for analytical glucose and choline detections. Anal Chim Acta 792:101–109
Deng K, Zhou J, Li X (2013) Noncovalent nanohybrid of ferrocene with chemically reduced graphene oxide and its application to dual biosensor for hydrogen peroxide and choline. Electrochim Acta 95:18–23
Pundir S, Chauhan N, Narang J, Pundir CS (2012) Amperometric choline biosensor based on multiwalled carbon nanotubes/zirconium oxide nanoparticles electrodeposited on glassy carbon electrode. Anal Biochem 427:26–32
Sajjadi S, Ghourchian H, Rafiee-Pour HA, Rahimi P (2012) Accelerating the electron transfer of choline oxidase using ionic-liquid/NH2-MWCNTs nano-composite. J Iran Chem Soc 9:111–119
Kanik FE, Rende E, Timur S, Toppare L (2012) A novel functional conducting polymer: synthesis and application to biomolecule immobilization. J Mater Chem 22:22517–22525
Zhang H, Yin Y, Wu P, Cai C (2012) Indirect electrocatalytic determination of choline by monitoring hydrogen peroxide at the choline oxidase-prussian blue modified iron phosphate nanostructures. Biosens Bioelectron 31:244–250
Cevik S, Timur S, Anik Y (2012) Biocentri-voltammetric biosensor for acetylcholine and choline. Microchim Acta 179:299–305
Wu B, Ou Z, Ju X, Hou S (2011) Carbon nanotubes/gold nanoparticles composite film for the construction of a novel amperometric choline biosensor. J Nanomater 2011:1–6
Zhang Z, Wang X, Yang X (2011) A sensitive choline biosensor using Fe3O4 magnetic nanoparticles as peroxidase mimics. Analyst 136:4960–4965
Zhao JJ, Wu MS, Feng TY (2011) Development of electrochemiluminescent biosensor for choline based on carbon nanotube modified platinum electrode. Chin J Anal Chem 39:985–989
Qin X, Wang H, Wang X, Miao Z, Chen L, Zhao W, Shan M, Chen Q (2010) Amperometric biosensors based on gold nanoparticles-decorated multiwalled carbon nanotubes-poly(diallyldimethylammonium chloride) biocomposite for the determination of choline. Sens Actuators B-Chem 147:593–598
Wang XF, Zhou Y, Xu JJ, Chen HY (2009) Signal-on electrochemiluminescence biosensors based on CdS–carbon nanotube nanocomposite for the sensitive detection of choline and acetylcholine. Adv Funct Mater 19:1444–1450
Ren X, Tang F, Liao R, Zhang L (2009) Using gold nanorods to enhance the current response of a choline biosensor. Electrochim Acta 54:7248–7253
Bai YH, Zhang H, Xu JJ, Chen HY (2008) Relationship between nanostructure and electrochemical/biosensing properties of MnO2 nanomaterials for H2O2/choline. J Phys Chem C 112:18984–18990
Bai YH, Du Y, Xu JJ, Chen HY (2007) Choline biosensors based on a bi-electrocatalytic property of MnO2 nanoparticles modified electrodes to H2O2. Electrochem Commun 9:2611–2616
Song Z, Zhao Z, Qin X, Huang J, Shi H, Wu B, Chen Q (2007) Highly sensitive choline biosensor based on carbon nanotube-modified Pt electrode combined with sol-gel immobilization. Front Chem China 2:146–150
Song Z, Huang JD, Wu BY, Shi HB, Anzai JI, Chen Q (2006) Amperometric aqueous sol–gel biosensor for low-potential stable choline detection at multi-wall carbon nanotube modified platinum electrode. Sens Actuators B-Chem 115:626–633
Wang J, Liu G, Lin Y (2006) Amperometric choline biosensor fabricated through electrostatic assembly of bienzyme/polyelectrolyte hybrid layers on carbon nanotubes. Analyst 131:477–483
Qu F, Yang M, Jiang J, Shen G, Yu R (2005) Amperometric biosensor for choline based on layer-by-layer assembled functionalized carbon nanotube and polyaniline multilayer film. Anal Biochem 344:108–114
Rao H, Lu Z, Ge H, Liu X, Chen B, Zou P, Wang X, He H, Zeng X, Wang Y (2017) Electrochemical creatinine sensor based on a glassy carbon electrode modified with a molecularly imprinted polymer and a Ni@polyaniline nanocomposite. Microchimica Acta 184:261–269
Kacar C, Erden PE, Pekyardımci S, Kilic E (2013) A Fe3O4-nanoparticles-based amperometric biosensor for creatine determination. Artif Cell Nanomed Nanotech 41:2–7
Kumar P, Jaiwal R, Pundir CS (2017) An improved amperometric creatinine biosensor based on nanoparticles of creatininase, creatinase and sarcosine oxidase. Anal Biochem 537:41–49
Serafin V, Hernandez P, Agui L, Yanez-Sedeno P, Pingarron JM (2013) Electrochemical biosensor for creatinine based on the immobilization of creatininase, creatinase and sarcosine oxidase onto a ferrocene/horseradish peroxidase/gold nanoparticles/multi-walled carbon nanotubes/Teflon composite electrode. Electrochim Acta 97:175–183
Chen P, Peng Y, He M, Yan XC, Zhang Y, Liu YN (2013) Sensitive electrochemical detection of creatinine at disposable screen-printed carbon electrode mixed with ferrocenemethanol. Int J Electrochem Sci 8:8931–8939
Yadav S, Devi R, Bhar P, Singhla S, Pundir CS (2012) Immobilization of creatininase, creatinase and sarcosine oxidase on iron oxide nanoparticles/chitosan-g-polyaniline modified Pt electrode for detection of creatinine. Enzym Microb Tech 50:247–254
Wei F, Cheng S, Korin Y, Reed EF, Gjertson D, Ho CM, Gritsch HA, Veale J (2012) Serum creatinine detection by a conducting-polymer-based electrochemical sensor to identify allograft dysfunction. Anal Chem 84:7933–7937
Yadav S, Kumar A, Pundir CS (2011) Amperometric creatinine biosensor based on covalently coimmobilized enzyme onto carboxylated multiwalled carbon nanotubes/polyaniline composite film. Anal Biochem 419:277–283
Yadav S, Devi R, Kumar A, Pundir CS (2011) Tri-enzyme functionalized ZnO-NPs/CHIT/c-MWCNT/PANI composite film for amperometric determination of creatinine. Biosens Bioelectron 28:64–70
Yadav DK, Gupta R, Ganesan V, Sonkar PK (2017) Individual and simultaneous voltammetric determination of ascorbic acid, uric acid and folic acid by using a glassy carbon electrode modified with gold nanoparticles linked to bentonite via cysteine groups. Microchimica Acta 184:1951–1957
Taleb M, Ivanov R, Bereznev S, Kazemi SH, Hussainova I (2017) Ultra-sensitive voltammetric simultaneous determination of dopamine, uric acid and ascorbic acid based on a graphene-coated alumina electrode. Microchimica Acta 184:4603–4610
Liu J, Xie Y, Wang K, Zeng Q, Liu R, Liu X (2017) A nanocomposite consisting of carbon nanotubes and gold nanoparticles in an amphiphilic copolymer for voltammetric determination of dopamine, paracetamol and uric acid. Microchimica Acta 184:1739–1745
