Abstract
The authors describe an antibody-aptamer based hetero-sandwich amperometric biosensor for the foodborne pathogen Vibrio parahaemolyticus. Antibody on the surface of a gold electrode first captures the target pathogen, and then the immunocomplex is incubated with an aptamer consisting of an ssDNA probe. A hetero-sandwich structure is formed which allows for signal enhancement by rolling circle amplification. Following addition of Methylene Blue as an electrochemical DNA probe, the amperometric signal, best measured at 0.28 V (vs. Ag/AgCl), covers the 2.2 to 2.2*108 cfu per mL concentration range, and the limit of detection is as low as 2 cfu per mL. The method was successfully applied to the quantification of V. parahaemolyticus in spiked fish samples. In our perception, this hetero-sandwich electrochemical biosensor provides an ultrasensitive tool for the quantitation of a variety of pathogen if appropriate antibodies and aptamers are available.

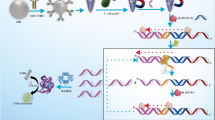
Schematic image of signal amplified hetero-sandwich electrochemical biosensor for Vibrio parahaemolyticus detection. Rolling circular amplification was adopted as the signal amplification strategy on the hetero-sandwich electrochemical biosensor for sensitive and accurate detection of Vibrio parahaemolyticus by simultaneous using of antibody and aptamer as the recognition probes. Excellent improvement of sensitivity and satisfied results were achieved for Vibrio parahaemolyticus in food samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of pathogen microorganisms, such as Escherichia coli, Salmonella, Listeria, Vibrio and Staphylococcus aureus in foods can be serious threatens to public health [1, 2]. V. parahaemolyticus is a Gram-negative halophilic microorganism and a major food-borne pathogen that causes diarrheal illness in humans through consumption of various seafood throughout the world [3,4,5]. Undoubtedly, effective and rapid detection, identification and quantification of microbial pathogens including V. parahaemolyticus are crucial for food safety and human wellness.
Common and traditional diagnostic and surveillance methods including the culture-based methods, enzyme-linked immunosorbent assays (ELISA), and molecular biological methods have been widely used [6,7,8]; and such methods meet or partially meet the urgent needs for sensitive and specific detection of V. parahaemolyticus. Unfortunately, limitations of these traditional and molecular methods, including the labor-intensive and time-consuming properties still hamper their applications [9]. The inability to differentiate viable and dead microorganisms and the false-positive-prone results of molecular and biological methods require additional means to complete the detection process. Also, molecular methods base on the detection of specific genetic markers of pathogens meticulously require specialized instrumentation and highly trained personnel [10]. Finally, despite the immuno methods have been widely used, the availability of antibody and the sensitivity of these methods indicate a need for rapid and sensitive assay protocols for foodborne pathogen detection as well.
Aptamers are artificial nucleic acids or peptides screened by systematic evolution of ligands by exponential enrichment (SELEX) [11,12,13], which can bind to their molecular targets with high affinity and specificity, similar to antibodies. Aptamers exhibit distinct advantages over antibodies such as easy large-scale preparation, simple chemical modification, low cost, target versatility and thermal stability [14, 15]. Aptamers have been used for the detection of microbes including L. acidophilus [16], S. aureus [17], V. parahaemolyticus [18], S. typhimurium [19], and S. dysenteriae [20, 21]. To remedy the hurdle raised due to the scarcity of antibody and to improve the sensitivity and specificity of the detection scheme, aptamers to V. parahaemolyticus were adopted as one of the recognition probes for signal reporting.
In addition, to improve the sensitivity of the detection methods, RCA can be adopted for signal amplification [22, 23]. RCA is an isothermal amplification technology that generates long ssDNA with repetitive sequence units complementary to the circle template. This technology possesses the advantages as a rapid and isothermal amplification process without requiring special laboratory instruments, which makes it a useful and economic tool over other amplification methodologies. Zhou et al. have constructed an aptamer-based RCA platform for electrochemical detection of protein [24]. In addition, Bi et al. developed a chemiluminescence imaging array for detection of cancer cells by using dual-aptamer recognition and bio bar-code nano-probe-based RCA [25]. Also, our group has adopted a similar approach by using aptamer-based probe and RCA for signal enhancement [23]. Together with RCA, protocols based on gold nanoparticles (AuNPs) labeling have also been extensively used as ideal candidates for both the electron transfer and probe immobilization substrate due to their excellent affinity with biomolecules, large surface/volume ratio and good conductivity [26].
