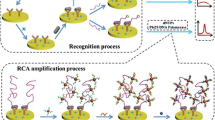
Abstract
A simple, rapid and ultrasensitive visual sensing method for the detection of Cronobacter sakazakii (C. sakazakii) based on a biohybrid interface was established. During the entire sensing process, quadruple-cascade amplification showed its superior sensing performance. First, the prepared immunomagnetic beads (IMB) were used to isolate and enrich specific targets from the food matrix. After adding the fusion aptamer, the aptamer sequence specifically recognized the target and formed the immune sandwich structure of antibody-target-fusion aptamer. In addition, the fusion aptamer also included the template sequence of exponential amplification reaction (EXPAR), which contained the antisense sequence of the G-rich sequence. Therefore, a large number of G-rich sequences can be generated after EXPAR can be triggered in the presence of Bst. DNA polymerase, nicking endonuclease, cDNA, and dNTP. They were self-assembled into G-quadruplex structures and then combined with hemin to form G4/hemin DNAzyme, resulting in visible coloration and measuring absorbance at 450 nm for quantitative detection. The assay showed a limit of detection (LOD) of 2 CFU/mL in pure culture and 12 CFU/g in milk powder in optimal conditions. This method provides a promising strategy for rapid and point-of-care testing (POCT) since it does not require DNA extraction, medium culturing, and expensive instrumentation.
Graphical abstract

Key points
•Single-cell level detection of C. sakazakii with ultrasensitive and rapidness
•The fusion aptamer integrated recognition and amplification
•Sensing analysis of C. sakazakii based on cascade amplification of biohybrid interface
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cronobacter sakazakii is a Gram-negative bacillus with flagella, producing yellow pigment that is a leading cause of neonatal infection (Bar-Oz et al. 2001; Tian et al. 2021a, b). It is a conditional food-borne pathogenic bacteria mainly existing in milk powder and is associated with necrotizing enterocolitis, septicemia, and infant meningitis, which is not conducive to the healthy growth of infants (Zhou et al. 2011; Fakruddin et al. 2013). In addition, adults with low immunity, especially older people, may also be infected (Nazarowec White & Farber 1997).
At present, the detection of microorganisms mainly depends on the traditional culture identification technology, which is relatively accurate but extraordinarily laborious and time-consuming. Specifically, the traditional method used for detecting C. sakazakii requires 3 to 7 days to complete the analysis, and the detection needs to be completed under different culture conditions, while the detection process must be performed in different culture conditions, significantly limiting its application scope (Healy et al. 2010). Therefore, developing rapid, reliable, and sensitive methods to detect C. sakazakii in food is essential.
In recent years, molecular biology technology has been applied for microbial detection. An electrochemical immunoassay for detecting C. sakazakii based on a screen-printed carbon array, and the recognition ability of different antibody-modified working electrodes for pathogenic species were compared (Dou et al. 2013). Zhu et al. (2018) developed a method to amplify the C. sakazakii target gene via polymerase chain reaction (PCR) and analyzed the products using microchip electrophoresis. Unfortunately, a significant drawback of these techniques is the increased detection cost, resulting from the need for complex equipment and well-trained operational personnel. Moreover, it is also critical to rapidly enrich pathogens in samples, reducing the time required for pathogen identification to a certain extent (Zhu & Wang 2016).
Immunomagnetic separation (IMS) is a technique that relies on the antigen–antibody reaction and uses magnetic beads (MB) to capture targets from complex samples (Cudjoe et al. 1994; Safarik & Safarikova 1999). On the one hand, it can fully ensure high binding specificity. On the other hand, the direct enrichment of target pathogens via magnetic separation may significantly reduce the enrichment period and detection time. This method is typically used to examine pathogens, such as Escherichia coli (E. coli) (Pappert et al. 2010; Qiao et al. 2021), Staphylococcus aureus (S. aureus) (Kim et al. 2018; Shi et al. 2021), and Vibrio parahaemolyticus (V. parahaemolyticus) (Zhao et al. 2020). However, the enriched of C. sakazakii from complex matrices is relatively rare, which is particularly critical in practical applications.
In addition, the performance of the biosensor can be improved by integrating recognition substances with higher affinity (Zhang et al. 2019a, b). Antibody-dependent detection is typically used to identify C. sakazakii (Hochel & Skvor 2009; Park et al. 2012; Zhang et al. 2012). Moreover, the aptamer proposed by Ellington and Tuerk in 1990 has rapidly become a powerful tool for detecting various target molecules due to its high affinity, quick synthesis, and strong stability, which is also known as the antibody of chemists (Ellington & Szostak 1990; Tuerk & Gold 1990). C. sakazakii detection using aptamers has shown significant progress (Kim et al. 2017; Liu et al. 2020; Peng et al. 2019), highlighting their potential in constructing of efficient and reliable analytical strategies for identifying C. sakazakii in food samples. To a certain degree, simultaneously using aptamers and antibodies recognition elements renders the immune sandwich mode reliable and straightforward. Introducing specific aptamers using traditional antigen–antibody strategies allows for the synergistic recognition of targets, while improving the specificity in biological recognition events. Therefore, the binding efficiency and selectivity of biosensors are enhanced (Huang et al. 2021).
