Abstract
A nanocomposite modified with dibenzo-18-crown-6 was synthesized and applied as a new sorbent for the preconcentration of thallium(I) via ultrasound assisted-solid phase extraction. This extraction step was combined with electrothermal atomic absorption spectrometry to determine ultra-trace amounts of thallium(I). The nanocomposite was characterized by Fourier transform infrared spectroscopy, X-ray diffraction spectrometry, field emission scanning electron microscopy and transmission electron microscopy. Under the optimized conditions, a dynamic linear range from 7.0 to 435 ng L−1, a detection limit of 1.8 ng L−1 and a quantification limit of 6.0 ng L−1 were obtained. Also, the intra- and inter-day relative standard deviations for 20.0 ng mL−1 Tl(I) were calculated as ±4.8% and ±5.1%, respectively. The adsorbent was applied to the determination of thallium(I) in the environmental, biological and standard samples with satisfactory results.

A magnetic nanocomposite was synthesized as adsorbent from halloysite nanotubes and a crown ether. Tl(I) ions were extracted selectively and determined by electrothermal atomic absorption spectrometry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thallium (Tl), is a bluish white, inelastic, soft and easily fusible metal [2]. The United States Environmental Protection Agency has located thallium in the list of important pollutants in drinking water, specifying 0.5 μg L−1 Tl as maximum allowed concentration. Tl mainly presents in trivalent [Tl(III)] and monovalent [Tl(I)] forms and each oxidation state is different in terms of toxicity; Tl(III) is more toxic than Tl(I) [3].
Tl has many applications, and the large number of Tl’s applications can cause human exposure to toxic thallium compounds. Therefore, the development of selective and sensitive analytical techniques for the determination of this metal is very important [4]. Different analytical techniques have been applied for the determination of thallium amounts including ICP-MS [5], spectrophotometry [6], electrolyte cathode discharge atomic emission spectrometry [7] and electrochemical methods [8].
The abundance of thallium is extremely low in environmental compounds. Tl concentration in non-polluted soils is in the range of 0.3–0.55 mg kg−1 [9] and in surface waters between 10 to 100 ng L−1 [10]. According to the above, determination of Tl is difficult because of its low levels (below the detection limit of the analytical apparatus) and the high amounts of interfering components. Therefore, a preconcentration and separation step is often required. Various techniques have been applied for the separation of Tl from different matrices such as solidified floating organic drop microextraction [11], cloud point extraction [12] and dispersive liquid-liquid microextraction [13].
Solid phase extraction (SPE) is a favorite technique due to its benefits including high recovery, low cost, high preconcentration factor, ease of automation, fast phase separation, low consumption of toxic organic solvents and simplicity of combination with different detection instruments [14]. Several types of adsorbents such as octadecyl silica membrane disk [15], ion imprinted polymer [16], silica gel [17], chromosorb 105 [18], alumina [19], nitrate resin [20] and some other solid substances, have been utilized for the preconcentration and separation of thallium from diverse samples.
The purpose of the present work was to develop a simple and novel ultrasound assisted-solid phase extraction (USA-SPE) system for Tl preconcentration. In the first step, a crown ether modified magnetic nanocomposite was synthesized and characterized by FT-IR, FE-SEM, TEM and XRD. Then, it was applied in USA-SPE process of ultra-trace levels of thallium(I) ions in some environmental and biological samples. The main parameters influencing the extraction efficiency of thallium(I) such as pH, type, concentration and volume of eluent, sorption and desorption times, adsorbent reusability, temperature, sample volume and adsorption capacity were studied and optimized. Also, the accuracy of the suggested method was checked by analyzing the standard reference material and spiked real samples.