In this research, we present a hetero-sandwich recognition model based aptamer/immuno hybrid electrochemical biosensor for rapid and sensitive detection of V. parahaemolyticus with RCA as the signal enhancement strategy. The immobilized antibody on surface of the electrode acts as the capture probe of V. parahaemolyticus while the designed aptamers serve as the signal reporting probe. Aptamer induced RCA products produce extraordinary electrochemical sensing currents for ultrasensitive and accurate detection of V. parahaemolyticus. Additionally, the aptamer/immuno hybrid electrochemical biosensor was successfully employed to evaluate its applicability in the determination of V. parahaemolyticus in food samples.
Experimental section
Chemicals and materials
All the oligonucleotides were synthesized and purified by Sangon Inc., (http://www.sangon.com/, Shanghai, China). Phi29 DNA polymerase, T4 DNA ligase, dNTPs and cysteamine hydrochloride were also ordered from Sangon Inc. Gold (III) chloride trihydrate (HAuCl4·3H2O) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich, (www.sigmaaldrich.com, St. Louis, MO, USA). Sodium citrate dehydrate (C6H5Na3O7·2H2O) was obtained from Alfa Aesar, USA. 1-ethyl3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were from Thermo Scientific (https://www.thermofisher.com, USA). Antibody against V. parahaemolyticus and V. parahaemolyticus ATCC 17802 were both purchased from Prajna Biology Tech. Ltd. (http://www.sangon.com/, Shanghai, China). V. parahaemolyticus ATCC 17802 samples were supplied by Jiangsu Entry-Exit Inspection and Quarantine Bureau (Nanjing, China). Typically, V. parahaemolyticus ATCC 17802 was cultured in alkaline peptone with 3% (w/v) NaCl overnight past the logarithmic phase. Then, 100 μL bacteria was diluted with medium and coated on the agar plates and culture at 37 for 24 h to determine the colony-forming units (cfu mL−1). V. parahaemolyticus ATCC 17802 solutions at different concentrations was used as received without further cultivation. The detailed sequences of the ssDNA probes used in this research are as follows:
-
Vp-aptamer included primer: 5′-TCT AAA AAT GGG CAA AGA AAC AGT GAC TCG TTG AGA TAC TAA AAA AAA ACA GGG CTG GGC ATA GAA GTC AGG GCA GA-3′ (under lined sequence is the aptamer sequence of Vp);
-
circle template: 5′-P-TAT GCC CAG CCC TGT AAG ATG AAG ATA GCG CAG AAT GGT CGG ATT CTC AAC TCG TAT CTG CCC TGA CTT C-3′;
-
labeling ssDNA probe (ssDNA): 5′-SH-(CH2)6-GCG CAG AAT GGT-3′;
-
complementary ssDNA probe (csDNA):5′-ACC ATT CTG CGC-3′.
All other chemicals were of analytical grade and used as received without further purifications. The water used in the experiments was deionized water. The antibody to V. parahaemolyticus was diluted with 0.1 M phosphate-buffered saline (PBS). V. parahaemolyticus powder was dissolved in the binding buffer of 50 mM Tris-HCl buffer (pH 7.4) including 100 mM NaCl, 5 mM KCl, and 1 mM MgCl2.
Cyclic voltammetry (CV), differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS) were conducted with a CHI 650D electrochemical workstation (Chen Hua CH Instruments, China), which contains a conventional three-electrode system: platinum wire as auxiliary, Ag/AgCl electrode as the reference, and a 3 mm-diameter gold electrode as the working electrode. The morphology of Au nanoparticles was tracked by a transmission electron microscope (TEM) (JEOL2100 HR, JEOL Instrument, Japan).