Isothermal temperature amplification technology can further amplify the detection signal to achieve more ideal results. Exponential amplification reaction (EXPAR) can achieve an ultra-high amplification capacity of > 106 times in a short time at 55 °C (Zhang et al. 2019a, b). Instead of PCR that converts dsDNA to ssDNA at high temperatures, it separates dsDNA or DNA-RNA hybrids into single-strand forms via polymerase with strand displacement activity (Reid et al. 2018). This technology is promising for POCT detection and field analysis since it requires fewer enzymes and a shorter amplification time.
Colorimetric reading is considered a potential candidate for the visual detection of target molecules without using special equipment (Liao et al. 2018). G-quadruplex/hemin DNAzyme is an excellent alternative to proteases that due to its peroxidase-mimicking activity and high stability, and is often used as a catalytic amplifier (Gan et al. 2017). Unlike natural enzymes or nanomaterials with catalytic activity, DNAzyme displays superior robusticity due to the excellent stability and easy preparation of G-quadruplex. Naked eye observation can be achieved by catalyzing 4,4’-diamino-3,3’,5,5’-tetra-methylbiphenyl (TMB)-H2O2, rendering it a preferred strategy for designing biosensors (Li et al. 2019; Tang et al. 2021).
Herein, we attempt to propose simple and highly sensitive multiple amplification detection strategies for detecting C. sakazakii. Magnetic immune separation realized the separation of the target from the complex matrix. The high specificity of the immune sandwich structure composed of antibodies and aptamers and the efficient amplification performance of EXPAR played a vital role in the sensing system. Finally, the continuous generation of the conversion signal readout element (G-quadruplex sequence) was achieved via the high catalytic activity of the DNAzyme. In optimal conditions, the products were analyzed via visual observation within 2 h, while the limits of detection of the C. sakazakii were as low as 2 CFU/mL. Applying of this IMS technology combined with the isothermal amplification method in milk samples also achieved satisfactory results, confirming that this method presented the advantages such as rapidly, low sample consumption, high selectivity, and high sensitivity.
Materials and methods
Reagents and apparatus
All HPLC-purified DNA oligonucleotides (listed in Table S1) were synthesized by Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China). These oligonucleotides were dissolved in ultrapure water and then stored at 4 °C. Carboxy MB (particle diameter 300 nm) was obtained from BioMag Scientific Inc. The Nt.BstNBI nicking endonuclease and Bst 2.0 DNA polymerase were obtained from New England Biolabs (Ipswich, MA) and the deoxynucleotide triphosphates (dNTPs) mixture (10 mM) was purchased from TaKaRa (Beijing, China). The TMB and hemin were obtained from MACKLIN (Shanghai, China), prepared in dimethyl sulfoxide (DMSO) and stored at − 20 °C as 50 mM and 5 mM stock solutions. All other reagents were at least of analytical reagent grade. C. (ATCC 29,544), V. parahaemolyticus (ATCC 17,802), S. aureus (CICC 21,600), E. coli (CICC 10,389), and Salmonella (CMCC 50,115) were supplied by the Key Laboratory of Agricultural Transgenic Biosafety Assessment (Food) of the Ministry of Agriculture at China Agricultural University. The C. sakazakii antibody was provided by Northwest A&F University (Shanxi, China). The absorbance was measured at room temperature using a multi-functional microplate reader (Thermo Fisher Scientific). Dynamic light scattering (DLS) analysis was performed using on a laser particle analyzer (Malvern Panalytical, China).
Strain culture
C. was cultured according to the traditional microbial culture method. After the logarithmic phase, the bacteria were collected and 4000 rpm centrifuge for 5 min, discarded, and PBS was prepared and stored at 4 °C.
The other negative control strains were treated in the same way as mentioned above after culturing the Luria–Bertani (LB) liquid medium.
Construction of immunomagnetic bead target
The IMB was prepared according to the instructions of the manufacturer with some modifications (BioMag Scientific Inc., Wuxi), and characterized using DLS. The prepared IMB were vibrated at 200 rpm for 30 min at room temperature, with different C. sakazakii concentrations to obtain the sandwich structure. Finally, the unbound targets were removed after four magnetic separations.
EXPAR assay
-
(1)
Recognition: The 0.3-μM fusion aptamer was allowed to fully reacted with the IMB-target for 30 min, after which magnetic separation was performed several times to remove the uncombined fusion aptamer.
-
(2)
Signal amplification: EXPAR reaction total 100 μL. In the previous step, dNTPs (4 μL, 10 mM), Bst 2.0 DNA polymerase (0.75 μL, 8 U/μL), Thermpol Reaction Buffer (15 μL, 10 ×), Nt.BstNBI (6 μL, 10 U/μL), NEB 3.1 buffer (7.5 μL, 10 ×), cDNA (3 μL, 10 μM), and water were added. After mixing the above solutions, we incubated them at 55 °C for 30 min and inactivated them at 95 °C for 10 min.