Experimental
Materials and reagents
Halloysite nanotubes (HNTs) were purchased from Auckland Company (New Zealand, www.imerys-ceramics.com). The thallium(I) standard solution (1000.0 mg L−1) was prepared from Merck (Germany, www.merckmillipore.com). Working standard solutions of thallium(I) were prepared freshly at various concentrations by diluting the stock solutions with deionized water. Dibenzo-18-crown-6 (98%) as modifier was purchased from Sigma-Aldrich (USA, www.sigmaaldrich.com). Ethanol (96%), toluene (99.9%), FeCl2.4H2O (99%), FeCl3.6H2O (99%), HNO3 (69%), HCl (37%), H2SO4 (98%), HClO4 (70%), NH3 (25%), NaOH (99%), tetrahydrofuran (THF) (99.8%) and dimethylformamide (DMF) (99.5%) were purchased from Merck (Germany, www.merckmillipore.com). Also, deionized water (pH: 5.5, resistivity: 18.4 MΩ·cm, conductivity: 4.1 μs cm−1, TOC: 42 μg L−1, sodium: 0.8 μg L−1, chloride: 0.9 μg L−1 and silica: 2.7 μg L−1) was used throughout.
Apparatus
Thallium measurements were performed with an atomic absorption spectrometer (Varian Spectra AA 220, Australia, www.varianinc.com) with a deuterium lamp background correction, equipped with graphite furnace (GTA-110 series). FT-IR spectra were recorded with a Bruker spectrometer (Tensor-27, Germany, www.bruker.com). A Hitachi field emission-scanning electron microscope (FE-SEM) (S-4160, Japan, www.hitachi-hightech.com) and Omega transmission electron microscopy (TEM) (LEO-912 AB, Germany, www.zeiss.com) were used for the morphological information. A Sonorex Digitec ultrasonic water bath (DT 255H, Germany, www.bandelin.com) with temperature control was applied for the preparation of nanocomposite and preconcentration process. This device features are as follows: capacity: 5.5 lit, frequency: 35 kHz, max peak output: 640 W/Per., HF-Power: 160 Weff, heating power: 280 W, current consumption: 2.0 A. Also, X-ray diffraction (XRD) images were prepared with a PANalytical instrument (X'Pert3, Holland, www.panalytical.com). Also, the pH measurements were carried out with a Metrohm pH meter (827 pH lab, Switzerland, www.metrohm.com) supplied with a combined glass–calomel electrode.
Samples preparation
Water samples
Two tap water samples (Kerman and Baft drinking water, Kerman, Iran) and a well water (Shahid Bahonar University of Kerman, Kerman, Iran) were selected and filtered. Then, pH was adjusted to pH = 2.0 with HNO3 to prevent the adsorption of the ions onto the flask walls, stored at 4 °C in a refrigerator [21] and the recommended method was applied.
Human hair
Hair samples with different lengths were collected from a men’s barber shop in Kerman (Iran) at the end of a working day (about 45 customers). The combined sample was immersed in acetone for 20 min, washed with deionized water and then dried in an oven. An exact amount of sample (0.5 g) was weighed and 30.0 mL of a mixture solution (HClO4 and HNO3, 1:8 v/v) was added to digest it. The resulted solution was dried and several drops of H2SO4 (50%) were added to the residue [22]. The final solution was transferred into a 100.0 mL measuring flask, diluted to the mark with deionized water. For Tl determination, an aliquot of last solution was chosen and the assay was performed according to the mentioned method.
Standard reference material
SRM 1643d–Trace Elements in Water: 5.0 mL sample solution was poured into a 100.0 mL measuring flask and diluted to the mark with deionized water. Then, an aliquot of this solution was taken and the thallium content was determined by the suggested method.
Preparation of modified magnetic nanocomposite
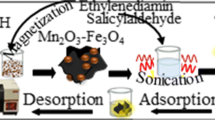
In order to prepare the magnetic nanocomposite, 2.5 g HNT and 150 mL deionized water were poured into a beaker. Then, 2.91 g FeCl3.6H2O and 0.99 g FeCl2.4H2O were added and stirred for 8 h at 70 °C. Its pH was adjusted in the range of 9–10 with ammonia. In the next step, the final solution was placed in the hot water bath (60 °C) for 12 h to complete the reaction. Finally, the prepared magnetic nanocomposite (MNC) were separated by using a Nd magnet, washed 3 times with 50 mL deionized water and 30 mL ethanol and dried in an oven (80 °C) for 8 h.