Preparation of AuNPs and AuNP-detection probe (AuNP-ssDNA) conjugates
Gold nanoparticles with the uniform diameters were prepared based on our previously reported method [26, 27]. Briefly, 850 μL of 5 g L−1 HAuCl4 solution was added into 60 mL of ultrapure water, and then heated to boiling under magnetic stirring. After quickly injecting 850 μL of 1% trisodium citrate solution, the mixture was stirred for 10 min until the solution became wine red and kept stirring for another 5 min. After gradual cooling to room temperature, the colloidal Au NPs with the size of 15 nm was stored in a refrigerator at 4 °C until needed. For preparation of AuNP-ssDNA conjugates, the disulfide bond of the ssDNA was initially reduced by incubating with 15 μL of 5 μM aptamer with 5 μL of 1 mM TCEP and 3 mL of acetate buffer for 1 h in the dark at room temperature. Then, 100 μL of AuNPs solution was added to the activated aptamers and kept for 1 h for assembling the aptamer onto the surface of AuNPs. Twenty μL of 100 μM dATP was added to the mixture and reacted for 30 min, followed by adding of 20 μL of NaCl (0.1 M) solution. Afterward, the AuNP-ssDNA conjugates were centrifuged at 10000 g for 7 min and washed twice with suspension buffer. The sediment was suspended in 100 μL of suspension buffer and ready for use or stored at 4 °C.
Preparation of circularization mixture and RCA
RCA was carried out based on our previous studies with few modifications [23]. Briefly, 10 μL of phosphorylated padlock DNA probe (5 μM) was mixed with 10 μL of aptamers (5 μM). Then, 2 μL of PEG [50%(w/v)) polyethylene glycol 4000] and 10 μL of 1× ligation buffer [40 mM Tris-HCl, 10 mM MgCl2, 10 mM dithiothreitol (DTT), and 0.5 mM pH 7.8 ATP at 25 °C] were added into the above mentioned mixture, and incubated at 37 °C for 1 h. Ligation was performed by adding 3 μL of T4 DNA ligase (5 U per μL) to the mixture and incubating at 22 °C for 1 h. Then, the T4 ligase was inactivated by heating at 65 °C for 10 min. The resulting mixture was ready for use or stored at 4 °C.
Fabrication of the sensing interface of the electrochemical biosensor
Gold electrode was polished to mirror-like surface with wet alumina power (0.1 and 0.03 μm respectively) and rinsed thoroughly with DI water. Then, the polished electrode was successively cleaned ultrasonically in ethanol and DI water, followed by immersing it in a piranha solution [concentrated sulfuric acid to 30% hydrogen peroxide (3:1 volume ratio)] for 5 min and rinsed with DI water. Then, the electrode was electrochemically cleaned by performing CV in 0.5 M H2SO4 solution between −0.3 V and 1.5 V until the curve was stable. The electrode was rinsed again and ready for use.
The cleaned gold electrode was placed into a 30 mM cystamine dihydrochloride solution overnight and then rinsed with PBS to remove physically adsorbed dithiols followed by reacting with 2.5% glutaraldehyde aqueous solution at 37 °C for 1 h. Then the electrode was incubated with 10 μL of antibody (Ab) solution (0.05 μg μL−1) in PBS (pH 7.4) at 37 °C for 1 h in a humidity chamber. After sufficiently rinsing with PBS, the electrode was dipped into a 0.1% BSA solution at 37 °C for 30 min to block the remaining adsorption reactive sites and to avoid the nonspecific adsorption effect during the sensing process.
The antibody modified electrode was incubated with the target pathogen V. parahaemolyticus at 37 °C for 0.5 h and thoroughly rinsed with PBS. In order to investigate the concentration-dependent V. parahaemolyticus sensing, the modified electrode was incubated with different concentrations of bacteria (10-time serial dilution 2.2 × 100–108 cfu mL−1) in borate buffer (pH 7.4) at 37 °C for 0.5 h followed by PBS rinsing. The reference bacterial strains used as controls were also processed in parallel with V. parahaemolyticus to test the specificity of the assay.