-
(3)
Signal output: 10 μL of the amplification products was mixed with 80 μL G4 buffer (100 mM Tris, 120 nM NaCl, 10 mM MgCl2·6H2O, and 10 mM KCl, pH = 8.4) and 10 μL 40 μM hemin, and incubated at 37 °C for 20 min, allowing the short G-rich ssDNA (EXPAR product) to properly form the G4/hemin DNAzyme. Next, a freshly prepared TMB-H2O2 solution was added and incubated at 37 °C for 20 min, avoiding direct light exposure, after which the reaction was terminated by adding 30 μL 2 M H2SO4. Finally, this solution was detected at 450 nm using a multi-functional microplate reader.
Detection of C. sakazakii in milk powder
Milk powder purchased from local supermarkets was diluted at 1:10 and inoculated with C. sakazakii at different concentrations after pasteurization. After the mixture was fully mixed, the experimental group was obtained. The milk powder matrix without a bacterial solution was the control group, and the test was carried out using the method given in Fig. 1.
Results
Principle of the scheme
The proposed novel biosensor strategy was illustrated in Fig. 1. First, the classic N-(3-dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS) method was used to couple the MB with the antibody. In the presence of the target, antibodies could be used as capture recognition elements, while magnetic separation removed the unconjugated targets. Adding the fusion aptamer, we achieved a sandwich type-based recognition via the specific binding of the target with the antibody and aptamer to ensure high specificity. In addition, since the fusion aptamer sequence included the EXPAR template, which contained the nicking enzyme recognition site and the G-rich antisense sequence, EXPAR was triggered under the action of Bst. DNA polymerase, Nt.BstNBI, and cDNA. The subsequent products contained a large number of G-rich sequences that combined with hemin to form G4/hemin DNAzyme.
DNAzyme with catalytic functionality can catalyze TMB coloration in the presence of H2O2, allowing the target quantification via a visual colorimetric signal. Therefore, the EXPAR can realize the exponential multiplication of reporter elements (G4) in a short time, achieving substantial signal readout growth. Using this method to amplify the effective signal of the target was expected to significantly improve the detection sensitivity of the biosensor, allowing for the evaluation of experimental results via the color or absorbance differences between the negative and positive samples.
Characterization of IMB
The successful synthesis of the IMB was verified to ensure the feasibility of the design in this study. DLS was used to characterize the relative MB changes regarding particle size and zeta potential before and after coupling with the antibody (Fig. S1). As shown in Fig. 2, the MB particle size was about 300 nm, increasing significantly to approximately 420 nm after coupling with the antibody, indicating successful IMB were synthesized. The zeta potential decreased from − 20.1 to − 32.5 mV on the surface of the coupled antibody. Studies showed that the antibody generally had negative charges (Bankert & Mayers 1980; Goyon et al. 2017; Suzuki et al. 2004), so the successful synthesis was verified again to a certain extent.
Feasibility analysis of the proposed strategy
Figure 3 showed the feasibility study of the biosensor. First, direct proof of the EXPAR mechanism was acquired via gel electrophoresis analysis. As depicted in Fig. 3a, the distinct bands in lane 1 represented the fusion aptamer (88 mer), while cDNA (lane 2) displayed no bands due to their single strands. Lane 3 denoted the analytical result of combining the fusion aptamer and cDNA. Compared with lane 1, a band with slightly lower mobility appeared in lane 2, indicating the hybridization reaction between the fusion aptamer and cDNA. The incubation of the template, cDNA, nicking endonuclease, polymerase, and dNTPs, resulted in a new band displaying the highest mobility in lane 8, suggesting short DNA production. Pronounced bands cannot be produced without the fusion aptamer, endonuclease, polymerase, or cDNA (lanes 4, 5, 6).
a Gel electrophoresis image for the EXPAR systems. Lane 1: fusion aptamer; lane 2: cDNA; lane 3: fusion aptamer + cDNA; lane 4: cDNA + Bst. DNA polymerase + Nt.BstNBI + dNTPs; lane 5: fusion aptamer + cDNA + Bst. DNA polymerase + dNTPs; lane 6: fusion aptamer + cDNA + Nt.BstNBI + dNTPs; lane 7: fusion aptamer + Bst. DNA polymerase + Nt.BstNBI + dNTPs; lane 8: fusion aptamer + cDNA + Bst. DNA polymerase + Nt.BstNBI + dNTPs. b UV–vis absorption spectra and the corresponding colors of different substances. Sample 1: non-target + EXPAR + hemin + TMB + H2O2; sample 2 ~ 5: the target after four consecutive fivefold gradient dilution + EXPAR + hemin + TMB + H2O2; sample 6: non-target + EXPAR + TMB + H2O2; sample 7: target + EXPAR + TMB + H2O2
In order to verify the possibility of the product as a signal output, the spectral response and corresponding color of the reaction solution were recorded by controlling the different reaction conditions, as shown in Fig. 3b. It is well known that hemin can catalyze TMB to produce blue products in the presence of H2O2 (Huge et al. 2014). After adding H2SO4, the solution became yellow, displaying a characteristic absorption peak at 450 nm (sample 1). When different target concentrations were added, the color of the solution gradually deepened as the concentration increased, while the 450-nm absorbance value gradually became higher (samples 2 ~ 5). In addition, the impact of the G-quadruplex (sample 6) and hemin (sample 7) in the solution on matrix TMB oxidation was also investigated. The results showed that their absorption spectra were almost overlapped, while no absorption peak was evident at 450 nm, indicating that other substances did not affect the catalytic results. These results provided direct evidence for the success of the biosensor design, indicating that this method could achieve significant signal amplification in immunoassays.