Song H, Xue G, Zhang J, Wang G, Ye BC, Sun S, Tian L, Li Y (2017) Simultaneous voltammetric determination of dopamine and uric acid using carbon-encapsulated hollow Fe3O4 nanoparticles anchored to an electrode modified with nanosheets of reduced graphene oxide. Microchimica Acta 184:843–853
Tsierkezos NG, Ritter U, Thaha YN, Downing C, Szroeder P, Scharff P (2016) Multi-walled carbon nanotubes doped with boron as an electrode material for electrochemical studies on dopamine, uric acid, and ascorbic acid. Microchimica Acta 183:35–47
Yan S, Li X, Xiong Y, Wang M, Yang L, Liu X, Li X, Alshahrani LAM, Liu P, Zhang C (2016) Simultaneous determination of ascorbic acid, dopamine and uric acid using a glassy carbon electrode modified with the nickel(II)-bis(1,10-phenanthroline) complex and single-walled carbon nanotubes. Microchimica Acta 183:1401–1408
Li Y, Lin H, Peng H, Qi R, Luo C (2016) A glassy carbon electrode modified with MoS2 nanosheets and poly(3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Microchimica Acta 183:2517–2523
Mei LP, Feng JJ, Wu L, Chen JR, Shen L, Xie Y, Wang AJ (2016) A glassy carbon electrode modified with porous Cu2O nanospheres on reduced graphene oxide support for simultaneous sensing of uric acid and dopamine with high selectivity over ascorbic acid. Microchimica Acta 183:2039–2046
Dai H, Wang N, Wang D, Zhang X, Ma H, Lin M (2016) Voltammetric uric acid sensor based on a glassy carbon electrode modified with a nanocomposite consisting of polytetraphenylporphyrin, polypyrrole, and graphene oxide. Microchimica Acta 183:3053–3059
Xing L, Ma Z (2016) A glassy carbon electrode modified with a nanocomposite consisting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchimica Acta 183:257–263
Wu F, Huang T, Hu Y, Yang X, Ouyang Y, Xie Q (2016) Differential pulse voltammetric simultaneous determination of ascorbic acid, dopamine and uric acid on a glassy carbon electrode modified with electroreduced graphene oxide and imidazolium groups. Microchimica Acta 183:2539–2546
Deng K, Li X, Huang H (2016) A glassy carbon electrode modified with a nickel(II) norcorrole complex and carbon nanotubes for simultaneous or individual determination of ascorbic acid, dopamine, and uric acid. Microchimica Acta 183:2139–2145
Li Y, Zhai X, Wang H, Liu X, Guo L, Ji X, Wang L, Qiu H, Liu X (2015) Non-enzymatic sensing of uric acid using a carbon nanotube ionic-liquid paste electrode modified with poly(β-cyclodextrin). Microchimica Acta 182:1877–1884
Zhao D, Fan D, Wang J, Xu C (2015) Hierarchical nanoporous platinum-copper alloy for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Microchimica Acta 182:1345–1352
Tian L, Zhang B, Sun D, Chen R, Wang B, Li T (2014) A thin poly(acridine orange) film containing reduced graphene oxide for voltammetric simultaneous sensing of ascorbic acid and uric acid. Microchimica Acta 181:589–595
Rafati AA, Afraz A, Hajian A, Assari P (2014) Simultaneous determination of ascorbic acid, dopamine, and uric acid using a carbon paste electrode modified with multiwalled carbon nanotubes, ionic liquid, and palladium nanoparticles. Microchimica Acta 181:1999–2008
Zhao Y, Wei X, Peng N, Wang J, Jiang Z (2017) Study of ZnS nanostructures based electrochemical and photoelectrochemical biosensors for uric acid detection. Sensors 17:1235
Ahmad R, Tripathy N, Ahn MS, Hahn YB (2017) Solution process synthesis of high aspect ratio ZnO nanorods on electrode surface for sensitive electrochemical detection of uric acid. Sci Reports 7:1–8
da Cruz FS, de Souza PF, Franco DL, dos Santos WTP, Ferreira LF (2017) Electrochemical detection of uric acid using graphite screen-printed electrodes modified with Prussian blue/poly(4-aminosalicylic acid)/Uricase. J Electroanal Chem 806:172–179
Jindal K, Tomar M, Gupta V (2017) A novel low-powered uric acid biosensor based on arrayed p-njunction heterostructures of ZnO thin film and CuO microclusters. Sens Actuators B-Chem 253:566–575
Omar MN, Salleh AB, Lim HN, Tajudin A (2016) Electrochemical detection of uric acid via uricase-immobilized graphene oxide. Anal Biochem 509:135–141
Cheng C, Kao CY (2016) An electrochemical biosensor with uricase immobilized on functionalized gold coated copper wire electrode for urinary uric acid assay. Electroanalysis 28:695–703
Ahmad R, Tripathy N, Jang NK, Khang G, Hahn YB (2015) Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens Actuators B-Chem 206:146–151
Erden PE, Kacar C, Ozturk F, Kilic E (2015) Amperometric uric acid biosensor based on poly(vinylferrocene)-gelatin-carboxylated multiwalled carbon nanotube modified glassy carbon electrode. Talanta 134:488–495
Ghosh T, Sarkar P, Turner APF (2015) A novel third generation uric acid biosensor using uricase electro-activated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry 102:1–9
Sun L, Liu J, Zhang P, Meng Y, Liu C, Ma Y, Xie Q, Meng W (2015) An amperometric biosensor and a biofuel cell of uric acid based on a chitosan/uricase-poly(furan-3-boronic acid)-Pd nanoparticles/plated Pd/multiwalled carbon nanotubes/Au electrode. J Electroanal Chem 739:187–196
Arora K, Tomar M, Gupta V (2014) Reagentless uric acid biosensor based on Ni microdiscs-loaded NiO thin film matrix. Analyst 139:4606–4612
Devi R, Pundir CS (2014) Construction and application of an amperometric uric acid biosensorbased on covalent immobilization of uricase on iron oxide nanoparticles/chitosan-g-polyaniline composite film electrodeposited on Pt electrode. Sens Actuators B-Chem 193:608–615
Numnuam A, Thavarungkui P, Kanatharana P (2014) An amperometric uric acid biosensor based on chitosan-carbon nanotubes electrospun nanofiber on silver nanoparticles. Anal Bioanal Chem 406:3763–3772
Huang Y, Bu L, Wang W, Qin X, Li Z, Huang Z, Fu Y, Su X, Xie Q, Yao S (2013) One-pot preparation of uricase-poly(thiophene-3-boronic acid)–Ptnano composites for high-performance amperometric biosensing of uric acid. Sens Actuators B-Chem 177:116–123
Jindal K, Tomar M, Gupta V (2013) Nitrogen-doped zinc oxide thin films biosensor for determination of uric acid. Analyst 138:4353–4362
Liu Y, Yuan M, Liu L, Guo R (2013) A facile electrochemical uricase biosensor designed from gold/amino acid nanocomposites. Sens Actuators B-Chem 176:592–597
Zhao Y, Yan X, Kang Z, Lin P, Fang X, Lei Y, Ma S, Zhang Y (2013) Highly sensitive uric acid biosensor based on individual zinc oxide micro/nanowires. Microchim Acta. 180:759–766
