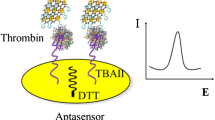
Abstract
A regenerable and ultrasensitive voltammetric biosensor is described for the determination of thrombin. It relies on a combination of (a) enzymatic catalysis, (b) a G-quadruplex/hemin DNAzyme system, and (c) AuPd nanoparticles for signal amplification. Poly(o-phenylenediamine) was decorated with AuPd nanoparticles and loaded with horseradish peroxidase (HRP) and thrombin aptamer (TBA), and the mixture was allowed to interact with hemin to form the G-quadruplex/hemin/HRP/AuPd/poly(o-phenylenediamine) bioconjugates. In the presence of thrombin, the bioconjugates remain immobilized on the surface of the modified glassy carbon electrode through a sandwich reaction. Poly(o-phenylenediamine) also acts as a redox mediator, and the electrochemical reaction of poly(o-phenylenediamine) in the presence of H2O2 is efficiently catalyzed by HRP, AuPd nanoparticles and G-quadruplex/hemin as the peroxidase mimics. Thus, a remarkably amplified electrochemical signal is obtained by the triple catalytic amplification. The biosensor has a dynamic range that spans the 100 f. to 20 nM thrombin concentration range, and the detection limit is 20 fM. The biosensor can be regenerated by applying an electrochemical desorption technique that breaks the gold-thiol bond and releases the components from the surface. In our perception, the mediator-free and signal-amplified biosensor demonstrated here has a large potential with respect to the quantitation of thrombin in clinical samples.

Schematic of the assay: The peroxidase-like AuPd nanoparticles, G-quadruplex/hemin DNAzyme and horseradish peroxidase synergistically catalyze the electrochemical reaction of poly(o-phenylenediamine) in the presence of H2O2, thereby amplifying the electrochemical detection signal of thrombin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thrombin, a type of proteinase, is extremely crucial in life processes such as haemostasis, thrombosis and the blood coagulation cascade [1, 2]. In addition, thrombin has a pronounced proinflammatory characteristic, which may have an impact on the development of atherosclerosis [3]. An abnormal concentration of thrombin in the blood can lead to thrombotic diseases or even death. Therefore, it is important in clinical diagnoses to detect thrombin sensitively and specifically. Many methods, such as fluorescence [4], colorimetry [5], electrochemiluminescence [6], surface plasmon resonance [7], and electrochemistry [8], have been exploited for the detection of thrombin. Compared with other detection methods, electrochemical approaches have become a more promising method due to their advantages of high sensitivity, simplicity, portability and low-cost instrumentation.
The detection sensitivity of a biosensor is expected to be increased through signal amplification [9]. Special attention has been paid to signal amplification strategies, such as the use of nanomaterials [10], the hybridization chain reaction [11], rolling circle amplification [12], etc. Wang’s group constructed an electrochemical biosensor for the detection of glutathione based on signal amplification by the hybridization chain reaction [13]. Wei’s group put forward a non-enzymatic electrochemical sensor for carcinoembryonic antigen using Fe3O4@MnO2@Pt nanocomposites for the signal amplification [10]. Yuan’s group designed an electrochemical aptasensor for mucin1 based on exonuclease-assisted amplification [14]. Among the various amplification strategies, nanomaterials have received a great deal of attention not only because of their large surface area for loading reporter molecules, but also because they have the advantage of distinct electronic and catalytic properties. It is difficult for various nanomaterials, such as metal nanoparticles, magnetic nanoparticles, or graphene, to directly attach to bio-macromolecules after they are used to load redox-active species [15]. This difficulty is an issue for biosensor fabrication. Thus, it is necessary to develop a nanomaterial that can easily load redox-active species and proteins. Therefore, the redox-active polymer has received a great deal of attention in the field of electrochemical biosensor fabrication. O-phenylenediamine is a type of redox-active material, and it can form conjugated polymers by radical polymerization for the structure of a planar aromatic [15]. Poly(o-phenylenediamine), a conjugated polymer of o-phenylenediamine, has been demonstrated to be a functional polymer in many studies [15, 16] due to its homogeneity in electrochemical deposition, positively charged surface and strong adherence to the electrode surface. In addition, poly(o-phenylenediamine) can combine with noble metal nanoparticles due to the presence of amino groups in the polymer. Bimetallic nanoparticles have also attracted a great deal of attention in the applications of various electrochemical biosensors. The bimetallic nanoparticle is an interesting system because it shows a high activity towards many electrochemical reactions and enhances the electrocatalytic oxidation of the substrates. AuPd, a bimetallic nanoparticle, conjugates the merits of AuNP and PdNP. AuPd nanoparticles have shown excellent catalytic performance as mimics of natural peroxidases [17]. G-quadruplex/hemin, a complex of hemin with a guanine-rich single-stranded nucleic acid, can also function as an HRP-mimicking DNAzyme [18]. Chen’s group designed a signal-amplified electrochemical aptasensor via super-sandwich G-quadruplex DNAzyme for cancer cell detection [19].