Optimization of reaction conditions
To achieve a satisfying sensing performance, the experimental conditions of the amplification reaction and color development were optimized, including the amplification time, Bst. DNA polymerase and Nt.BstNBI nicking endonuclease concentration (Fig. S2), as well as hemin concentration dosages and hemin incubation time (Fig. S3).
Amplification time is essential for determining the sensing performance. As shown in Fig. S2a, the color signals generated at 0 min, 10 min, 20 min, 30 min, and 40 min were quantitatively evaluated in the same conditions. The results showed a gradual absorbance increase in conjunction with an extended reaction time from 0 to 40 min, and remained unchanged after 30 min, indicating that the amplification was completed after 30 min. Therefore, 30 min was selected as the optimal IMB-EXPAR reaction time for reducing the detection time while maintaining an adequate color. In addition, 0.06 U/μL Bst. DNA enzyme (Fig. S2b), 0.6 U/μL Nt.BstNBI nicking endonuclease (Fig. S2c), 40 μM hemin (Fig. S3a), and a 20-min incubation period were selected (Fig. S3b) as the optimal reaction conditions for subsequent hemin experiments.
Application of the method for detecting C. sakazakii in pure culture
Under the above optimal conditions, the quantitative detection performance of the biosensor for C. sakazakii in pure culture and milk powder matrix was investigated (Fig. 4). The biosensor was applied to the detection of C. sakazakii containing 2 ~ 103 CFU/mL and was recorded the absorption of value after the completion of TMB-H2O2 coloration. The relationship between the absorbance value and the number of targets was determined according to the sensing strategy shown in Fig. 1, indicating that the termination reaction color increased with a rise in the target concentration (Fig. 4a). Therefore, the target concentrations could be quantified according to the absorbance value. The standard curve was relatively linear up to 103 CFU/mL with a regression coefficient of 0.9921, and its LOD was 2 CFU/mL.
Selectivity of this assay for C. sakazakii
To evaluate the selectivity of the proposed assay, four common pathogenic microorganisms (V. parahaemolyticus, S. aureus, E. coli, and Salmonella) that may exist in milk powder were selected as negative references. As shown in Fig. 5, the results showed that only C. sakazakii could produce a high value of A450. Even when the non-target concentration was high, the signals they produced were relatively weak. At the same concentration, the absorbance signal ratio of the target and non-target was between 4 and 10, which was sufficient to distinguish the target and non-target, indicating that this method exhibited high specificity for the C. sakazakii detection.
Analysis of C. sakazakii in spiked milk powder samples
Finally, the application of the proposed method to milk powder was discussed. The first detection process of the milk powder samples yielded no positive results. Therefore, to assess the accuracy of the biosensor, the samples were spiked with three different C. sakazakii concentrations (103 CFU/g, 102 CFU/g, 10 CFU/g), while the recovery was achieved in a range of 91.7 ~ 120% and the RSDs were within 1.3 ~ 2.12% (n = 3). Moreover, the sensitivity of the artificially inoculated milk powder was investigated, as shown in Fig. 4b. The linear equation was fitted as follows: y = 0.8313lgC − 0.661 (R2 = 0.9859), with a LOD of was 12 CFU/g. These findings indicated that the designed sensing platform was suitable for C. sakazakii detection in actual samples.
Discussion
Cronobacter sakazakii is one of the most harmful pathogens to newborns at present (Tian et al., 2021a, b). According to the standards specified by the International Organization for Standardization on C. sakazakii, this bacterium cannot be detected (ISO/TS 22,964:2006(E)). Unfortunately, it has been detected in many dairy products in recent years, which is not conducive to the healthy growth of infants. Although the state attaches great importance to food safety, the traditional microbial culture method has been used at the method level, which makes the detection efficiency lag and high resource cost (Greenhalgh & Amund, 2019). With the development of molecular student matter technology, other detection technologies are gradually derived, but there are many problems such as low sensitivity, weak specificity, and complex technical methods. Therefore, the prevention and detection of C. sakazakii urgently need an efficient, sensitive, and realizable technical method for POCT.