Dey RS, Raj CR (2013) Redox-functionalized graphene oxide architecture for the development of amperometric biosensing platform. ACS Appl Mater Interfaces 5:4791–4798
Piermarini S, Migliorelli D, Volpe G, Massoud R, Pierantozzi A, Cortese C, Palleschi G (2013) Uricase biosensor based on a screen-printed electrode modified with Prussian blue for detection of uric acid in human blood serum. Sens Actuators B-Chem 179:170–174
Puri N, Sharma V, Tanwar V, Singh N, Biradar AM, Rajesh (2013) Enzyme-modified indium tin oxide microelectrode array-based electrochemical uric acid biosensor. Prog Biomater 2:1–7
Chu H, Wei X, Wu M, Yan J, Tu Y (2012) An electrochemiluminescent biosensor based on polypyrrole immobilized uricase for ultrasensitive uric acid detection. Sens Actuators B-Chem 163:247–252
Lei Y, Liu X, Yan X, Song Y, Kang Z, Luo N, Zhang Y (2012) Multicenter uric acid biosensor based on tetrapod-shaped ZnO nanostructures. J Nanosci Nanotechnol 12:513–518
Kanyong P, Pemberton RM, Jackson SK, Hart JP (2012) Development of a sandwich format, amperometric screen-printed uric acid biosensor for urine analysis. Anal Biochem 428:39–43
Rawal R, Chawla S, Chauhan N, Dahiya T, Pundir CS (2012) Construction of amperometric uric acid biosensor based on uricase immobilized on PBNPs/cMWCNT/PANI/Au composite. Int J Biol Macromol 50:112–118
Usman Ali SM, Ibupoto ZH, Kashif M, Hashim U, Willander M (2012) A potentiometric indirect uric acid sensor based on ZnO nanoflakes and immobilized uricase. Sensors 12:2787–2797
Ahuja T, Tanwar VK, Mishra SK, Kumar D, Biradar AM, Rajesh (2011) Immobilization of uricase enzyme on self-assembled gold nanoparticles for application in uric acid biosensor. J Nanosci Nanotechnol 11:4692–4701
Usman Ali SM, Ibupoto ZH, Chey CO, Nur O, Willander M (2011) Functionalized ZnO nanotube arrays for the selective determination of uric acid with immobilized uricase. Chem Sensors 1:19
Usman Ali SM, Alvi NH, Ibupoto ZH, Nur O, Willander M, Danielsson B (2011) Selective potentiometric determination of uric acid with uricase immobilized on ZnO nanowires. Sens Actuators B-Chem 152:241–247
Arora K, Tomar M, Gupta V (2011) Highly sensitive and selective uric acid biosensor based on RF sputtered NiO thin film. Biosens Bioelectron 30:333–336
Jena BK, Raj CR (2011) Enzyme integrated silicate-Pt nanoparticle architecture: A versatile biosensing platform. Biosens Bioelectron 26:2960–2966
Chauhan N, Pundir CS (2011) An amperometric uric acid biosensor based on multiwalled carbon nanotube-gold nanoparticle composite. Anal Biochem 413:97–103
Ahuja T, Rajesh KD, Tanwar VK, Sharma V, Singh N, Biradar AM (2010) An amperometric uric acid biosensor based on Bis[sulfosuccinimidyl] suberate crosslinker/3-aminopropyltriethoxysilane surface modified ITO glass electrode. Thin Solid Film 519:1128–1134
Bhambi M, Sumana G, Malhotra BD, Pundir CS (2010) An amperomertic uric acid biosensor based on immobilization of uricase onto polyaniline-multiwalled carbon nanotube composite film. Artif Cell Blood Sub 38:178–185
Tao H, Wang X, Wang X, Hu Y, Ma Y, Lu Y, Hu Z (2010) Construction of uric acid biosensor based on biomimetic titanate nanotubes. J Nanosci Nanotechnol 10:860–864
Wang Y, Yu L, Zhu Z, Zhang J, Zhu J (2009) Novel uric acid sensor based on enzyme electrode modified by ZnO nanoparticles and multiwall carbon nanotubes. Anal Lett 42:775–789
Arslan F (2008) An amperometric biosensor for uric acid determination prepared from uricase immobilized in polyaniline-polypyrrole film. Sensors 8:5492–5500
Luo YC, Do JS, Liu CC (2006) An amperometric uric acid biosensor based on modified Ir–C electrode. Biosens Bioelectron 22:482–488
Zhang F, Li C, Li X, Wang X, Wan Q, Xian Y, Jin L, Yamamoto K (2006) ZnS quantum dots derived a reagentless uric acid biosensor. Talanta 68:1353–1358
Cete S, Yasar A, Arslan F (2006) An amperometric biosensor for uric acid determination prepared from uricase immobilized in polypyrrole film. Artif Cells Blood Substit Immobil Biotechnol 34:367–380
Kanner L (1943) Autistic disturbances of affective contact. Nerv Child 2:217–250
Xiao Z, Qiu T, Ke X, Xiao X, Xiao T, Liang F, Zou B, Huang H, Fang H, Chu K, Zhang J, Liu Y (2014) Autism spectrum disorder as early neurodevelopmental disorder: Evidence from the brain imaging abnormalities in 2–3 years old toddlers. J Autism Dev Disord 44:1633–1640
Al-Mosalem OA, El-Ansary A, Attas O, Al-Ayadhi L (2009) Metabolic biomarkers related to energy metabolism in Saudi autistic children. Clin Biochem 42:949–957
Essa MM, Subash S, Braidy N, Al-Adawi S, Lim CK, Manivasagam T, Guillemin GJ (2013) Role of NAD+, oxidative stress and tryptophan metabolism in autism spectrum disorders. Int J Tryptophan Res 6:15–28
Kaluzna-Czaplinska J, Jozwik-Pruska J, Chirumbolo S, Bjørklund G (2017) Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab Brain Dis 32:1585–1593
Boccuto L, Chen CF, Pittman AR, Skinner CD, McCartney HJ, Jones K, Bochner BR, Stevenson RE, Schwartz CE (2013) Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol Autism 4:16–19
Wang G, Su X, Xu Q, Xu G, Lin J, Luo X (2018) Antifouling aptasensor for the detection of adenosine triphosphate in biological media based on mixed self-assembled aptamer and zwitterionic peptide. Biosens Bioelectron 101:129–134
Li X, Yang J, Xie J, Jiang B, Yuan R, Xiang Y (2018) Cascaded signal amplification via target-triggered formation of aptazyme for sensitive electrochemical detection of ATP. Biosens Bioelectron 102:296–300
Cui L, Lu M, Li Y, Tang B, Zhang CY (2018) A reusable ratiometric electrochemical biosensor on the basis of the binding of methylene blue to DNA with alternating AT base sequence for sensitive detection of adenosine. Biosens Bioelectron 102:87–93
Ding X, Wang Y, Cheng W, Mo F, Sang Y, Xu L, Ding S (2017) Aptamer based electrochemical adenosine triphosphate assay based on a target-induced dendritic DNA nanoassembly. Microchim Acta 184:431–438
Gopinath SCB, Perumal V, Balakrishnan SR, Md Arshad MK, Lakshmipriya T, Haarindraprasad R, Hashim U (2017) Aptamer-based determination of ATP by using a functionalized impedimetric nanosensor and mediation by a triangular junction transducer. Microchim Acta 184:4425–4431
Shi P, Zhang Y, Yu Z, Zhang S (2017) Label-free electrochemical detection of ATP based on amino-functionalized metal-organic framework. Sci Reports 7:1–7