Herein, an ultrasensitive aptamer-based thrombin biosensor was designed by combining poly(o-phenylenediamine) with G-quadruplex/hemin and HRP immobilized AuPd bioconjugates. It is worthwhile to note that poly(o-phenylenediamine) not only served as a type of electron mediator but also as a support for the AuPd particles. The electroactive polymer poly(o-phenylenediamine) [15, 20] which acted as the electron mediator was first applied to combine the AuPd particles. Then, the hybrid AuPd/poly(o-phenylenediamine) nanocomposites were used to immobilize thiolated TBA and HRP. The conjugated AuPd/poly(o-phenylenediamine) nanocomposites integrated the advantages of the excellent redox-activity and electrocatalytic ability. Then, to form G-quadruplex/hemin structures (which exhibit excellent peroxidase-mimicking properties [21, 22]), the AuPd/poly(o-phenylenediamine) nanocomposites labeled with the HRP and TBA were intercalated with hemin. This resulted in the formation of G-quadruplex/hemin/HRP/AuPd/poly(o-phenylenediamine) hybrid nanocomposites with good electrocatalytic properties and electroactive ability. The peak current of poly(o-phenylenediamine) in the presence of H2O2 was remarkably amplified based on the triplex-amplified system of the biosensor, in which HRP, HRP-mimicking DNAzyme and AuPd can simultaneously amplify the signal response. This electrochemical biosensor shows an outstanding analytical performance for the quantitative determination of thrombin because of the aforementioned amplification factors. Furthermore, the surface of the AuNP/Glassy carbon electrode (GCE) can be regenerated at least once by electrochemical reductive desorption to re-assemble the sensing layer and reuse the biosensor. Additionally, the removal of biomolecules, such as enzymes, from the electrode surfaces may be coupled to a microfluidic device for the automation of the sample analysis in the future [23, 24].
Experimental
Chemicals and materials
Thrombin was purchased from Sigma-Aldrich Chem. Co. (St. Louis, MO, USA, http://www.sigmaaldrich.com/china-mainland.html). O-phenylenediamine (98 wt%), tetrachloroauric acid (HAuCl4), hexadecylpyridinium chloride monohydrate (HDPC), tris-(2-carboxyethyl)-phosphine hydrochloride (TCEP), ascorbic acid (AA), hemin, potassium tetrachloropalladate(II) (K2PdCl4), horseradish peroxidase (HRP) were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China, http://www.aladdin-e.com/). Thiolated thrombin aptamer (SH-TBA): 5′-SH-(CH2)6-GGTTGGTGTGGTTGG-3′ was synthesized and purified from Sangon Biotech. Co., Ltd. (Shanghai, China, http://www.sangon.com/). TE buffer was also purchased from Sangon Biotech. Co., Ltd. (Shanghai, China, http://www.sangon.com/). 6-mercapto-1-hexanol (MCH) was purchased from J&K Chemical Ltd. (Guangzhou, China, http://www.jkchemical.com/index.aspx). Acetic acid-buffered saline (ABS) solution was prepared with HAc, NaAc and KCl. All reagents were analytical grade, and solutions were prepared using ultrapure water (18.2 MΩ⋅cm resistivity, Millipore).
Apparatus
Thermal Field Emission Environmental Scanning Electron Microscopy (SEM; Quanta 400F, France, https://www.fei.com/home/), Transmission Electron Microscopy (TEM; FEI Tecnai G2 Spirit, Netherlands, https://www.fei.com/home/) and X-ray photoelectron spectroscopy (XPS, ESCALab250, Thermo Fisher Scientific, USA, https://www.thermofisher.com/cn) were applied to characterize the morphologies and structures of the samples.