A rapid, highly specific, ultrasensitive, and label-free colorimetric method based on quadruple-cascade amplification was developed for the detection of C. sakazakii. Compared with the traditional microbial culture method, this method takes less time (< 2 h). In addition, due to the high requirements of the microbial culture method for the culture environment and operation technology, a little carelessness will lead to false-positive results, that is, the specificity may be affected. In contrast, this experiment has multiple guarantees for specificity. On the one hand, the immune sandwich structure can provide high specificity, and on the other hand, it is due to the ingenious design of the fusion nucleic acid aptamer sequence. Once the base is mismatched or there is no template, the subsequent reaction will not be carried out. Therefore, the specific detection performance is superior to the traditional microbial culture method. And, this experiment introduced the EXPAR process, in terms of sensitivity than microbial culture method is higher. Its product self-assembly formed G4/hemin DNAzyme, which can catalyze TMB to produce colored products, and finally realize visual detection.
At present, a great deal of structures of double antibody sandwich immunity have been reported (Her et al., 2017; Lu et al., 2020; Ranjbar & Hafezi-Moghadam, 2016; Shkembi et al., 2021). However, most of these strategies are limited by slow reaction speed, insufficient stability, device dependence, unsuitable for onsite detection, and so on. In order to solve these problems, based on the hetero-sandwich structure, this manuscript transformed the fusion aptamer to realize the integration of recognition and amplification and gave it dual-functional characteristics. On this basis, the direct formation of subsequent EXPAR products (G-rich sequences) ensured the simplicity of cascade amplification. In addition, since it contains quadruple-cascade amplification (magnetic enrichment, target-aptamer one-to-many recognition, EXPAR amplification, and DNAzyme signal accumulation), compared with other detection methods for C. sakazakii (Akineden et al. 2020; Fu et al. 2018; Hu et al. 2015; Chai et al. 2020; Xu et al. 2018; Ruan et al. 2013; Zhang et al. 2017; Zhu & Wang 2016), it has also effectively improved the sensitivity (Table S2), reduced the detection limit to 2 CFU/mL (Fig. 4a), and realized single-cell level detection.
Hemin has the activity of horseradish peroxidase due to it takes metalloporphyrin as the reaction active center. In the presence of H2O2, it can catalyze the redox reaction of the chromogenic substrate (TMB) and change the color of the solution (Guo et al., 2011). The results of Fig. 3b showed that the catalytic activity of hemin itself was weak, which was not conducive to the ultrasensitive detection of the target. Furthermore, the catalytic activity of G4/hemin DNAzyme is much lower than that of horseradish peroxidase (HRP), and the sensitivity directly related to the detectable concentration of G-quadruplex sequence may be limited (Frasson et al., 2022). However, the complex formed by hemin and G-quadruplex in this strategy showed good peroxidase catalytic activity (Fig. 3b). The reason is that we have introduced the cascade amplification strategy, using the one-to-many binding of target-fusion aptamer, each fusion aptamer can trigger EXPAR, and its product can catalyze TMB to finally realize the visual index amplification of the signal. At the same time, we also optimized the color development conditions and amplification conditions, which is particularly important for reducing the background value and improving the reaction efficiency in a complex environment.
After the successful construction of the biosensor, we also detected the actual samples and selected the milk powder matrix that is most likely to pollute C. sakazakii as the detection sample. The results were also impressive, and the detection limit is reduced to 12 CFU/g, indicating its practical application of rapid response to pathogen pollution investigation. Moreover, the comparison with the traditional culturing method showed that the results were similar (Table 1). The pretreatment of the whole process is simple, there is no need to extract the target genome, easy to operate, and meets the needs of onsite detection. Finally, our sensing system can select different antibodies and aptamers according to different targets, which makes the colorimetric detection method developed in this study show the possibility of detecting other risk factors without designing primers for each target, which reduces the technical difficulty of detection.