Mashhadizadeh MH, Naseri N, Mehrgardi MA (2017) A simple non-enzymatic strategy for adenosine triphosphate electrochemical aptasensor using silver nanoparticle-decorated graphene oxide. J Iran Chem Soc 14:2007–2016
Wang G, Xu Q, Liu L, Su X, Lin J, Xu G, Luo X (2017) Mixed self-assembly of polyethylene glycol and aptamer on polydopamine surface for highly sensitive and low-fouling detection of adenosine triphosphate in complex media. ACS Appl Mater Interfaces 9:31153–31160
Hu T, Wen W, Zhang X, Wang S (2016) Nicking endonuclease-assisted recycling of target-aptamer complex for sensitive electrochemical detection of adenosine triphosphate. Analyst 141:1506–1511
Jia J, Feng J, Chen HG, Luo HQ, Li NB (2016) A simple electrochemical method for the detection of ATP usingtarget-induced conformational change of dual-hairpin DNA structure. Sens Actuators B-Chem 222:1090–1095
Liang Y, Su J, Huang Y, Li X, Tao Y, Lu C, Zhu J, Bai Z, Meng J, Lu X, Zhao Y (2016) An ATP aptasensor based on the peroxidase-like activity of hemin/graphene oxide nanosheets. Anal Sci 32:565–569
Su S, Sun H, Cao W, Chao J, Peng H, Zuo X, Yuwen L, Fan C, Wang L (2016) Dual-target electrochemical biosensing based on DNA structural switching on gold nanoparticle-decorated MoS2 nanosheets. ACS Appl Mater Interfaces 8:6826–6833
Tang D, Hou L (2016) Aptasensor for ATP based on analyte-induced dissociation of ferrocene-aptamer conjugates from manganese dioxide nanosheets on a screen-printed carbon electrode. Microchim Acta 183:2705–2711
Zhao T, Lin C, Yao Q, Chen X (2016) A label-free electrochemiluminescent sensor for ATP detection based on ATP-dependent ligation. Talanta 154:492–497
Zhou Q, Lin Y, Lin Y, Wei Q, Chen G, Tang D (2016) In situ amplified electrochemical aptasensing for sensitive detection of adenosine triphosphate by coupling target-induced hybridization chain reaction with the assembly of silver nanotags. Talanta 146:23–28
Huang X, Li Y, Zhang X, Zhang X, Chen Y, Gao W (2015) An efficient signal-on aptamer-based biosensor for adenosine triphosphate detection using graphene oxide as both electrochemical and electrochemiluminescence signal indicator. Analyst 140:6015–6024
Wu D, Ren X, Hu L, Fan D, Zheng Y, Wei Q (2015) Electrochemical aptasensor for the detection of adenosine by using PdCu@MWCNTs-supported bienzymes as labels. Biosens Bioelectron 74:391–397
Zhu L, Liu Y, Yang P, Liu B (2015) Label-free aptasensor based on electrodeposition of gold nanoparticles on graphene and its application in the quantification of adenosine triphosphate. Electrochim Acta 172:88–93
Lu L, Si JC, Zhang Y, Lei JL, Luo HQ, Li NB (2015) Highly selective and sensitive electrochemical biosensor for ATP based on the dual strategy integrating the cofactor-dependent enzymatic ligation reaction with self-cleaving DNAzyme-amplified electrochemical detection. Biosens Bioelectron 63:14–20
Kong Q, Li M, Ma C, Yang H, Ge S, Yan M, Yu J (2015) Ultrasensitive electrochemiluminescence aptasensor based on a graphene/polyaniline composite film modified electrode and CdS quantum dot coated platinum nanostructured networks as labels. RSC Adv 5:70345–70351
Feng L, Zhang Z, Ren J, Qu X (2014) Functionalized graphene as sensitive electrochemical label in target-dependent linkage of split aptasensor for dual detection. Biosens Bioelectron 62:52–58
Li M, Yang H, Ma C, Zhang Y, Ge S, Yu J, Yan M (2014) A sensitive signal-off aptasensor for adenosine triphosphatebased on the quenching of Ru(bpy)3 2+-doped silica nanoparticleselectrochemiluminescence by ferrocene. Sens Actuators B-Chem 191:377–383
Chen H, Chen Q, Zhao Y, Zhang F, Yang F, Tang J, He P (2014) Electrochemiluminescence aptasensor for adenosine triphosphate detection using host-guest recognition between metallocyclodextrin complex and aptamer. Talanta 121:229–233
Shahdost-Fard F, Salimi A, Khezrian S (2014) Highly selective and sensitive adenosine aptasensor based on platinum nanoparticles as catalytical label for amplified detection of biorecognition events through H2O2 reduction. Biosens Bioelectron 53:355–362
Liu Y, Lei J, Huang Y, Ju H (2014) Off-On electrochemiluminescence system for sensitive detection of ATP via target-induced structure switching. Anal Chem 86:8735–8741
Liu S, Lin Y, Liu T, Cheng C, Wei W, Wang L, Li F (2014) Enzyme-free and label-free ultrasensitive eelectrochemical detection of DNA and adenosine triphosphate by dendritic DNA concatamer-based signal amplification. Biosens Bioelectron 56:12–18
Wen W, Bao T, Yang J, Zhang MZ, Chen W, Xiong HY, Zhang XH, Zhao YD, Wang SF (2014) A novel amperometric adenosine triphosphate biosensor by immobilizing graphene/dual-labeled aptamers complex onto poly(o-phenylenediamine) modified electrod. Sens Actuators B-Chem 114:1052–1058
Lu J, Yan M, Ge L, Ge S, Wang S, Yan J, Yu J (2013) Electrochemiluminescence of blue-luminescent graphene quantum dots and its application in ultrasensitive aptasensor for adenosine triphosphate detection. Biosens Bioelectron 47:271–277
Kashefi-Kheyrabadi L, Mehrgardi MA (2013) Aptamer based electrochemical biosensor for detection of adenosine triphosphate using a nanoporous gold platform. Bioelectrochemistry 94:47–52
Liu S, Wang Y, Zhang C, Lin Y, Li F (2013) Homogeneous electrochemical aptamer-based ATP assay with signal amplification by exonuclease III assisted target recycling. Chem Commun 49:2335–2337
Sanghavi BJ, Sitaula S, Griep MH, Karna SP, Ali MF, Swami NS (2013) Real-time electrochemical monitoring of adenosine triphosphate in the picomolar to micromolar range using graphene-modified electrodes. Anal Chem 85:8158–8165
Wu L, Zhang X, Liu W, Xiong E, Chen J (2013) Sensitive electrochemical aptasensor by coupling “Signal-on” and “Signal-off” strategies. Anal Chem 85:8397–8402
Kashefi-Kheyrabadi L, Mehrgardi MA (2012) Aptamer-conjugated silver nanoparticles for electrochemical detection of adenosine triphosphate. Biosens Bioelectron 37:94–98
Zhang H, Han Y, Guo Y, Dong C (2012) Porphyrin functionalized graphene nanosheets-based electrochemical aptasensor for label-free ATP detection. J Mater Chem 22:23900–23905
Wang L, Xu M, Han L, Zhou M, Zhu C, Dong S (2012) Graphene enhanced electron transfer at aptamer modified electrode and its application in biosensing. Anal Chem 84:7301–7307
Tian CY, Xu JJ, Chen HY (2012) A novel aptasensor for the detection of adenosine in cancer cells by electrochemiluminescence of nitrogen doped TiO2 nanotubes. Chem Commun 48:8234–8236