Electrochemical measurements
All electrochemical measurements were performed with a CHI660E electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd. Shanghai, China, http://www.chinstr.com/). A conventional three-electrode system was employed using the following: a platinum wire counter electrode, an Ag/AgCl reference electrode (the KCl concentration in the reference electrode is saturated solution) and a bare modified GCE (3 mm in diameter) as the working electrode (Gaoss Union Technology Co., Ltd. Wuhan, China, http://gaossunion.cn.china.cn/). Electrochemical impedance spectroscopy (EIS) measurements were performed with the frequency range of 0.01 Hz to 100 kHz and an amplitude of a 5 mV in 5 mM K3Fe(CN)6/K4Fe(CN)6 solution containing 0.5 M KCl. Cyclic voltammetry (CV) measurements were conducted at a potential range of −0.2 V to 0.6 V with a scan rate of 100 mV s−1 in 5 mM K3Fe(CN)6/K4Fe(CN)6 solution containing 0.5 M KCl. Differential pulse voltammetry (DPV) measurements were performed in acetic acid-buffered saline solution (a buffer solution containing 0.1 M HAc, 0.1 M NaAc and 0.1 M KCl, PH 4.5) containing 3 mM H2O2. The parameters of DPV were as follows: potential range, −0.6 ~ −0.2 V; pulse amplitude, 0.05 V; pulse width, 0.05 s; and quiet time, 2 s.
Synthesis of poly(o-phenylenediamine) microspheres and AuPd nanoparticles
The poly(o-phenylenediamine) microspheres were prepared using a previously reported method [25] with a slight modification. First, an o-phenylenediamine (200 μL, 0.1 M) aqueous solution was diluted to 3 mL with ultrapure water. Then, an aqueous solution of K2Cr2O7 (150 μL, 0.1 M) was rapidly added to the mixture. Subsequently, the solution was stirred gently overnight at room temperature. The final products were collected by centrifuging and washing with ultrapure water several times, which were then resuspended in 1.0 mL of ultrapure water.
The AuPd nanoparticles were synthesized according to previous work [17, 26] with some modifications. Briefly, 0.1 g HDPC, K2PdCl4 solution (10 mM, 2 mL) and HAuCl4 solution (10 mM, 0.5 mL) were added to 25 mL ultrapure water to form a homogeneous solution with the aid of ultrasound. Next, the AA solution (0.1 M, 1.5 mL) was quickly mixed with the homogeneous solution; and, the mixture was kept at 35 °C for 3 h. Subsequently, the black product was centrifuged and washed thoroughly three times with ultrapure water and ethanol. Lastly, the precipitate was re-dispersed in 10 mL water and stored at 4 °C.
Preparation of G-quadruplex/hemin/HRP/AuPd/poly(o-phenylenediamine) nanoprobes
Initially, the poly(o-phenylenediamine) colloids were added to 10 mL of AuPd colloids. Subsequently, the mixture was softly shaken at room temperature overnight. AuPd nanoparticles were linked onto poly(o-phenylenediamine) microspheres through the amino groups [25, 27] on the polymer. Finally, the AuPd/poly(o-phenylenediamine) nanocomposites were collected by centrifugation (Scheme 1a). In total, 50 μL TBA (5 μM) and 50 μL HRP (1 mg mL−1) were added to the colloidal solution of AuPd/poly(o-phenylenediamine) nanocomposites. The resulting mixture was shaken at 4 °C for 22 h. Subsequently, 0.1 mg of hemin was added, and the mixture was incubated at 4 °C for 2 h to form the G-quadruplex/hemin configuration. After centrifugation and washing with water, the G-quadruplex/hemin/HRP/AuPd/poly(o-phenylenediamine) nanoprobes were stored in ultrapure water at 4 °C until further use.