Due to its visualization ability, isothermal qualities, specificity, and sensitivity, this strategy shows promise for broad application in future food safety approaches, environmental monitoring endeavors, and clinical diagnoses. Of course, novel and appropriate materials and other biotechnology should also be considered for better application.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Akineden O, Wittwer T, Geister K, Plötz M, Usleber E (2020) Nucleic acid lateral flow immunoassay (NALFIA) with integrated DNA probe degradation for the rapid detection of Cronobacter sakazakii and Cronobacter malonaticus in powdered infant formula. Food Control 109:106952. https://doi.org/10.1016/j.foodcont.2019.106952
Bankert R, Mayers G (1980) Use of hybridoma technology to assess the antibody repertoire to a negatively charged hapten. Transpl P 12:409–412. https://doi.org/10.1080/10630739208724463
Bar-Oz B, Preminger A, Peleg O, Block C, Arad I (2001) Enterobacter sakazakii infection in the newborn. Acta Paediatr 90(3):356–358. https://doi.org/10.1080/080352501300067857
Chai X, Hao Y, Wang J, Li S (2020) Rapid detection of Enterobacter sakazakii in infant milk powder based on PAMAM dendrimer nano immunosensor. J Chinese Institute Food Sci Technol 20(8):264–269. https://doi.org/10.16429/j.1009-7848.2020.08.032
Cudjoe KS, Krona R, Olsen E (1994) IIMS: a new selective enrichment technique for detection of Salmonella in foods. Int J Food Microbiol 23(2):159–165. https://doi.org/10.1016/0168-1605(94)90049-3
Dou W, Tang W, Zhao G (2013) A disposable electrochemical immunosensor arrays using 4-channel screen-printed carbon electrode for simultaneous detection of Escherichia coli O157:H7 and Enterobacter sakazakii. Electrochim Acta 97:79–85. https://doi.org/10.1016/j.electacta.2013.02.136
Ellington A, Szostak J (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822. https://doi.org/10.1038/346818a0
Fakruddin M, Rahaman M, Ahmed M, Hoque M (2013) Cronobacter sakazakii (Enterobacter sakazakii): an emerging food borne pathogen. Int J Biomedical and Advance Research 4(6):349–359. https://doi.org/10.7439/ijbar.v4i6.356
Frasson I, Pirota V, Richter SN, Doria F (2022) Multimeric G-quadruplexes: a review on their biological roles and targeting. Int J Biological Macromolecules 204:89–102. https://doi.org/10.1016/j.ijbiomac.2022.01.197
Fu S, Jiang Y, Jiang X, Zhao Y, Chen S, Yang X, Man C (2018) Probe-free label system for rapid detection of Cronobacter genus in powdered infant formula. AMB Express 8(1):155. https://doi.org/10.1186/s13568-018-0689-x
Gan C, Wang B, Huang J, Qileng A, He Z, Lei H, Liu W, Liu Y (2017) Multiple amplified enzyme-free electrochemical immunosensor based on G-quadruplex/hemin functionalized mesoporous silica with redox-active intercalators for microcystin-LR detection. Biosens Bioelectron 98:126–133. https://doi.org/10.1016/j.bios.2017.06.038
Guo Y, Deng L, Li J, Guo S, Wang E, Dong S (2011) Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano 5(2):1282–1290. https://doi.org/10.1021/nn1029586
Greenhalgh J, Amund D (2019) Examining the presence of Cronobacter spp. in ready-to-eat edible insects. Food safety (Tokyo Japan) 7(3):74–78. https://doi.org/10.14252/foodsafetyfscj.D-19-00004
Her J, Jo H, Ban C (2017) Enzyme-linked antibody aptamer assays based colorimetric detection of soluble fraction of activated leukocyte cell adhesion molecule. Sens Actuators B Chem 242:529–534. https://doi.org/10.1016/j.snb.2016.11.070
Healy B, Cooney S, O’Brien S, Iversen C, Whyte P, Nally J, Callanan J, Fanning S (2010) Cronobacter (Enterobacter sakazakii): an opportunistic foodborne pathogen. Foodborne Pathog Dis 7(4):339–350. https://doi.org/10.1089/fpd.2009.0379
Hochel, I Skvor, J (2009) Characterisation of antibodies for the immunochemical detection of Enterobacter sakazakii. Czech J Food Sci, 27(Special Iss. (2)), S2–66-S62–74 https://doi.org/10.17221/206/2009-CJFS
Hu X, Dou W, Zhao G (2015) Electrochemical immunosensor for Enterobacter sakazakii detection based on electrochemically reduced graphene oxide-gold nanoparticle/ionic liquid modified electrode. J Electroanal Chem 756:43–48. https://doi.org/10.1016/j.jelechem.2015.08.009
Huang Z, Chen H, Ye H, Chen Z, Jaffrezic-Renault N, Guo Z (2021) An ultrasensitive aptamer-antibody sandwich cortisol sensor for the noninvasive monitoring of stress state. Biosens Bioelectron 190:113451–113451. https://doi.org/10.1016/j.bios.2021.113451
Huge B, Flaherty R, Dada O, Dovichi N (2014) Capillary electrophoresis coupled with automated fraction collection. Talanta 130:288–293. https://doi.org/10.1016/j.talanta.2014.07.018
ISO/TS 22964:2006(E): Milk and milk products—detection of Enterobacter sakazakii.