Lin Z, Luo F, Liu Q, Chen L, Qiu B, Cai Z, Chen G (2011) Signal-on electrochemiluminescent biosensor for ATP based on the recombination of aptamer chip. Chem Commun 47:8064–8066
Zhang H, Jiang B, Xiang Y, Zhang Y, Chai Y, Yuan R (2011) Aptamer/quantum dot-based simultaneous electrochemical detection of multiple small molecules. Anal Chim Acta 688:99–103
Wang X, Dong P, He P, Fang Y (2010) A solid-state electrochemiluminescence sensing platform for detection of adenosine based on ferrocene-labeled structure-switching signaling aptamer. Anal Chim Acta 658:128–132
Huang H, Tan Y, Shi J, Liang G, Zhu JJ (2010) DNA aptasensor for the detection of ATP based on quantum dots electrochemiluminescence. Nanoscale 2:606–612
Deng C, Chen J, Nie L, Nie Z, Yao S (2009) Sensitive bifunctional aptamer-based electrochemical biosensor for small molecules and protein. Anal Chem 81:9972–9978
Li W, Nie Z, Xu X, Shen Q, Deng C, Chen J, Yao S (2009) A sensitive, label free electrochemical aptasensor for ATP detection. Talanta 78:954–958
Liu Z, Li Z, Shen G, Yu R (2009) Reagentless aptamer based impedance biosensor for monitoring adenosine. Electroanalysis 21:1781–1785
Feng K, Sun C, Kang Y, Chen J, Jiang JH, Shen GL, Yu RQ (2008) Label-free electrochemical detection of nanomolar adenosine based on target-induced aptamer displacement. Electrochem Commun 10:531–535
Zhang S, Xia J, Li X (2008) Electrochemical biosensor for detection of adenosine based on structure-switching aptamer and amplification with reporter probe DNA modified Au nanoparticles. Anal Chem 80:8382–8388
Li Z, Su W, Liu S, Ding X (2015) An electrochemical biosensor based on DNA tetrahedron/graphene composite film for highly sensitive detection of NADH. Biosens Bioelectron 69:287–293
Deng L, Zhang L, Shang L, Guo S, Wen D, Wang F, Dong S (2009) Electrochemiluminescence detection of NADH and ethanol based on partial sulfonation of sol-gel network with gold nanoparticles. Biosens Bioelectron 24:2273–2276
Ghica ME, Brett CMA (2006) Development of novel glucose and pyruvate biosensors at poly(Neutral red) modified carbon film electrodes; Application to natural samples. Electroanalysis 18:748–756
Hashkavayi AB, Raoof JB, Ojani R (2017) Construction of a highly sensitive signal-on aptasensor based on gold nanoparticles/functionalized silica nanoparticles for selective detection of tryptophan. Anal Bioanal Chem 409:6429–6438
Majidi MR, Omidi Y, Karimi P, Johari-Ahar M (2016) Reusable potentiometric screen-printed sensor and label-free aptasensor with pseudo-reference electrode for determination of tryptophan in the presence of tyrosine. Talanta 150:425–433
Majidi MR, Karami P, Johari-Ahar M, Omidi Y (2016) Direct detection of tryptophan for rapid diagnosis of cancer cell metastasis competence by an ultrasensitive and highly selective electrochemical biosensor. Anal Methods 8:7910–7919
Zor E, Patir IH, Bingol H, Ersoz M (2013) An electrochemical biosensor based on human serum albumin/graphene oxide/3-aminopropyltriethoxysilane modified ITO electrode for the enantioselective discrimination of D- and L-tryptophan. Biosens Bioelectron 42:321–325
Huizenga DE, Szostak JW (1995) A DNA aptamer that binds adenosine and ATP. Biochemistry 34:656–665
Deng Q, German I, Buchanan D, Kennedy RT (2001) Retention and separation of adenosine and analogues by affinity chromatography with an aptamer stationary phase. Anal Chem 73:5415–5421
Yang X, Han Q, Zhang Y, Wu J, Tang X, Dong C, Liu W (2015) Determination of free tryptophan in serum with aptamer-Comparison of two aptasensors. Talanta 131:672–677
Amantonico A, Urban PL, Zenobi R (2010) Analytical techniques for single-cell metabolomics: state of the art and trends. Anal Bioanal Chem 398:2493–2504
Zhang A, Sun H, Yan G, Han Y, Ye Y, Wang X (2013) Urinary metabolic profiling identifies a key role for glycocholic acid in human liver cancer by ultra-performance liquid-chromatography coupled with high-definition mass spectrometry. Clin Chim Acta 418:86–90
Munshi SU, Taneja S, Bhavesh NS, Shastri J, Aggarwal R, Jameel S (2011) Metabonomic analysis of hepatitis E patients shows deregulated metabolic cycles and abnormalities in amino acid metabolism. J Viral Hepat 18:e591–e602
Beyoglu D, Idle JR (2013) The metabolomic window into hepatobiliary disease. J Hepatol 59:842–858
Sterling SA, Puskarich MA, Jones AE (2015) The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study. Clin Exp Emerg Med 2:197–202
Wu CY, Lin HL, Chen CW, Lin JN, Kuo LC, Cheng YC, Lin TY, Lee WC (2010) Relationship between blood alcohol concentration and hepatic enzymes in an emergency department. Tzu Chi Med J 22:24–28
Shan C, Yang H, Han D, Zhang Q, Ivaska A, Niu L (2010) Electrochemical determination of NADH and ethanol based on ionic liquid-functionalized graphene. Biosens Bioelectron 25:1504–1508
Schmitt RE, Molitor HR, Wu T (2012) Voltammetric method for the determination of lactic acid using a carbon paste electrode modified with cobalt phthalocyanine. Int J Electrochem Sci 7:10835–10841
Jaime J, Rangel G, Munoz-Bonilla A, Mayoral A, Herrasti P (2017) Magnetite as a platform material in the detection of glucose, ethanol and cholesterol. Sens Actuators B-Chem 238:693–701
Volkan Ozdokur K, Demir B, Atman E, Yalin Tatli A, Yilmaz B, Odaci Demirkol D, Kocak S, Timur S, Nil Ertas F (2016) A novel ethanol biosensor on pulsed deposited MnOx-MoOx electrodedecorated with Pt nanoparticles. Sens Actuators B-Chem 237:291–297
Bilgi M, Ayranci E (2016) Biosensor application of screen-printed carbon electrodes modified with nanomaterials and a conducting polymer: Ethanol biosensors based on alcohol dehydrogenase. Sens Actuators B-Chem 237:849–855
Chinnadayyala SR, Santhosh M, Singh NK, Goswami P (2015) Alcohol oxidase protein mediated in-situ synthesized and stabilized gold nanoparticles for developing amperometric alcohol biosensor. Biosens Bioelectron 69:155–161
Gómez-Anquela C, García-Mendiola T, Abad JM, Pariente MPF, Lorenzo E (2015) Scaffold electrodes based on thioctic acid-capped gold nanoparticles coordinated alcohol dehydrogenase and Azure A films for high performance biosensor. Bioelectrochemistry 106:335–342
Jafari F, Salimi A, Navaee A (2014) Electrochemical and photoelectrochemical sensing of NADH and ethanol based on immobilization of electrogenerated chlorpromazine sulfoxide onto graphene-CdS quantum dot/ionic liquid nanocomposite. Electroanalysis 26:530–540