For preparation of modified magnetic nanocomposite (MMNC), 3.0 g of prepared nanocomposite (as described above) and 300 mL toluene were poured into a 250.0 mL measuring flask and sonicated for 3 min to disperse the MNCs. Then, 1.404 g dibenzo-18-crown-6 was added and refluxed for 24 h at 110 °C. After cooling, the prepared adsorbent was washed 3 times with 50 mL deionized water and 30 mL ethanol and dried in an oven.
General procedure
For thallium preconcentration and extraction step, 10.0 mL solution containing known amount of Tl(I) was poured into a 100 mL beaker and its pH was adjusted in 10 by NaOH solution (0.1 mol L−1) and HNO3 (0.1 mol L−1). A 40.0 mg prepared MMNC was added to the beaker, put in an ultrasonic bath, sonicated for 4 min and then, the adsorbent was collected and removed with a Nd magnet.
For the desorption step, 2.0 mL HNO3 (3.0 mol L−1) was added to the MMNC and sonicated for 5 min. Finally, the eluted adsorbent was removed from the solution with a magnet and the resulted solution was introduced to ETAAS directly for thallium determination.
Results and discussion
Some adsorbents such as carbon active, silica gel, zeolite, carbon nanotube and HNT were examined to Tl(I) determination, but the best extraction recovery was performed by using modified magnetic nanocomposite with HNT and dibenzo-18-crown-6.
FT-IR spectra (Figs. S1(a-d)) confirmed that dibenzo-18-crown-6 was settled on the surface of adsorbent. These spectra have some backbones including; 431.17 cm−1 for Fe-O, 539.14 cm−1 for Fe-O-Fe, 750–1100 cm−1 for Si-O or Al-O of HNT, 1384.01 cm−1 for CH2, 1631 cm−1 for C = C, 2800–3000 cm−1 for C-H and 3100–3500 cm−1 for stretching O-H. The comparison between the FT-IR spectra of halloysite nanotubes (Fig. S1(a)), magnetic nanocomposite (Fig. S1(b)), dibenzo-18-crown-6 (Fig. S1(c)) and modified magnetic nanocomposite by dibenzo-18-crown-6 (Fig. S1(d)) show the adsorbent synthesis process was done successfully.
Also in XRD pattern (Fig. S2(a)) of modified magnetic nanocomposite and its interpretation (Fig. S2(b)), the peaks of Fe3O4 nanoparticles and dibenzo-18-crown-6 are clearly visible. FE-SEM images of magnetic nanocomposite (Fig. S3(a)) and modified nanocomposite by dibenzo-18-crown-6 (Fig. S3(b)) show the surface morphology and size of nanoparticles. In TEM image (Fig. S4), the tubular structure of HNTs, Fe3O4 and dibenzo-18-crown-6 can be seen clearly and confirmed the successful synthesis of desirable adsorbent.
Optimization of method
After characterization and confirmation of MMNC synthesis, some preconcentration and extraction parameters including pH, type, concentration and volume of eluent, sorption and desorption times, adsorbent reusability, temperature, sample volume and adsorption capacity were investigated and optimized. The related explanations were presented in Electronic Supplementary Material file, but the results are summarized below:
-
a)
Sample pH value: pH = 10 was chosen as optimized pH.
-
b)
Type, concentration and volume of eluent: 2.0 mL HNO3 (3.0 mol L−1) was applied as the best eluent.
-
c)
Sorption and desorption times: For quantitative sorption and desorption processes, 4 and 5 min were sufficient, respectively.
-
d)
Adsorbent reusability: The thallium preconcentration efficiency decreased slightly when MMNC was reused 6 times.
-
e)
Temperature: 25 °C was selected as optimum temperature.
-
f)
Sample volume: The preconcentration process can perform quantitatively until 550 mL.
-
g)
Adsorption capacity: The maximum adsorption capacity of 16.7 mg g −1 was obtained for the synthesized MMNC.
Effect of interferences
Crown ethers let only some ions to enter and complex formation that their sizes are equal to ether cavity. So, use of crown ether strongly improves selectivity. The interference effect of different ions including anions and cations, those can affect on the method performance was tested by using a solution containing 100.0 ng mL−1 thallium(I) and addition of diverse concentrations of the interferences. The tolerance level was described as the maximum quantity of interferences those can produce a ± 5% error in thallium determination. The tolerance level of every interference ion was studied and if interference happened, the ratio was decreased until it stopped. Table 1 shows the studied species and their maximum tolerable quantities. As can be seen, several ions did not have any interference even at high levels and this means the suggested method can be applied to the analysis of thallium in different samples.