Electrochemical biosensor fabrication
First, the GCE was polished repeatedly with 0.05 μm alumina powder, followed by successive sonication in ultrapure water and ethanol for 2 min. Then gold nanoparticles were deposited on the cleaned GCE by electrodeposition under a potential of −0.2 V for 60 s. Before being assembled onto the electrode, the thiolated aptamer was treated with 1 mM TCEP for 1 h to reduce the disulfide bonds [11, 28]. Then, the cleaned AuNP/GCE was soaked in the treated TBA solution (1 μM, 50 μL) for 14 h at room temperature. After thorough rinsing, the aptamer/AuNP/GCE was incubated with the MCH solution (1 mM, 50 μL) for 40 min to block the remaining active sites. The modified electrode was incubated with thrombin solution (20 μL) for 50 min at 4 °C. Finally, the previously prepared G-quadruplex/hemin/HRP/AuPd/poly(o-phenylenediamine) solution (20 μL) was dropped on the surface of the electrode and incubated for 50 min to form the sandwich biosensor for the electrochemical measurement.
Results and discussion
Choice of materials and biosensor design
It is very important for the fabrication of a biosensor to choose suitable nanomaterials because suitable nanomaterials can amplify the signal and improve the performance of a biosensor. In this work, the aim is to design a mediator-free and triple signal-amplified thrombin biosensor. Poly(o-phenylenediamine), a conjugated polymer of o-phenylenediamine, is a redox-active material that has received wide interest in the area of biosensors fabrication [15, 20]. Due to its strong electrochemical properties, poly(o-phenylenediamine) can be applied as a mediator of electron transfer. AuPd nanoparticles have excellent catalytic performance as peroxidase mimics because they are bimetallic nanoparticles, which have proven to improve catalytic performance by the electronics effect and a synergistic effect [17]. AuPd nanoparticles combine the desirable properties of AuNP and PdNP. To develop nanomaterials that can easily load proteins and redox-active species, a conjugated polymer and metallic nanoparticles were combined, and the composites were used to immobilize the enzyme and aptamer in this work. Thus, poly(o-phenylenediamine) was used as a support for the AuPd nanoparticles. To obtain signal amplification, enzyme was immobilized on the AuPd/poly(o-phenylenediamine) nanocomposites. To specifically bind with the target thrombin, thrombin aptamer was also loaded on the AuPd/poly(o-phenylenediamine) nanocomposites. It is worthwhile to note that the thrombin aptamer can react with hemin to form a G-quadruplex/hemin DNAzyme system [9, 22]. A voltammetric aptamer-based thrombin biosensor exploiting signal amplification via synergetic catalysis by DNAzyme and enzyme decorated AuPd nanoparticles on a poly(o-phenylenediamine) support is displayed in Scheme 1. First, the G-quadruplex/hemin/HRP/AuPd/poly(o-phenylenediamine) nanoprobes were synthesized (Scheme 1a). The thiolated thrombin aptamer was attached on the surface of AuNP/GCE through Au-S interaction. Then the aptamer can recognize and capture thrombin after being incubated with thrombin. The G-quadruplex/hemin/HRP/AuPd/poly(o-phenylenediamine) nanoprobes can be immobilized on the electrode only in the presence of the captured thrombin through the specific recognition and high binding affinity between the thrombin and aptamer. Then, the HRP, G-quadruplex/hemin DNAzyme system and AuPd particles can catalyze the electrochemical reaction of poly(o-phenylenediamine) in the presence of H2O2. The peak current was related to the amount of nanoprobes on the AuNP/GCE surface, so thus, it reflects the amount of thrombin immobilized on the AuNP/GCE surface. Finally, an electrochemical desorption technique that causes the break of the gold-thiol bond and the release of the components from the surface was applied to the electrode for regenerate of the bare AuNP/GCE surface (Scheme 1b).
Characterizations of poly(o-phenylenediamine) and AuPd/poly(o-phenylenediamine) nanocomposites
The morphologies of poly(o-phenylenediamine) and AuPd/poly(o-phenylenediamine) were characterized by TEM and SEM. Figure 1a is the SEM image of poly(o-phenylenediamine). It clearly shows many spherical colloids with smooth surfaces ranging from 200 to 300 nm. As Fig. 1b shows, plenty of AuPd particles were attached to the poly(o-phenylenediamine), which indicates that the AuPd/poly(o-phenylenediamine) were successfully prepared. The TEM micrograph of poly(o-phenylenediamine) and AuPd are shown in Fig. 1c, d, respectively. These images show that the AuPd nanoparticles possess porous nanostructures with highly branched subunits and homogeneous dispersion. The typical TEM images of AuPd/poly(o-phenylenediamine) conjugates are shown in Fig. 1e. AuPd particles were scattered on the poly(o-phenylenediamine), which also suggests that AuPd were successfully loaded onto poly(o-phenylenediamine).