Then, the electrode was placed into an aptamer-included primer solution at room temperature for 45 min and washed for subsequent RCA. For the RCA process, basically, it was followed our previous studies [22]. Briefly, 1.5 μL of Phi29 DNA polymerase (10 U μL−1), 5 μL of dNTPs (10 mM) and 10 μL of 1× Phi29 buffer [33 mM Tris-HCl (pH 7.9), 10 mM MgCl2, 66 mM KCl, 0.1%(v/v) Tween 20, and 1 mM DTT] were sequentially added into the polymerization mixture. The polymerization reaction was carried out at 37 °C for 1 h. Finally, the detection probe (DNA-AuNP) was added to the mixture to hybridize with the RCA products at 37 °C for 30 min. Then, csDNA probe at 0.5 μM was added and hybridized with labeling ssDNA probe on the surface of AuNPs at 37 °C for 30 min to form the numerous double stranded DNA structure for MB labeling. After hybridization, the electrode was cultured in MB solution for 5 min for electrochemical measurement. After washing, the electrochemical signal was measured in 0.1 M PBS as the working buffer including 0.1 M Na2HPO4, 0.1 M NaH2PO4, 2 mM MgCl2. 6H2O, and10 mM KCl (0.1 M, pH 7.4) by using DPV (−0.6 V to 0.1 V) with 4 mV of potential incremental, 50 mV of amplitude, 0.05 s of pulse width, and 0.0167 s of sample width.
Results and discussions
Design of hetero-sandwich aptamer/immuno hybrid electrochemical biosensor
Our detection protocol initially is designed with two types of recognition probes targeting V. parahaemolyticus as shown in Scheme 1. It depicts the complete detection process and the sensing principle of this hetero-sandwich based electrochemical biosensor. First, the Ab is immobilized onto the electrode chemically, and the functionalized electrode acts as the capture probe for V. parahaemolyticus. Second, the aptamer probe of V. parahaemolyticus recognizes and binds to V. parahaemolyticus to form a HSS. The reasons for using Ab and aptamers as the capture probe and signal reporting probe are as follows: i) using Ab as the capture probe on the electrode can minimize the background current signal generated from the nonspecific adsorption between methylene blue (MB) and DNA probe; ii) using aptamer as the signal reporting probe provides the base for further signal amplification by in vitro isothermal nucleic amplification protocol, e.g., RCA was adopted to increase the sensitivity; and iii) using aptamer enables the gold nanoparticle-ssDNA conjugates to label the RCA products, so that the amplified electrochemical signal of MB can be measured for quantitative detection of V. parahaemolyticus. Of note, due to the duplex DNA formed as the amplification products, MB is adopted as the electrochemical redox probe for its prior affinity to dsDNA. Thus, the hetero-sandwich recognition model based signal amplification protocol provides a novel sensing platform for ultrasensitive and accurate detection of V. parahaemolyticus.
Characterization of the development and optimization of the electrochemical aptasensor for V. parahaemolyticus detection
The RCA-based signal amplification strategy is confirmed before the development of hetero-sandwich electrochemical aptasensor for V. parahaemolyticus detection. In comparison of the images in the dark and bright fields of the agarose gel electrophoresis, it is clearly demonstrated that RCA was successful, which can be verified by the presence of the product of high molecular weight in lane 3 in the dark field of the gel (Fig. 1a); the RCA products are well labeled with the Au-ssDNA conjugates in lane 4 in the bright field of the gel (Fig. 1b). Also, the RCA products and the product labeling are confirmed by the TEM results (Fig. 1c-e). A critical assessment is made from the TEM results as shown in Fig. 1e that AuNPs with the diameter of about 18 ± 3 nm (Fig. 1c) are well assembled into a linear structure in high density after the hybridization induced labeling with the Au-ssDNA conjugates (Fig. 1d), which greatly increases the amount of the electrochemical redox tag of MB, and thus improves the electrochemical sensing signal. Due to the macro gold electrode adopted, therefore, SEM is not applied for direct characterization of electrode surface. Besides, the RCA time is also considered and 60 min is adopted as the optimal time for amplification (See details in Fig. S3). Consequentially, excellent signal amplification is achieved for V. parahaemolyticus detection, which is the core mechanism for the signal amplification in this detection scheme.