Goyon A, Excoffier M, Janin-Bussat M, Bobaly B, Fekete S, Guillarme D (2017) Determination of isoelectric points and relative charge variants of 23 therapeutic monoclonal antibodies. J CHROMATOGR B 1065:119–128. https://doi.org/10.1016/j.jchromb.2017.09.033
Kim H, Kim Y, Chon J, Kim D, Yim J, Kim H, Seo K (2017) Two-stage label-free aptasensing platform for rapid detection of Cronobacter sakazakii in powdered infant formula. Sens Actuators B Chem 239:94–99. https://doi.org/10.1016/j.snb.2016.07.173
Kim, J., Yoo, J., Ham, J., Oh, M. (2018). Direct detection of Escherichia coli, Staphylococcus aureus, and Salmonella spp. in animal-derived foods using a magnetic bead-based immunoassay. Korean J Food Sci An, 38(4), 727–736. https://doi.org/10.5851/kosfa.2018.e11
Li R, Liu Q, Jin Y, Li B (2019) G-triplex/hemin DNAzyme: an ideal signal generator for isothermal exponential amplification reaction-based biosensing platform. Anal Chim Acta 1079:139–145. https://doi.org/10.1016/j.aca.2019.06.002
Liao A, Pan W, Benson J, Wong A, Rose B, Caltagirone G (2018) A simple colorimetric system for detecting target antigens by a three-stage signal transformation-amplification strategy. Biochemistry 57(34):5117–5126. https://doi.org/10.1021/acs.biochem.8b00523
Z Liu Y Yuan X Wu Q Ning S Wu L Fu 2020 A turn-off colorimetric DNAzyme-aptasensor for ultra-high sensitive detection of viable Cronobactersakazakii Sens Actuators B Chem 322. https://doi.org/10.1016/j.snb.2020.128646
Lu L, Liu B, Leng J, Ma X, Peng H (2020) Electrochemical mixed aptamer-antibody sandwich assay for mucin protein 16 detection through hybridization chain reaction amplification. Anal Bioanal Chem 412(26):7169–7178. https://doi.org/10.1007/s00216-020-02849-5
NazarowecWhite M, Farber J (1997) Enterobacter sakazakii: a review. Int J Food Microbiol 34(2):103–113. https://doi.org/10.1016/s0168-1605(96)01172-5
Pappert G, Rieger M, Niessner R, Seidel M (2010) Immunomagnetic nanoparticle-based sandwich chemiluminescence-ELISA for the enrichment and quantification of E. coli. Microchimica Acta 168(1–2):1–8. https://doi.org/10.1007/s00604-009-0264-x
Park S, Shukla S, Kim Y, Oh S, Kim SH, Kim M (2012) Development of sandwich enzyme-linked immunosorbent assay for the detection of Cronobacter muytjensii (formerly called Enterobacter sakazakii). Microbiol Immunol 56(7):472–479. https://doi.org/10.1111/j.1348-0421.2012.00466.x
Peng H, Hui Y, Ren R, Wang B, Song S, He Y, Zhang F (2019) A sensitive electrochemical aptasensor based on MB-anchored GO for the rapid detection of Cronobacter sakazakii. J Solid State Electr 23(12):3391–3398. https://doi.org/10.1007/s10008-019-04426-y
Qiao, Z Cai, Q Fu, Y Lei, C Yang, W (2021). Visual and quantitative detection of E. coli O157:H7 by coupling immunomagnetic separation and quantum dot-based paper strip. Anal. Bioanal. Chem 413(17), 4417–4426 https://doi.org/10.1007/s00216-021-03395-4
Ranjbar, R., Hafezi-Moghadam, M. (2016). Design and construction of a DNA origami drug delivery system based on MPT64 antibody aptamer for tuberculosis treatment. Electron. physician, 8(2), 1857–1864. https://doi.org/10.19082/1857
Reid M, Le X, Zhang H (2018) Exponential isothermal amplification of nucleic acids and assays for proteins, cells, small molecules, and enzyme activities: An EXPAR example. Angew Chem Int Ed Engl 57(37):11856–11866. https://doi.org/10.1002/anie.201712217
Ruan J, Li M, Liu Y, Li Y, Li Y (2013) Rapid and sensitive detection of Cronobacter spp. (previously Enterobacter sakazakii ) in food by duplex PCR combined with capillary electrophoresis–laser-induced fluorescence detector. J CHROMATOGR B 921–922:15–20. https://doi.org/10.1016/j.jchromb.2013.01.008
Safarik I, Safarikova M (1999) Use of magnetic techniques for the isolation of cells. J Chromatogr B 722(1–2):33–53. https://doi.org/10.1016/s0378-4347(98)00338-7
Shi X, Yu L, Lin C, Li K, Chen J, Qin H (2021) Biotin exposure-based immunomagnetic separation coupled with sodium dodecyl sulfate, propidium monoazide, and multiplex real-time PCR for rapid detection of viable Salmonella Typhimurium, Staphylococcus aureus, and Listeria monocytogenes in milk. J Dairy Sci 104(6):6588–6597. https://doi.org/10.3168/jds.2020-19887
Shkembi X, Skouridou V, Svobodova M, Leonardo S, Bashammakh A, Alyoubi A (2021) Hybrid antibody-aptamer assay for detection of tetrodotoxin in pufferfish. Anal Chem 93(44):14810–14819. https://doi.org/10.1021/acs.analchem.1c03671
Suzuki M, Takayanagi A, Shimizu N (2004) Targeted gene delivery using humanized single-chain antibody with negatively charged oligopeptide tail. Cancer Sci 95(5):424–429. https://doi.org/10.1111/j.1349-7006.2004.tb03226.x