Additionally, X-ray photoelectron spectroscopy (XPS) was performed to obtain an elemental analysis of AuPd/poly(o-phenylenediamine). Fig. S1 shows the fully scanned spectra of AuPd/poly(o-phenylenediamine). The result suggests the existence of carbon, nitrogen, chromium, oxygen, gold, and palladium elements in AuPd/poly(o-phenylenediamine). A detailed discussion is shown in the Electronic Supplementary Material ( Fig. S2 ). According to the results of the elemental analysis, the formation of AuPd/poly(o-phenylenediamine) was further confirmed.
Electrochemical characterization of the biosensor
EIS and CV were applied to confirm the stepwise modification process of the electrode. The results and detailed discussion of EIS and CV are shown in the Electronic Supplementary Material ( Fig. S3 ).
Comparison of different signal amplification strategies
To highlight the advantages of the electrochemical behavior and the amplification properties of the biosensor, a comparative study of the changes of the electrochemical signal was carried out using different labeled probes. G-quadruplex/hemin/AuPd/poly(o-phenylenediamine) bioconjugates and TBA/HRP/AuPd/poly(o-phenylenediamine) bioconjugates were used. From Fig. 2, the biosensor with G-quadruplex/hemin/HRP/AuPd/poly(o-phenylenediamine) bioconjugates (Fig. 2c) shows much greater electrochemical signal variation in contrast with the other two bioconjugates (Fig. 2a, b). The results demonstrate that the presence of the G-quadruplex/hemin, HRP and AuPd provides significant signal amplification. The amplified performance of the biosensor may be ascribed to the synergetic catalysis of HRP, AuPd, and G-quadruplex/hemin. The biosensor remarkably amplified the peak current of poly(o-phenylenediamine) in the presence of H2O2 based on the triplex-amplified system.
DPV responses of different strategies in the absence a and in the presence with b 10 nM thrombin in 5 mL ABS (0.1 M, pH 4.5) containing 3 mM H2O2 using: (a) G-quadruplex/hemin/AuPd/poly(o-phenylenediamine) probe. (b) TBA/HRP/AuPd/poly(o-phenylenediamine) probe, (c) G-quadruplex/hemin/HRP/AuPd/poly (o-phenylenediamine) probe
Analytical performance of the biosensor
To investigate the analytical performance of the electrochemical biosensor, the biosensor was used to detect different concentrations of thrombin under optimum experimental conditions. The pH of the working buffer was 4.5, the incubation time of thrombin and probe was 50 min. A detailed discussion of conditions optimization is supplied in the Electronic Supplementary Material ( Fig. S4 ). As seen in Fig. 3a, the DPV signal increases with an increase of thrombin concentration from 0 to 50 nM. The DPV peak current response without thrombin is relatively low, indicating inappreciable unspecific binding. Figure 3b shows the relationship between peak currents and thrombin concentration. As shows in the inset, the DPV peak current is proportional to the logarithm of thrombin concentration in the range from 100 f. to 20 nM. The linear regression equation was I = 6.5478 + 0.8054 log C (the unit of I is μA) with a correlation coefficient of R2 = 0.9913. A detection limit of 20 f. for thrombin was obtained according to the 3σ method. The linear range and detection limit of the biosensor can be compared with other techniques for thrombin, such as colorimetry, photoelectrochemical, electrochemical impedance spectroscopy, metal enhanced fluorescence, and fluorescence resonance energy transfer, etc. (Table 1). Compared with previous work on thrombin sensors, the biosensor in this work showed a wider response range and lower limit of detection. The results are attributed to the triple amplification system based on the synergetic catalysis of DNAzyme, HRP and AuPd nanoparticles.
a DPV response of the biosensor for the detection of different concentrations of thrombin (from a to l: 0 fM, 100 fM, 500 fM, 1 pM, 10 pM, 100 pM, 500 pM, 1 nM, 5 nM, 20 nM, 40 nM, 50 nM). b Variation of the current to the concentrations of thrombin. Inset: Calibration curve of currents to different concentrations of thrombin in the range from 100 f. to 20 nM. Error bars show the standard deviation of three experiments
Specificity and reproducibility of the electrochemical biosensor
The selectivity of the biosensor toward thrombin was evaluated by measuring the DPV current responses of systems to other non-target molecules, including bovine serum albumin (BSA), alpha fetal protein (AFP), and arginine (Arg) under the same experimental conditions. The results are shown in Fig. 4. The detection response of BSA (100 nM), Arg (100 nM), and AFP (100 nM) exhibits no major signal change in comparison with the blank group. After incubation with thrombin (10 nM) and its mixture with the above three interferences (100 nM), the value of current response increases distinctly revealing that the biosensor has a high specific affinity for thrombin detection.