The confirmation results of using RCA for signal amplification. The results of the RCA products in the dark field (a) and bright field (b) of an agarose gel electrophoresis; (c), the TEM result of AuNPs; (d), the TEM result of the detection probe-AuNPs conjugates; (e), the TEM result of the RCA products labeled with AuNPs; (f), DPV results of RCA based signal amplification
The step-by-step modification of the sensing interface of the electrode is characterized by EIS and CV tests, respectively. Compared with the bare electrode (curve a in Fig. 2), the Ab immobilized electrode shows a larger electron transfer impedance Ret (curve b) due to the poor conductivity of antibody. Similarly, blocking with BSA and recognition of V. parahaemolyticus leads to an increase of Ret due to the insulativity of protein (curves c and d). Meanwhile, the amount of the aptamer-included DNA probe, the recognition time of antibody, the incubation time of MB labeling are also confirmed to get the best sensing results. And 1.4 M of aptamer-included DNA probe, 15 min of antibody recognition and 5 min for MB labeling are adopted as the optimal conditions for the final determination (See detailed results in Fig. S4-S6).
For signal reporting and amplification, the binding of aptamer to the target V. parahaemolyticus further increases the Ret for the negative charge of the DNA (curve e in Fig. 2). Based on this immobilized aptamers, RCA is carried out and the elongated RCA products made the repulsion of [Fe(CN)6]3−/4- stronger and Ret larger (curve f). Finally, both the labeling of RCA products with AuNP-ssDNA conjugate and hybridization of complementary DNA probe onto AuNPs conversely and obviously decreases the Ret (curves g and h in Fig. 2). The reason for this noticeable decrease of Ret is the superior conductivity of AuNPs, which can greatly enhance the transfer of electron. In addition, the CV tests are also performed to characterize the development of the electrode and sensing system. Similar results are achieved as those of EIS (detailed CV results in Fig. S1). Overall, the results of EIS and CV tests well document a successful modification and development of the hetero-sandwich structure based electrochemical aptasensor for sensitive detection of V. parahaemolyticus.
Another important aspect of this research is to improve the sensitivity of the hetero-sandwich recognition electrochemical aptasensor for accurate detection of V. parahaemolyticus and to deepen the insight into the amplified electrochemical signal with RCA. The amplified electrochemical signal with RCA is characterized and the comparison analysis is performed with different settings. In comparison with the blank signal of the bare electrode control (curve A in Fig. 1f), the traditional measuring signal of V. parahaemolyticus without signal amplification treatment (curve B in Fig. 1f) is easily distinguished from the blank one with the characteristic peak at −0.27 V. Of great importance, is the great enhancement in measuring signal of V. parahaemolyticus with RCA (curve C in Fig. 1f) compared with the signal derived from the control (bare electrode) and traditional testing without RCA (curves A and B in Fig. 1f), further demonstrating the extraordinary signal amplification performance of our designed hetero-sandwich aptasensor.
Analytical performance of V. parahaemolyticus with the hetero-sandwich recognition electrochemical aptasensor
Under optimal conditions (detailed optimization results were not shown here), the analytical performance of the our aptasensor is evaluated by measuring different concentrations of V. parahaemolyticus in 0.1 M PBS (pH 7.4) containing 10 mM KCl and 2 mM MgCl2.6H2O. The results in Fig. 3a demonstrate that the characteristic peak currents increases in proportion with the increase of the concentrations of V. parahaemolyticus ranging from 2.2 to 2.2*108 cfu/mL compared the current from the bare electrode control. In addition, a corresponding calibration curve is constructed (Fig. 3b), which showed a good linear relationship between the peak currents and the concentrations of V. parahaemolyticus. Not only is sensing performance of the hetero-sandwich recognition based electrochemical aptasensor excellent, but also the LOD achieved as low as 2 cfu/mL, indicating that V. parahaemolyticus even can be detected at this concentration.