Analytical figures of merit
Under the optimized conditions, the performance was studied using the thallium(I) standard solutions. The results showed that the calibration graph for seven points was linear in the concentration range of 7.0–435 ng L−1 in the initial solution (Fig. 1). The regression equation can be expressed as Y = 2.8015X + 0.0137, with the correlation coefficient R2=0.9974. As can be seen from this plot, the slope is sharp and this shows that the sensitivity is high. In the regression equation, in addition to the slope, there is another parameter: intercept. Whatever the intercept is closer to zero, the method’s sensitivity is higher.
The repeatability (intra-day) and reproducibility (inter-day) were evaluated by carrying out eight replicate extractions and determinations of thallium at a concentration level of 20.0 ng mL−1 during a day (intra-day) and five replicates in five subsequent days (inter-day). Intra- and inter-day precisions were satisfactory with a relative standard deviation (RSD) of ±4.8% and ±5.1%, respectively. The detection limit based on 3sb/m (where sb = blank standard deviation and m = slope of the calibration curve) and quantification limit based on 10sb/m were found to be 1.8 ng L−1 and 6.0 ng L−1 (RSD = 5.0%). Also, 275 was obtained as the preconcentration factor.
Analytical applications
In order to survey the credibility of the suggested technique, it was used for the preconcentration of thallium(I) in water samples and human hair. The trustiness was tested by analysis of the spiked environmental and biological samples with known amount of Tl. The data in Table 2 show that recovery at 95% confidence level is satisfactory.
To consider accuracy, this method was utilized for the thallium(I) determination in a certified reference material; SRM 1643d–Trace Elements in Water. The obtained analytical data are shown in Table S1. As can be seen, the presented results are in good agreement with the registered amounts and there is no remarkable discrepancy between the obtained results and the accepted values. So, the suggested system is valid for analysis of thallium in diverse samples.
Comparison with previously reported methods
In Table 3, figures of merit were compared with other methods for thallium determination. As can be seen from this Table, the recommended method has a wide linear graph, suitable RSD. and high sensitivity. This method has also the lowest detection limit except only one reported literature [28] and best preconcentration factor. Moreover, due to the non-toxicity of method (without application of toxic and organic solvent), this technique can be regarded as a green method.
Conclusion
A modified magnetic nanocomposite was synthesized and applied for the preconcentration and determination of thallium(I) in combination with ETAAS. The aim of this work is to introduce a green technique that utilizes of magnetic nanocomposite for the preconcentration and determination of thallium ions in environmental, biological and standard samples. The suggested method has many advantages such as environmentally-friendly property, inexpensive, ease of adsorbent collection and enhanced sensitivity. Also, because of application of crown ether as modifier and limitation of other ions (except the Tl ion) entering to its cavities, the method’s selectivity was improved significantly. This method has only one weakness and that is the required long time of MMNC preparation (about 52 h), but this time is necessary just for once and all needed nanocomposites are made at the same time. The performance characteristics of the method such as detection limit, linear range and preconcentration factor are superior and comparable in comparison with previously Tl reported analytical protocols. Also, this method was shown to be a good alternative for the determination of ultra-trace amount of thallium in different and complicated matrices.