The reproducibility of the biosensor was examined via analysis with the same concentration of thrombin (10 nM) using six biosensors under the same experimental conditions, and the relative standard deviation (RSD) of the observed electrochemical current response is 4.5%. The result demonstrates that the reproducibility of our protocol is acceptable.
Detection of thrombin in human blood serum sample
The potential applicability of the electrochemical biosensor was investigated by determining the recoveries of different concentrations of thrombin in healthy human blood serum by the standard addition method. In the experiment, serum samples were obtained from The Third Affiliated Hospital of Sun Yat-sen University. All the experiments were performed according to the institutional guidelines and the relevant laws, and the Ethics Committee of Hospital has approved the experimentation with human subjects) were diluted 10-fold. The results show that the recoveries and relative standard deviation from 95.0% to 101.0% and 3.7% to 7.7%, respectively (Electronic Supplementary Material Table S1 ). The results demonstrate that the biosensor has a promising potential application in real biological samples.
Regeneration of the biosensor system
To regenerate the surface of the AuNP/GCE, an electrochemical desorption method that breaks the Au-S bond was used, allowing for reconstruction of the biosensor surface. The regeneration assessment of biosensor was investigated by CV and EIS. The results are shown in Fig. 5. In comparison with the original bare AuNP/GCE, there is no obvious peak current, and the impedance spectra changes are observed for the regenerated AuNP/GCE, demonstrating the successful removal of the components on the electrode surface. This result is consistent with the literature [26, 29]. The regenerated AuNP/GCE surfaces still retained 86% of their initial DPV current value for a tested concentration of 10 nM thrombin after one time desorption and self-assembly. This result indicates that the regenerated AuNP/GCE surface is sufficient for reuse in electrochemical applications.
Conclusions
In conclusion, an electrochemical biosensor that amplified the detection signal of thrombin by the synergistic catalysis of HRP and porous AuPd nanostructures and HRP-mimicking DNAzyme was successfully constructed. The combination of poly(o-phenylenediamine) spheres and AuPd nanoparticles exhibited excellent electroactivity and electrocatalytic activity. AuPd nanoparticles with excellent catalytic performance as peroxidase mimics were used to immobilize a large amount of TBA and HRP, thus, greatly enhancing the sensitivity of the biosensor. The biosensor performed well in the detection of thrombin with wide linear range, good selectivity and a low detection limit. In addition, regeneration of the surface of the AuNP/GCE was performed successfully via electrochemical desorption. In the future, it is an attractive concept to integrate the electrochemical detection with electrochemical desorption for further biosensor research. Because removing biomolecules from the surfaces of electrode may be integrated with a microfluidic device for the upstream sample analysis and automation. However, the fabrication procedure of the biosensor is somewhat complicated and time-consuming. And there is still much effort needed to use the biosensor for clinical sample analysis.