Soylemez S, Kanik FE, Uzun SD, Hacioglu SO, Toppare L (2014) Development of an efficient immobilization matrix based on a conducting polymer and functionalized multiwall carbon nanotubes: synthesis and its application to ethanol biosensors. J Mater Chem B 2:511–521
Chinnadayyala SR, Kakoti A, Santhosh M, Goswami P (2014) A novel amperometric alcohol biosensor developed in a 3rd generation bioelectrode platform using peroxidase coupled ferrocene activated alcohol oxidase as biorecognition system. Biosens Bioelectron 55:120–126
Sharifi E, Salimi A, Shams E (2013) Electrocatalytic activity of nickel oxide nanoparticles as mediatorless system for NADH and ethanol sensing at physiological pH solution. Biosens Bioelectron 45:260–2666
Das M, Goswami P (2013) Direct electrochemistry of alcohol oxidase using multiwalled carbon nanotube as electroactive matrix for biosensor application. Bioelectrochemistry 89:19–25
Gao W, Chen Y, Xi J, Lin S, Chen Y, Lin Y, Chen Z (2013) A novel electrochemiluminescence ethanol biosensor based on tris(2,2'-bipyridine) ruthenium(II) and alcohol dehydrogenase immobilized in graphene/bovine serum albumin composite film. Biosens Bioelectron 41:776–782
Li L, Lu H, Deng L (2013) A sensitive NADH and ethanol biosensor based on graphene-Au nanorods nanocomposites. Talanta 113:1–6
Zhang M, Gorski W (2011) Amperometric ethanol biosensors based on chitosan-NAD+-alcohol dehydrogenase films. Electroanalysis 23:1856–1862
Asav E, Akyilmaz E (2010) Preparation and optimization of a bienzymic biosensor based on self-assembled monolayer modified gold electrode for alcohol and glucose detection. Biosens Bioelectron 25:1014–1018
Jia T, Cai Z, Chen X, Lin Z, Huang X, Chen X, Chen G (2009) Electrogenerated chemiluminescence ethanol biosensor based on alcohol dehydrogenase functionalized Ru(bpy)3 2+ doped silica nanoparticles. Biosens Bioelectron 25:263–267
Yang DW, Liu HH (2009) Poly(brilliant cresyl blue)-carbonnanotube modified electrodes for determination of NADH and fabrication of ethanol dehydrogenase-based biosensor. Biosens Bioelectron 25:733–738
Jiang X, Zhu L, Yang D, Mao X, Wu Y (2009) Amperometric ethanol biosensor based on integration of alcohol dehydrogenase with Meldola's blue/ordered mesoporous carbon electrode. Electroanalysis 21:1617–1623
Lee CA, Tsai YC (2009) Preparation of multiwalled carbon nanotube-chitosan-alcohol dehydrogenase nanobiocomposite for amperometric detection of ethanol. Sens Actuators B-Chem 138:518–523
Gouveia-Caridade C, Pauliukaite R, Brett CMA (2008) Development of electrochemical oxidase biosensors based on carbon nanotube-modified carbon film electrodes for glucose and ethanol. Electrochim Acta 53:6732–6739
Luo P, Xie G, Liu Y, Xu H, Deng S, Song F (2008) Electrochemical detection of blood alcohol concentration using a disposable biosensor based on screen-printed electrode modified with nafion and gold nanoparticles. Clin Chem Lab Med 46:1641–1647
Manso J, Mena M, Yanez-Sedeno P, Pingarron JM (2008) Alcohol dehydrogenase amperometric biosensor based on a colloidal gold-carbon nanotubes composite electrode. Electrochim Acta 53:4007–4012
Choi HN, Lyu YK, Han JH, Lee WY (2007) Amperometric ethanol biosensor based on carbon nanotubes dispersed in sol-gel-derived titania-nafion composite film. Electroanalysis 19:1524–1530
Du P, Liu S, Wu P, Cai C (2007) Single-walled carbon nanotubes functionalized with poly(nile blue A) and their application to dehydrogenase-based biosensors. Electrochim Acta 53:1811–1823
Liu S, Cai C (2007) Immobilization and characterization of alcohol dehydrogenase on single-walled carbon nanotubes and its application in sensing ethanol. J Electroanal Chem 602:103–114
Tsai YC, Huang JD, Chiu CC (2007) Amperometric ethanol biosensor based on poly(vinyl alcohol)–multiwalled carbon nanotube-alcohol dehydrogenase biocomposite. Biosens Bioelectron 22:3051–3056
Zhang L, Xu Z, Sun X, Dong S (2007) A novel alcohol dehydrogenase biosensor based on solid-state electrogenerated chemiluminescence by assembling dehydrogenase to Ru(bpy)3 2+-Au nanoparticles aggregates. Biosens Bioelectron 22:1097–1100
Wu L, McIntosh M, Zhang X, Ju H (2007) Amperometric sensor for ethanol based on one-step electropolymerization of thionine-carbon nanofiber nanocomposite containing alcohol oxidase. Talanta 74:387–392
Liaw HW, Chen JM, Tsai YC (2006) Development of an amperometric ethanol biosensor based on a multiwalled carbon nanotube-nafion-alcohol dehydrogenase nanobiocomposite. J Nanosci Nanotechnol 6:2396–2402
Carelli D, Centonze D, De Giglio A, Quinto M, Zambonin PG (2006) An interference-free first generation alcohol biosensor based on a gold electrode modified by an overoxidised non-conducting polypyrrole film. Anal Chim Acta 565:27–35
Santos AS, Cesar Pereira A, Duran N, Kubota LT (2006) Amperometric biosensor for ethanol based on co-immobilization of alcohol dehydrogenase and Meldola’s Blue on multi-wall carbon nanotube. Electrochim Acta 52:215–220
Smutok O, Ngounou B, Pavlishko H, Gayda G, Gonchar M, Schuhmann W (2006) A reagentless bienzyme amperometric biosensor based on alcohol oxidase/peroxidase and an Os-complex modified electrodeposition paint. Sens Actuators B-Chem 113:590–598
Rohlen DL, Pilas J, Schöning MJ, Selmer T (2017) Development of an amperometric biosensor platform for the combined determination of L-malic, fumaric, and L-aspartic acid. Appl Biochem Biotechnol 183:566–581
Liang J, Chen Z, Guo L, Li L (2011) Electrochemical sensing of L-histidine based on structure-switching DNAzymes and gold nanoparticle-graphene nanosheet composites. Chem Commun 47:5476–5478
Li LD, Chen ZB, Zhao HT, Guo L (2011) Electrochemical real-time detection of l-histidine via self-cleavage of DNAzymes. Biosens Bioelectron 26:2781–2785
Chen Q, Sun T, Song X, Ran Q, Yu C, Yang J, Feng H, Yu L, Wei D (2017) Flexible electrochemical biosensors based on graphene nanowalls for the real-time measurement of lactate. Nanotechnology 28:1–8
Chan D, Barsan MM, Korpan Y, Brett CMA (2017) L-lactate selective impedimetric bienzymatic biosensor based on lactate dehydrogenase and pyruvate oxidase. Electrochim Acta 231:209–215
Dagar K, Pundir CS (2017) An improved amperometric L-lactate biosensor based on covalent immobilization of microbial lactate oxidase onto carboxylated multiwalled carbon nanotubes/copper nanoparticles/polyaniline modified pencil graphite electrode. Enzyme Microb Technol 96:177–186