References
Pandey GP, Singh AK, Prasad S, Deshmukh L, Asthana A (2015) Development of surfactant assisted kinetic method for trace determination of thallium in environmental samples. Microchem J 118:150–157
Parakudyil AS, Pillai AK, Mathew SB (2011) Sensitive spectrophotometric determination of thallium(I) using rhodamine B hydrazide in micellar medium. Anal Methods 3:1546–1551
Escudero LB, Wuilloud RG, Olsina RA (2013) Sensitive determination of thallium species in drinking and natural water by ionic liquid-assisted ion-pairing liquid-liquid microextraction and inductively coupled plasma mass spectrometry. J Hazard Mater 244-245:380–386
Fazelirad H, Taher MA (2013) Ligandless, ion pair-based and ultrasound assisted emulsification solidified floating organic drop microextraction for simultaneous preconcentration of ultra-trace amounts of gold and thallium and determination by GFAAS. Talanta 103:375–383
Nielsen SG, Lee CTA (2013) Determination of thallium in the USGS glass reference materials BIR-1G, BHVO-2G and BCR-2G and application to quantitative Tl concentrations by LA-ICP-MS. Geostand Geoanal Res 37:337–343
Fouladgar M, Ensafi AA (2010) A novel optical chemical sensor for thallium(III) determination using 4-(5-bromo-2-pyridylazo)-5-(diethylamino)-phenol. Sens Actuators B 143:590–594
Shekhar R, Madhavi K, Meeravali NN, Kumar SJ (2014) Determination of thallium at trace levels by electrolyte cathode discharge atomic emission spectrometry with improved sensitivity. Anal Methods 6:732–740
Cheraghi S, Taher MA, Fazelirad H (2013) Voltammetric sensing of thallium at a carbon paste electrode modified with a crown ether. Microchim Acta 180:1157–1163
Tremel A, Masson P, Garraud H, Donard OFX, Baize D, Mench M (1997) Thallium in French agrosystems-II. Concentration of thallium in field-grown rape and some other plant species. Environ Pollut 97:161–168
Lukaszewski Z, Zembrzuski W, Piela A (1996) Direct determination of ultratraces of thallium in water by flow-injection—differential-pulse anodic stripping voltammetry. Anal Chim Acta 318:159–165
Chamsaz M, Hossein-Poor-Zaryabi M, Arbab-Zavar MH, Darroudi A (2014) Dispersive liquid-liquid Microextraction based on solidification of floating organic drop combined with flame atomic absorption spectrometry for Preconcentration and determination of thallium(III) in water samples. Iran J Chem Chem Eng 33:59–66
Meeravali NN, Madhavi K, Kumar SJ (2013) Microwave assisted aqua regia extraction of thallium from sediment and coal fly ash samples and interference free determination by continuum source ETAAS after cloud point extraction. Talanta 104:180–186
Anthemidis AN, Ioannou KI (2012) Sequential injection ionic liquid dispersive liquid-liquid microextraction for thallium preconcentration and determination with flame atomic absorption spectrometry. Anal Bioanal Chem 404:685–691
Taher MA, Pourmohammad F, Fazelirad H (2015) Column preconcentration and electrothermal atomic absorption spectrometric determination of rhodium in some food and standard samples. J Sep Sci 38:4153–4158
Mashhadizadeh MH, Moatafavi A, Allah-Abadi H, Zadmehr MR (2004) Flame atomic absorption spectrometric determination of ultra traces of thallium(I) ion after solid phase extraction by Octadecyl silica membrane disk modified by a new Schiff Base. Bull Kor Chem Soc 25:1309–1313
Nasiri-Majd M, Taher MA, Fazelirad H (2015) Synthesis and application of nano-sized ionic imprinted polymer for the selective voltammetric determination of thallium. Talanta 144:204–209
Hassanien MM, Kenawy IM, Mostafa MR, El-Dellay H (2011) Extraction of gallium, indium and thallium from aquatic media using amino silica gel modified by gallic acid. Microchim Acta 172:137–145
Karatepe A, Soylak M, Elci L (2011) Selective preconcentration of thallium species as chloro and iodo complexes on Chromosorb 105 resin prior to electrothermal atomic absorption spectrometry. Talanta 85:1974–1979
Biaduń E, Sadowska M, Ospina-Alvarez N, Krasnodębska-Ostręga B (2016) Direct speciation analysis of thallium based on solid phase extraction and specific retention of a Tl(III) complex on alumina coated with sodium dodecyl sulfate. Microchim Acta 183:177–183