DPV results of different concentrations (10-time serial dilutions from 2.2 to 2.2*108 cfu/mL) of target V. parahaemolyticus (a) and a quantitative calibration curve of peak currents vs. logarithm of the concentrations of V. parahaemolyticus (b); the specificity confirmation results of the hetero-sandwich electrochemical biosensor for V. parahaemolyticus detection (c) the DPV results of the specificity testing and (d) the quantitative results of the specificity testing
To assess the specificity of this hetero-sandwich recognition electrochemical aptasensor for V. parahaemolyticus detection, exclusivity experiments are conducted with reference strains consisting of common foodborne pathogens, S.aureus, Shigella sonnie, E. coli O157:H7, Salmonella, C.jejuni and V. mimicus. The exclusivity test results clearly indicate that only the V. parahaemolyticus samples and the V. parahaemolyticus mixtures induced obvious redox current response (in Fig. 3c and d), whereas all the reference strains did not induce any redox current response. The excellent specificity of the electrochemical aptasensor can be attributed to the designed hetero-sandwich recognition structure which is composed of both antibody and aptamers. The reproducibility of the hetero-sandwich recognition electrochemical aptasensor is further evaluated by using the electrodes from different manufacture batches and a given concentration (2.2*105 cfu per mL) of V. parahaemolyticus. The results show that a variation coefficient (under 4.3%) is achieved among the detection replicates done with the electrodes of different manufacture batches. The stability of the developed hetero-sandwich recognition electrochemical aptasensor is assessed by the long-term storage test. After two-week storage at 4 °C, the fabricated electrochemical aptasensor retains the sensing current response to about 95.2% of its initial level. Therefore, the hetero-sandwich recognition electrochemical aptasensor demonstrates excellent reproducibility and stability, suggesting great potential for practical detection of V. parahaemolyticus in food (Table 1).
Moreover, the suitability of this hetero-sandwich recognition electrochemical aptasensor for foodborne pathogen detection is evaluated with V. parahaemolyticus spiked fish samples. All V. parahaemolyticus samples with known concentrations are all prepared and determined with the classic culture method, which is supplied by the Jiangsu Entry-Exit Inspection Guarantee. Different amount of V. parahaemolyticus are then added into the fish samples and determined with the fabricated electrochemical aptasensor. The detection results are used to assess the accuracy achieved with the fabricated electrochemical aptasensor. The recoveries of V. parahaemolyticus at different spiked concentrations reach from 93.7% to 108.3%, indicating a good accuracy for this hetero-sandwich recognition electrochemical aptasensor in V. parahaemolyticus detection. Besides, five different biosensors are used to determine the same sample at the concentration of and the related peak current are compared. The calculated relative standard deviation was about 4.92%, which reveals the good repeatability of the biosensor. Furthermore, the sensing performance of this amperometric aptamer/immuno hybrid biosensor is compared to other similar reported methods. Results in Table 2 demonstrate that the method reported in this research is comparable to other method in sensing performance and has the superior properties in sensitivity [28,29,30,31,32,33,34,35]. All these research indicate that this aptamer/immuno hybrid biosensor is a useful platform with potential for sensitive and accurate analysis of general food pathogens.
Conclusions
In summary, a hetero-sandwich recognition electrochemical aptasensor was reported for sensitive and accurate detection of V. parahaemolyticus. Both antibody and aptamers to V. parahaemolyticus were adopted as the recognition probes to form an antibody-analyte-aptamer hetero-sandwich structure. The antibody in the hetero-sandwich structure acted as the immobilized probe; while the aptamers served as the signal reporting probe. In addition, to further improve the sensing performance of the strategy, RCA was employed in the system to amplify the current signal response. The data in the present study clearly demonstrated that the sensing performance reached the LOD of 2 cfu•mL−1 in detection, representing a huge improvement. Using both antibody and aptamers in the sensor may have improved the specificity of the strategy; while the adaptation of RCA into the strategy may have increased the sensitivity of the assay. Moreover, we have successfully applied the detection strategy to spiked food samples with satisfactory sensitivity and specificity. This hetero-sandwich recognition electrochemical aptasensor technology not only provides a specific and ultrasensitive protocol for detection of V. parahaemolyticus in food, but also offers a powerful and versatile platform with the potential for rapid and sensitive detection of a variety of pathogens in the clinical diagnosis, food safety, and bio-threat detections. Further research to find a more friend antibody modification protocol and highly simplified fabrication procedures of the sensing electrode is ongoing is our group.