Hernández-Ibáñez N, García-Cruz L, Montiel V, Foster CW, Banks CE, Iniesta J (2016) Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens Bioelectron 77:1168–1174
Hickey DP, Reid RC, Milton RD, Minteer SD (2016) A self-powered amperometric lactate biosensor based on lactate oxidase immobilized in dimethylferrocene-modified LPEI. Biosens Bioelectron 77:26–31
Jiang D, Chu Z, Peng J, Jin W (2016) Screen-printed biosensor chips with Prussian blue nanocubes for the detection of physiological analytes. Sens Actuators B-Chem 228:679–687
Kacar C, Ozturk F, Erden PE, Kilic E (2016) Magnetite nanoparticles and Prussian blue modified amperometric biosensor for lactic acid determination. Nisan 42:17–29
Tu D, He Y, Rong Y, Wang Y, Li G (2016) Disposable L-lactate biosensor based on a screen-printed carbon electrode enhanced by graphene. Meas Sci Technol 27:1–8
Briones M, Casero E, Vazquez L, Pariente F, Lorenzo E, Petit-Domíngue MD (2016) Diamond nanoparticles as a way to improve electron transfer in sol-gel L-lactate biosensing platforms. Anal Chim Acta 908:141–149
Sun C, Wang D, Zhang M, Ni Y, Shen X, Song Y, Geng Z, Xu W, Liu F, Mao C (2015) Novel L-lactic acid biosensors based on conducting polypyrrole-block copolymer nanoparticles. Analyst 140:797–802
Azzouzi S, Rotariu L, Benito AM, Maser WK, Ali MB, Bala C (2015) A novel amperometric biosensor based on gold nanoparticles anchored on reduced graphene oxide for sensitive detection of L-lactate tumor biomarker. Biosens Bioelectron 69:280–286
Briones M, Casero E, Petit-Domínguez MD, Ruiz MA, Parra-Alfambra AM, Pariente F, Lorenzo E, Vázquez L (2015) Diamond nanoparticles based biosensors for efficient glucose and lactate determination. Biosens Bioelectron 68:521–528
Celic AC, Ozturk F, Erden PE, Kacar C, Kilic E (2015) Amperometric lactate biosensor based on carbon paste electrode modified with benzo[c]cinnoline and multiwalled carbon nanotubes. Electroanalysis 27:2820–2828
Loaiza OA, Lamas-Ardisana PJ, Añorga L, Jubete E, Ruiz V, Borghei M, Cabañero G, Grande HJ (2015) Graphitized carbon nanofiber-Pt nanoparticle hybrids as sensitive tool for preparation of screen printing biosensors. Detection of lactate in wines and ciders. Bioelectrochemistry 101:58–65
Pagán M, Suazo D, del Toro N, Griebenow K (2015) A comparative study of different protein immobilization methods for the construction of an efficient nano-structured lactate oxidase-SWCNT-biosensor. Biosens Bioelectron 64:138–146
Casero E, Alonso C, Petit-Domínguez MD, Vázquez L, Parra-Alfambra AM, Merino P, Álvarez-García S, de Andrés A, Suárez E, Pariente F, Lorenzo E (2014) Lactate biosensor based on a bionanocomposite composed of titanium oxide nanoparticles, photocatalytically reduced graphene, and lactate oxidase. Microchim Acta 181:79–87
Lamas-Ardisana PJ, Loaiza OA, Añorga L, Jubete E, Borghei M, Ruiz V, Ochoteco E, Cabañero G (2014) Disposable amperometric biosensor based on lactate oxidase immobilised on platinum nanoparticle-decorated carbon nanofiber and poly(diallyldimethylammoniumchloride) films. Biosens Bioelectron 56:345–351
Nesakumar N, Thandavan K, Sethuraman S, Krishnan UM, Rayappan JBB (2014) An electrochemical biosensor with nanointerface for lactate detection based on lactate dehydrogenase immobilized on zinc oxide nanorods. J Colloid Interface Sci 414:90–96
Zhao Y, Yan X, Kang Z, Fang X, Zheng X, Zhao L, Du H, Zhang Y (2014) Zinc oxide nanowires-based electrochemical biosensor for L-lactic acid amperometric detection. J Nanopart Res 16:2398
Casero E, Alonso A, Vazquez L, Petit-Dominguez MD, Parra-Alfambra AM, de la Fuente M, Merino P, Alvarez-Garcia S, de Andres A, Pariente F, Lorenzo E (2013) Comparative response of biosensing platforms based on synthesized graphene oxide and electrochemically reduced graphene. Electroanalysis 25:154–165
Jia W, Bandodkar AJ, Valdés-Ramírez G, Windmiller JR, Yang Z, Ramírez J, Chan G, Wang J (2013) Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal Chem 85:6553–6560
Labroo P, Cui Y (2013) Flexible graphene bio-nanosensor for lactate. Biosens Bioelectron 41:852–856
Nesakumar N, Sethuraman S, Krishnan UM, Rayappan JBB (2013) Fabrication of lactate biosensor based on lactate dehydrogenase immobilized on cerium oxide nanoparticles. J Colloid Interface Sci 410:158–164
Yu Y, Yang Y, Gu H, Zhou T, Shi G (2013) Size-tunable Pt nanoparticles assembled on functionalized ordered mesoporous carbon for the simultaneous and on-line detection of glucose and L-lactate in brain microdialysate. Biosens Bioelectron 41:511–518
Zanini VIP, Tulli F, Martino DM, de Mishima BL, Borsarelli CD (2013) Improvement of the amperometric response to L-lactate by using a cationic bioinspired thymine polycation in a bioelectrode with immobilized lactate oxidase. Sens Actuators B-Chem 181:251–258
Gamero M, Sosna M, Pariente F, Lorenzo E, Bartlett PN, Alonso C (2012) Influence of macroporous gold support and its functionalization on lactate oxidase-based biosensors response. Talanta 94:328–334
Ibupoto ZH, Shah SMUA, Khun K, Willander M (2012) Electrochemical L-lactic acid sensor based on immobilized ZnO nanorods with lactate oxidase. Sensors 12:2456–2466
Lei Y, Luo N, Yan X, Zhao Y, Zhang G, Zhang Y (2012) A highly sensitive electrochemical biosensor based on zinc oxide nanotetrapods for L-lactic acid detection. Nanoscale 4:3438–3443
Qin C, Chen C, Xie Q, Wang L, He X, Huang Y, Zhou Y, Xie F, Yang D, Yao S (2012) Amperometric enzyme electrodes of glucose and lactate based on poly(diallyldimethylammonium)-alginate-metal ion-enzyme biocomposites. Anal Chim Acta 720:49–56
Perez S, Sanchez S, Fabregas E (2012) Enzymatic strategies to construct L-lactate biosensors based on polysulfone/carbon nanotubes membranes. Electroanalysis 24:967–974
Shakir I, Shahid M, Yang HW, Cherevko S, Chung CH, Kang DJ (2012) α-MoO3 nanowire-based amperometric biosensor for L-lactate detection. J Solid State Electrochem 16:2197–2201
Shimomura T, Sumiya T, Ono M, Ito T, Hanaoka TA (2012) Amperometric L-lactate biosensor based on screen-printed carbon electrode containing cobalt phthalocyanine, coated with lactate oxidase-mesoporous silica conjugate layer. Anal Chim Acta 714:114–120
Teymourian H, Salimi A, Hallaj R (2012) Low potential detection of NADH based on Fe3O4 nanoparticles/multiwalled carbon nanotubes composite: Fabrication of integrated dehydrogenase-based lactate biosensor. Biosens Bioelectron 33:60–68