References
Tian R, Chen XJ, Li QW, Yao C (2016) An electrochemical aptasensor electrocatalyst for detection of thrombin. Anal Biochem 500:73–79
Meini N, Farre C, Chaix C, Kherrat R, Dzyadevych S, Jaffrezic-Renault N (2012) A sensitive and selective thrombin impedimetric aptasensor based on tailored aptamers obtained by solid-phase synthesis. Sensors Actuators B Chem 166:715–720
Politi J, Rea I, Nici F, Dardano P, Terracciano M, Oliviero G, Borbone N, Piccialli G, De Stefano L (2016) Nanogravimetric and Optical Characterizations of Thrombin Interaction with a Self-Assembled Thiolated Aptamer. J Sens:2016
Hao LH, Zhao Q (2016) Microplate based assay for thrombin detection using an RNA aptamer as affinity ligand and cleavage of a chromogenic or a fluorogenic peptide substrate. Microchim Acta 183(6):1891–1898
Chen ZB, Tan LL, Hu LY, Zhang YM, Wang SX, Lv FY (2016a) Real colorimetric thrombin Aptasensor by masking surfaces of catalytically active gold nanoparticles. ACS Appl Mater Interfaces 8(1):102–108
Wang J, Jiang XC, Han HY (2016a) Turn-on near-infrared electrochemiluminescence sensing of thrombin based on resonance energy transfer between CdTe/CdS core(small)/shell(thick) quantum dots and gold nanorods. Biosens Bioelectron 82:26–31
Li S, Zhang DM, Zhang Q, Lu YL, Li NT, Chen QW, Liu QJ (2016) Electrophoresis-enhanced localized surface plasmon resonance sensing based on nanocup array for thrombin detection. Sensors Actuators B Chem 232:219–225
Yi HY, Xu WJ, Yuan YL, Bai LJ, Wu YM, Chai YQ, Yuan R (2014) A pseudo triple-enzyme cascade amplified aptasensor for thrombin detection based on hemin/G-quadruplex as signal label. Biosens Bioelectron 54:415–420
Yang ZH, Zhuo Y, Yuan R, Chai YQ (2015) Amplified thrombin Aptasensor based on alkaline phosphatase and hemin/G-Quadruplex-catalyzed oxidation of 1-Naphthol. ACS Appl Mater Interfaces 7(19):10308–10315
Wu D, Ma HM, Zhang Y, Jia HY, Yan T, Wei Q (2015) Corallite-like magnetic Fe3O4@MnO2@Pt Nanocomposites as multiple signal amplifiers for the detection of Carcinoembryonic antigen. ACS Appl Mater Interfaces 7(33):18786–18793
Yu YY, Chen ZG, Jian WS, Sun DP, Zhang BB, Li XC, Yao MC (2015) Ultrasensitive electrochemical detection of avian influenza a (H7N9) virus DNA based on isothermal exponential amplification coupled with hybridization chain reaction of DNAzyme nanowires. Biosens Bioelectron 64:566–571
Chen AY, Ma SY, Zhuo Y, Chai YQ, Yuan R (2016b) In situ electrochemical generation of Electrochemiluminescent silver Naonoclusters on target-cycling synchronized rolling circle amplification platform for MicroRNA detection. Anal Chem 88(6):3203–3210
Wang YH, Jiang L, Leng QG, Wu YH, He XX, Wang KM (2016b) Electrochemical sensor for glutathione detection based on mercury ion triggered hybridization chain reaction signal amplification. Biosens Bioelectron 77:914–920
Wen W, Hu R, Bao T, Zhang XH, Wang SF (2015) An insertion approach electrochemical aptasensor for mucin 1 detection based on exonuclease-assisted target recycling. Biosens Bioelectron 71:13–17
Liu ZM, Rong QF, Ma ZF, Han HL (2015) One-step synthesis of redox-active polymer/AU nanocomposites for electrochemical immunoassay of multiplexed tumor markers. Biosens Bioelectron 65:307–313
Chen XJ, Zhang Q, Qian CH, Hao N, Xu L, Yao C (2015) Electrochemical aptasensor for mucin 1 based on dual signal amplification of poly(o-phenylenediamine) carrier and functionalized carbon nanotubes tracing tag. Biosens Bioelectron 64:485–492
Ge SG, Liu F, Liu WY, Yan M, Song XR, Yu JH (2014) Colorimetric assay of K-562 cells based on folic acid-conjugated porous bimetallic Pd@Au nanoparticles for point-of-care testing. Chem Commun 50(4):475–477
Zheng YN, Chai YQ, Yuan YL, Yuan R (2014) A pseudo triple-enzyme electrochemical aptasensor based on the amplification of Pt-Pd nanowires and hemin/G-quadruplex. Anal Chim Acta 834:45–50
Lu CY, Xu JJ, Wang ZH, Chen HY (2015) A novel signal-amplified electrochemical aptasensor based on supersandwich G-quadruplex DNAzyme for highly sensitive cancer cell detection. Electrochem Commun 52:49–52