References
Duan N, Wu S, Ma X, Yu X, Wang Z (2014) A universal fluorescent aptasensor based on AccuBlue dye for the detection of pathogenic bacteria. Anal Biochem 454:1–6
Suffredini E, Mioni R, Mazzette R, Bordin P, Serratore P, Fois F, Piano A, Cozzi L, Croci L (2014) Detection and quantification of Vibrio parahaemolyticus in shellfish from Italian production areas. Int J Food Microbiol 184:14–20
Sakata J, Kawatsu K, Iwasaki T, Kumeda Y (2015) Development of a rapid and simple immunochromatographic assay to identify Vibrio parahaemolyticus. J Microbiol Methods 116:23–29
Raghunath P, Acharya S, Bhanumathi A, Karunasagar I, Karunasagar I (2008) Detection and molecular characterization of Vibrio parahaemolyticus isolated from seafood harvested along the southwest coast of India. Food Microbiol 25:824–830
Garrido-Maestu A, Chapela MJ, Peñaranda E, Vieites JM, Cabado AG (2014) In-house validation of novel multiplex real-time PCR gene combination for the simultaneous detection of the main human pathogenic vibrios. Food Control 37:371–379
Rizvi AV, Bej AK (2010) Multiplexed real-time PCR amplification of tlh, tdh and trh genes in Vibrio parahaemolyticus and its rapid detection in shellfish and Gulf of Mexico water. Antonie Van Leeuwenhoek 98:279–290
Pechorsky A, Nitzan Y, Lazarovitch T (2009) Identification of pathogenic bacteria in blood cultures: comparison between conventional and PCR methods. J Microbiol Methods 78:325–330
Juvonen R, Koivula T, Haikara A (2008) Group-specific PCR-RFLP and real-time PCR methods for detection and tentative discrimination of strictly anaerobic beer-spoilage bacteria of the class clostridia. Int J Food Microbiol 125:162–169
Raghunath P, Karunasagar I, Karunasagar I (2009) Improved isolation and detection of pathogenic Vibrio parahaemolyticus from seafood using a new enrichment broth. Int J Food Microbiol 129:200–203
Santiago-Felipe S, Tortajada-Genaro LA, Puchades R, Maquieira A (2014) Recombinase polymerase and enzyme-linked immunosorbent assay as a DNA amplification-detection strategy for food analysis. Anal Chim Acta 811:81–87
Hamula CLA, Zhang HQ, Guan LL, Li XF, Le XC (2008) Selection of aptamers against live bacterial cells. Anal Chem 80:7812–7819
Ellington AD, Szostak JW (1990) In vitro selection RNA molecules bind specific ligands. Nature 346:818–822
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Teng J, Yuan F, Ye YW, Zheng L, Yao L, Xue F, Chen W, Li BG (2016) Aptamer-based Technologies in Foodborne Pathogen Detection. Front Microbiol 7(1426):1–11
Wu JJ, Zhu YY, Xue F, Mei ZL, Yao L, Wang X, Zheng L, Liu J, Liu GD, Peng CF, Chen W (2014) Recent trends in SELEX technique and its application to food safety monitoring. Microchim Acta 181:479–491
Yoo SM, Kim DK, Lee SY (2015) Aptamer-functionalized localized surface plasmon resonance sensor for the multiplexed detection of different bacterial species. Talanta 132:112–117
Wu W, Li J, Pan D, Li J, Song S, Rong M, Li Z, Gao J, Lu J (2014) Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of Salmonella typhimurium. ACS Appl Mater Interfaces 6:16974–16981
Wu S, Duan N, Shi Z, Fang C, Wang Z (2014) Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels. Anal Chem 86:3100–3107
Ma X, Jiang Y, Jia F, Yu Y, Chen J, Wang Z (2014) An aptamer-based electrochemical biosensor for the detection of Salmonella. J Microbiol Methods 98:94–98
Xie Y, Xu L, Wang Y, Shao J, Wang L, Wang H, Qian H, Yao W (2013) Label-free detection of the foodborne pathogens of Enterobacteriaceae by surface-enhanced Raman spectroscopy. Anal Methods 5:946–952
Paniel N, Baudart J, Hayat A, Barthelmebs L (2013) Aptasensor and genousensor methods for detection of microbes in real world samples. Methods 64:229–240