Tang Y, Huang X, Wang X, Wang C, Tao H, Wu Y (2021) G-quadruplex DNAzyme as peroxidase mimetic in a colorimetric biosensor for ultrasensitive and selective detection of trace tetracyclines in foods. Food Chem 366:130560. https://doi.org/10.1016/j.foodchem.2021.130560
L Tian X Wang R Liu D Zhang X Wang R Sun W Guo S Yang H Li G Gong 2021a Antibacterial mechanism of thymol against Enterobacter sakazakii Food Control 123. https://doi.org/10.1016/j.foodcont.2020.107716
Tian, L Wu, M Guo, W Li, H Gai, Z Gong, G (2021b) Evaluation of the membrane damage mechanism of chlorogenic acid against Yersinia enterocolitica and Enterobacter sakazakii and its application in the preservation of raw pork and skim milk. Molecules, 26(21) https://doi.org/10.3390/molecules26216748
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage El-T. Science 249(4968):505–510. https://doi.org/10.1126/science.2200121
Xu D, Ming X, Gan M, Wu X, Dong Y, Wang D, Wei H, Xu F (2018) Rapid detection of C. sakazakii in powdered infant formula by thermophilic helicase-dependent isothermal amplification combined with silica-coated magnetic particles separation. J Immunol Methods 462:54–58. https://doi.org/10.1016/j.jim.2018.08.008
Zhang L, Chen Y, Cheng N, Xu Y, Huang K, Luo Y, Wang P, Duan D, Xu W (2017) Ultrasensitive detection of viable Enterobacter sakazakii by a continual cascade nanozyme biosensor. Anal Chem 89(19):10194–10200. https://doi.org/10.1021/acs.analchem.7b01266
Zhang X, Dou W, Zhan X, Zhao G (2012) A novel immunosensor for Enterobacter sakazakii based on multiwalled carbon nanotube/ionic liquid/thionine modified electrode. Electrochim Acta 61:73–77. https://doi.org/10.1016/j.electacta.2011.11.092
Zhang X, Feng Y, Duan S, Su L, Zhang J, He F (2019) Mycobacterium tuberculosis strain H37Rv electrochemical sensor mediated by aptamer and AuNPs-DNA. ACS Sensors 4(4):849–855. https://doi.org/10.1021/acssensors.8b01230
Zhang Y, Cui Y, Li X, Du Y, Tang A, Kong D (2019) A modified exponential amplification reaction (EXPAR) with an improved signal-to-noise ratio for ultrasensitive detection of polynucleotide kinase. Chem Commun 55(53):7611–7614. https://doi.org/10.1039/c9cc03568k
L Zhao X Lv X Cao J Zhang X Gu H Zeng L Wang 2020 Improved quantitative detection of VBNC Vibrio parahaemolyticus using immunomagnetic separation and PMAxx-qPCR Food Control 110. https://doi.org/10.1016/j.foodcont.2019.106962
Zhou X, Fu S, Gao J, Chen H (2011) Enterobacter sakazakii: an emerging foodborne pathogenic bacterium. ANN MICROBIOL 62:1–5. https://doi.org/10.1007/s13213-011-0274-x
Zhu L, Zhang Y, He P, Zhang Y, Wang Q (2018) A multiplex PCR amplification strategy coupled with microchip electrophoresis for simultaneous and sensitive detection of three foodborne bacteria. J Chromatogr B Analyt Technol Biomed Life Sci 1093–1094:141–146. https://doi.org/10.1016/j.jchromb.2018.06.057
Zhu Y, Wang D (2016) Rapid detection of Enterobacter Sakazakii in milk powder using amino modified chitosan immunomagnetic beads. Int J Biol Macromol 93(Pt A):615–622. https://doi.org/10.1016/j.ijbiomac.2016.09.024
Funding
This work was financially supported by the National Natural Science Foundation of China (grant no. 31471700) and the 2115 Talent Development Program of China Agricultural University (no. 00109016).
Author information
Authors and Affiliations
Contributions
X. X.: conceptualization, data processing, visualization, writing—original draft; L. Z.: methodology, writing—review and editing; X. W.: writing—review and editing; X. L.: writing—review and editing; H. C.: writing—review and editing; H. T.: methodology, supervision, project administration; W. X.: funding acquisition, writing—review and editing, supervision. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Supporting information
The supporting information for this study includes the tables of the primers used in this work and the comparison of the performance of biosensor recently used for target analysis. Additional information includes the original DLS characterization diagram of MB and IMB, the optimization patterns and results for other reaction conditions.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, X., Zhu, L., Wang, X. et al. Sandwich capture ultrasensitive sensor based on biohybrid interface for the detection of Cronobacter sakazakii. Appl Microbiol Biotechnol 106, 4287–4296 (2022). https://doi.org/10.1007/s00253-022-11978-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11978-z