Xu TS, Li XY, Xie ZH, Li XG, Zhang HY (2015a) Poly(o-phenylenediamine) nanosphere-conjugated capture antibody immobilized on a glassy carbon electrode for electrochemical immunoassay of carcinoembryonic antigen. Microchim Acta 182:2541–2549
Stefan L, Denat F, Monchaud D (2011) Deciphering the DNAzyme activity of Multimeric Quadruplexes: insights into their actual role in the telomerase activity evaluation assay. J Am Chem Soc 133(50):20405–20415
Xu WJ, Yi HY, Yuan YL, Jing P, Chai YQ, Yuan R, Wilson GS (2015b) An electrochemical aptasensor for thrombin using synergetic catalysis of enzyme and porous Au@Pd core-shell nanostructures for signal amplification. Biosens Bioelectron 64:423–428
Lin DH, Tang T, Harrison DJ, Lee WE, Jemere AB (2015) A regenerating ultrasensitive electrochemical impedance immunosensor for the detection of adenovirus. Biosens Bioelectron 68:129–134
Majidi MR, Omidi Y, Karami P, Johari-Ahar M (2016) Reusable potentiometric screen-printed sensor and label-free aptasensor with pseudo-reference electrode for determination of tryptophan in the presence of tyrosine. Talanta 150:425–433
Chen HF, Cui YL, Zhang B, Liu BQ, Chen GN, Tang DP (2012) Poly(o-phenylenediamine)-carried nanogold particles as signal tags for sensitive electrochemical immunoassay of prolactin. Anal Chim Acta 728:18–25
Sun DP, Lu J, Zhong YW, Yu YY, Wang Y, Zhang BB, Chen ZG (2016) Sensitive electrochemical aptamer cytosensor for highly specific detection of cancer cells based on the hybrid nanoelectrocatalysts and enzyme for signal amplification. Biosens Bioelectron 75:301–307
Turkmen E, Bas SZ, Gulce H, Yildiz S (2014) Glucose biosensor based on immobilization of glucose oxidase in electropolymerized poly(o-phenylenediamine) film on platinum nanoparticles-polyvinylferrocenium modified electrode. Electrochim Acta 123:93–102
Sun DP, Lu J, Chen ZG, Yu YY, Mo MN (2015) A repeatable assembling and disassembling electrochemical aptamer cytosensor for ultrasensitive and highly selective detection of human liver cancer cells. Anal Chim Acta 885:166–173
Mahmoud AM, Tang T, Harrison DJ, Lee WE, Jemere AB (2014) A regenerating self-assembled gold nanoparticle-containing electrochemical impedance sensor. Biosens Bioelectron 56:328–333
Fan DW, Guo CJ, Ma HM, Zhao D, Li YN, Wu D, Wei Q (2016) Facile fabrication of an aptasensor for thrombin based on graphitic carbon nitride/TiO2 with high visible-light photoelectrochemical activity. Biosens Bioelectron 75:116–122
Ocana C, del Valle M (2016) Three different signal amplification strategies for the impedimetric sandwich detection of thrombin. Anal Chim Acta 912:117–124
Sui N, Wang LN, Xie FX, Liu FY, Xiao HL, Liu MH, Yu WW (2016) Ultrasensitive aptamer-based thrombin assay based on metal enhanced fluorescence resonance energy transfer. Microchim Acta 183(5):1563–1570
Wen QQ, Lu P, Yang PH (2016) A glassy carbon electrode modified with in-situ generated chromium-loaded CdS nanoprobes and heparin for ultrasensitive electrochemiluminescent determination of thrombin. Microchim Acta 183(1):123–132
Zou P, Liu YL, Wang HY, Wu J, Zhu FF, Wu H (2016) G-quadruplex DNAzyme-based chemiluminescence biosensing platform based on dual signal amplification for label-free and sensitive detection of protein. Biosens Bioelectron 79:29–33
Chen CK, Huang CC, Chang HT (2010) Label-free colorimetric detection of picomolar thrombin in blood plasma using a gold nanoparticle-based assay. Biosens Bioelectron 25(8):1922–1927
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21375152) and Guangdong Provincial Science and Technology Projects (Grant Nos. 2014A020221098 and 2016B030303002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Xiangyan Wang and Duanping Sun contributed equally to this work.
Electronic supplementary material
ESM 1
(DOC 1922 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Sun, D., Tong, Y. et al. A voltammetric aptamer-based thrombin biosensor exploiting signal amplification via synergetic catalysis by DNAzyme and enzyme decorated AuPd nanoparticles on a poly(o-phenylenediamine) support. Microchim Acta 184, 1791–1799 (2017). https://doi.org/10.1007/s00604-017-2160-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2160-0