Donolato M, Antunes P, Torre TZ, Hwu ET, Chen CH, Burger R, Rizzi G, Bosco FG, Stromme M, Boisen A, Hansen MF (2015) Quantification of rolling circle amplified DNA using magnetic nanobeads and a Blu-ray optical pick-up unit. Biosens Bioelectron 67:649–655
Huang L, Wu JJ, Zheng L, Qian HS, Xue F, Wu YC, Pan DD, Adeloju SB, Chen W (2013) Rolling chain amplification based signal-enhanced electrochemical aptasensor for ultrasensitive detection of ochratoxin a. Anal Chem 85:10842–10849
Zhou L, LJ O, Chu X, Shen GL, Yu RQ (2007) Aptamer-based rolling circle amplification: a platform for electrochemical detection of protein. Anal Chem 29:7492–7500
Bi S, Ji B, Zhang Z, Zhang S (2013) A chemiluminescence imaging array for the detection of cancer cells by dual-aptamer recognition and bio-bar-code nanoprobe-based rolling circle amplification. Chem Commun 49:3452–3454
Pincella F, Song Y, Ochiai T, Isozaki K, Sakamoto K, Miki K (2014) Square-centimeter-scale 2D-arrays of au@ag core–shell nanoparticles towards practical SERS substrates with enhancement factor of 107. Chem Phys Lett 605-606:115–120
Mei ZL, Qu W, Deng Y, Chu HQ, Cao JX, Xue F, Zheng L, El-Nezamic HS, Wu YC, Chen W (2013) One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol a (BPA) in aqueous samples. Biosens Bioelectron 49:457–461
Liu K, Yan X, Mao B, Wang S, Deng L (2016) Aptamer-based detection of Salmonella enteritidis using double signal amplification by Klenow fragment and dual fluorescence. Microchim Acta 183:63–649
Lan MB, Zhou Q, Zhao YH, Teng YJ, Chen C, Zhao HL, Yuan HH (2010) Electrochemical detection of a Vibrio parahaemolyticus sequence-specific gene based on a gold electrode modified with a single stranded probe. Sci China: Chem 53:1366–1370
Zhao GY, Xing FF, Deng SP (2007) A disposable amperometric enzyme immunosensor for rapid detection of Vibrio parahaemolyticus in food based on agarose/Nano-au membrane and screen-printed electrode Electrochem. Commun 9:1263–1268
Wang XY, Sun DP, Tong YL, Zhong YS, Chen ZG (2017) A voltammetric aptamer-based thrombin biosensor exploiting signal amplification via synergetic catalysis by DNAzyme and enzyme decorated AuPd nanoparticles on a poly(o-phenylenediamine) support. Microchim Acta 184:1791–1799
Rafati A, Gill P (2015) Microfluidic method for rapid turbidimetric detection of the DNA of mycobacterium tuberculosis using loop-mediated isothermal amplification in capillary tubes. Microchim Acta 182(3–4):523–530
Liu S, Leng X, Wang X, Pei Q, Cui X, Wang Y, Huang J (2017) Enzyme-free colorimetric assay for mercury (II) using DNA conjugated to gold nanoparticles and strand displacement amplification. Microchim Acta. doi:10.1007/s00604-017-2182-7
Wang X, Liu W, Yin B, Sang Y, Liu Z, Dai Y, Tao Z (2017) An isothermal strand displacement amplification strategy for nucleic acids using junction forming probes and colorimetric detection. Microchim Acta 184:1603–1610
Yamanaka ES, Tortajada-Genaro LA, Maquieira Á (2017) Low-cost genotyping method based on allele-specific recombinase polymerase amplification and colorimetric microarray detection. Microchim Acta. doi:10.1007/s00604-017-2144-0
Acknowledgements
This work is financially supported by the Science and Technology Ministry of China (2015BAD17B02-3), the NSFC Grant of 21475030, the S&T Research Project of Anhui Province 15czz03109, the National 10000 Talents-Youth Top-notch Talent Program, and the KC Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 540 kb)
Rights and permissions
About this article
Cite this article
Teng, J., Ye, Y., Yao, L. et al. Rolling circle amplification based amperometric aptamer/immuno hybrid biosensor for ultrasensitive detection of Vibrio parahaemolyticus . Microchim Acta 184, 3477–3485 (2017). https://doi.org/10.1007/s00604-017-2383-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2383-0