Haghighi B, Bozorgzadeh S (2011) Fabrication of a highly sensitive electrochemiluminescence lactate biosensor using ZnO nanoparticles decorated multiwalled carbon nanotubes. Talanta 85:2189–2193
Goran JM, Lyon JL, Stevenson KJ (2011) Amperometric detection of L-lactate using nitrogen-doped carbon nanotubes modified with lactate oxidase. Anal Chem 83:8123–8129
Parra-Alfambra AM, Casero E, Petit-Dominguez MD, Barbadillo M, Pariente F, Vazquez L, Lorenzo E (2011) New nanostructured electrochemical biosensors based on three-dimensional (3-mercaptopropyl)-trimethoxysilane network. Analyst 136:340–347
Wang YT, Yu L, Wang J, Lou L, Du WJ, Zhu ZQ, Peng H, Zhu JZ (2011) A novel L-lactate sensor based on enzyme electrode modified with ZnO nanoparticles and multiwall carbon nanotubes. J Electroanal Chem 661:8–12
Gamero M, Pariente F, Lorenzo E, Alonso C (2010) Nanostructured rough gold electrodes for the development of lactate oxidase-based biosensors. Biosens Bioelectron 25:2038–2044
Korneyeva LC, Borisova AV, Yashina YI, Karyakina EE, Voronin OG, Cosnier S, Karyakin AA (2010) Electrochemical polymerization of N-substituted pyrrols for the development of novel lactate biosensor. Moscow University Chem Bull 65:49–55
Piano M, Serban S, Biddle N, Pittson R, Drago CA, Hart JP (2010) Amperometric lactate biosensor for flow injection analysis based on a screen-printed carbon electrode containing Meldola’s Blue-Reinecke salt, coated with lactate dehydrogenase and NAD+. Talanta 82:34–37
Romero MR, Ahumada F, Garay F, Baruzzi AM (2010) Amperometric biosensor for direct blood lactate detection. Anal Chem 82:5568–5572
Rahman MM, Shiddiky MJA, Rahman MA, Shim YB (2009) A lactate biosensor based on lactate dehydrogenase/nictotinamide adenine dinucleotide (oxidized form) immobilized on a conducting polymer/multiwall carbon nanotube composite film. Anal Biochem 384:159–165
Rawson FJ, Purcell WM, Xu J, Pemberton RM, Fielden PR, Biddle N, Hart JP (2009) A microband lactate biosensor fabricated using a water-based screen-printed carbon ink. Talanta 77:1149–1154
Liu CX, Liu HM, Yang QD, Qing T, Cai XX (2009) Development of amperometric lactate biosensor modified with Pt-black nanoparticles for rapid assay. Chin J Anal Chem 37:624–628
Huang J, Li J, Yang Y, Wang X, Wu B, Anzai J, Osa T, Chen Q (2008) Development of an amperometric L-lactate biosensor based on L-lactate oxidase immobilized through silica sol-gel film on multi-walled carbon nanotubes/platinum nanoparticle modified glassy carbon electrode. Mater Sci Eng C 28:1070–1075
Yang M, Wang J, Li H, Zheng JG, Wu NN (2008) A lactate electrochemical biosensor with a titanate nanotube as direct electron transfer promoter. Nanotechnology 19:1–6
Cui X, Li CM, Zang J, Yu S (2007) Highly sensitive lactate biosensor by engineering chitosan/PVI-Os/CNT/LOD network nanocomposite. Biosens Bioelectron 22:3288–3292
Romero MR, Garay F, Baruzzi AM (2008) Design and optimization of a lactate amperometric biosensor based on lactate oxidase cross-linked with polymeric matrixes. Sens Actuators B-Chem 131:590–595
Huang J, Song Z, Li J, Yang Y, Shi H, Wu B, Anzai J, Osa T, Chen Q (2007) A highly-sensitive L-lactate biosensor based on sol-gel film combined with multi-walled carbon nanotubes (MWCNTs) modified electrode. Mater Sci Eng C 27:29–34
Jena BK, Raj CR (2007) Amperometric L-lactate biosensor based on gold nanoparticles. Electroanalysis 19:816–822
Lupu A, Valsesia A, Bretagnol F, Colpo P, Rossi F (2007) Development of a potentiometric biosensor based on nanostructured surface for lactate determination. Sens Actuators B-Chem 127:606–612
Pereira AC, Aguiar MR, Kisner A, Macedo DV, Kubota LT (2007) Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola blue coimmobilized on multi-wall carbon-nanotube. Sens Actuators B-Chem 124:269–276
Parra A, Casero E, Vazquez L, Jin J, Pariente F, Lorenzo E (2006) Microscopic and voltammetric characterization of bioanalytical platforms based on lactate oxidase. Langmuir 22:5443–5450
Wang K, Xu JJ, Chen HY (2006) Biocomposite of cobalt phthalocyanine and lactate oxidase for lactate biosensing with MnO2 nanoparticles as an eliminator of ascorbic acid interference. Sens Actuators B-Chem 114:1052–1058
Suman S, Singhal R, Sharma AL, Malthotra BD, Pundir CS (2005) Development of a lactate biosensor based on conducting copolymer bound lactate oxidase. Sens Actuators B-Chem 107:768–772
Naghib SM, Rabiee M, Omidinia E (2014) Electrochemical biosensor for L-phenylalanine based on a gold electrode modified with graphene oxide nanosheets and chitosan. Int J Electrochem Sci 9:2341–2353
Omidinia E, Shadjou N, Hasanzadeh M (2014) Electrochemical nanobiosensing of phenylalanine using phenylalanine dehydrogenase incorporated on amino-functionalized mobile crystalline material-41. IEEE Sensors J 14:1081–1088
Hasanzadeh M, Hassanpour S, Nahr AS, Shadjou N, Mokhtarzadeh A, Mohammadi J (2017) Proline dehydrogenase-entrapped mesoporous magnetic silica nanomaterial for electrochemical biosensing of L-proline in biological fluids. Enzyme Microb Technol 105:64–76
Wei J, Qiu J, Li L, Ren L, Zhang X, Chaudhuri J, Wang S (2012) A reduced graphene oxide based electrochemical biosensor for tyrosine detection. Nanotechnology 23:1–7
Ma Q, Ai S, Yin H, Chen Q, Tang T (2010) Towards the conception of an amperometric sensor of L-tyrosine based on Hemin/PAMAM/MWCNT modified glassy carbon electrode. Electrochim Acta 55:6687–6694
Cheng H, Chen C, Zhang S (2009) Electrochemical behavior and sensitive determination of L-tyrosine with a gold nanoparticles modified glassy carbon electrode. Anal Sci 25:1221–1225
Li C (2006) Voltammetric determination of tyrosine based on an L-serine polymer film electrode. Colloid Surf B: Biointerface 50:147–151
Acknowledgements
We greatly appreciate the support of this work by Research Councils of Nuclear Science and Technology Research Institute and Razi University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Farzin, L., Shamsipur, M., Samandari, L. et al. Advances in the design of nanomaterial-based electrochemical affinity and enzymatic biosensors for metabolic biomarkers: A review. Microchim Acta 185, 276 (2018). https://doi.org/10.1007/s00604-018-2820-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2820-8