Nukatsuka I, Seitoh H, Ohzeki K (2004) Solid-phase extraction with slurry injection of the resin into ETAAS for trace determination of thallium in natural water. Microchim Acta 148:177–182
Taher MA, Daliri Z, Fazelirad H (2014) Simultaneous extraction and preconcentration of copper, silver and palladium with modified alumina and their determination by electrothermal atomic absorption spectrometry. Chin Chem Lett 25:649–654
Fazelirad H, Taher MA (2014) Preconcentration of ultra-trace amounts of iron and antimony using ion pair solid phase extraction with modified multi-walled carbon nanotubes. Microchim Acta 181:655–662
George L, Varghese A, Nizam A (2015) Determination of thallium in environmental samples by surfactant assisted dispersive liquid-liquid microextraction combined with first order derivative spectrophotometry. Eur J Chem 6:183–188
Amin AS, El-Sharjawy AM, Kassem MA (2013) Determination of thallium at ultra-trace levels in water and biological samples using solid phase spectrophotometry. Spectrochim Acta A 110:262–268
Moghimi A (2008) Solid phase extraction of thallium(III) on micro crystalline naphthalene modified with N, N′-Bis (3-methylsalicylidene)-ortho-phenylenediamine and determination by spectrophotometry. Chin J Chem 26:1831–1836
Nadiki HH, Taher MA, Ashkenani H (2013) Ionic liquid ultrasound assisted dispersive liquid–liquid/micro-volume back extraction procedure for preconcentration and determination of ultra trace amounts of thallium in water and biological samples. Int J Environ Anal Chem 93:623–636
Mortada WI, Kenawy IM, Hassanien MM (2015) A cloud point extraction procedure for gallium, indium and thallium determination in liquid crystal display and sediment samples. Anal Methods 7:2114–2120
Taher MA, Mohammadi SZ, Mahani FS (2005) FAAS determination of thallium after preconcentration using nitroso-S and TDBA onto benzophenone. Bull Chem Soc Ethiop 19:17–22
Dadfarnia S, Assadollahi T, Haji Shabani AM (2007) Speciation and determination of thallium by on-line microcolumn separation/preconcentration by flow injection-flame atomic absorption spectrometry using immobilized oxine as sorbent. J Hazard Mater 148:446–452
Darroudi A, Arbab Zavar MH, Chamsaz M, Zohuri G, Ashraf N (2012) Ion-imprinted polymer mini-column for on-line preconcentration of thallium(III) and its determination by flame atomic absorption spectrometry. Anal Methods 4:3798–3803
Taher MA (2003) Flame atomic absorption spectrometric determination of trace amounts of thallium after solid-liquid extraction and preconcentration with use of 1, 10-phenanthroline onto benzophenone. Bull Kor Chem Soc 24:1177–1180
Gil RA, Pacheco PH, Smichowski P, Olsina RA, Martinez LD (2009) Speciation analysis of thallium using electrothermal AAS following on-line pre-concentration in a microcolumn filled with multiwalled carbon nanotubes. Microchim Acta 167:187–193
Chamsaz M, Arbab-Zavar MH, Darroudi A, Salehi T (2009) Preconcentration of thallium(I) by single drop microextraction with electrothermal atomic absorption spectroscopy detection using dicyclohexano-18-crown-6 as extractant system. J Hazard Mater 167:597–601
Arbab-Zavar MH, Chamsaz M, Zohuri G, Darroudi A (2011) Synthesis and characterization of nano-pore thallium(III) ion-imprinted polymer as a new sorbent for separation and preconcentration of thallium. J Hazard Mater 185:38–43
Asadpour S, Chamsaz M, Entezari MH, Haron MJ, Ghows N (2012) On-line preconcentration of ultra-trace thallium(I) in water samples with titanium dioxide nanoparticles and determination by graphite furnace atomic absorption spectrometry. Arab J Chem. doi:10.1016/j.arabjc.2012.05.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 354 kb)
Rights and permissions
About this article
Cite this article
Ashrafzadeh Afshar, E., Taher, M.A. & Fazelirad, H. Ultra-trace determination of thallium(I) using a nanocomposite consisting of magnetite, halloysite nanotubes and dibenzo-18-crown-6 for preconcentration prior to its quantitation by ET-AAS. Microchim Acta 184, 791–797 (2017). https://doi.org/10.1007/s00604-016-2040-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2040-z