Abstract
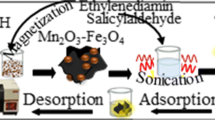
A magnetic nanocomposite (m-NC) was prepared from halloysite nanotubes and magnetite (Fe3O4) by chemical precipitation and used as an effective sorbent for preconcentration of trace quantities of cadmium(II). The m-NC was characterized by Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy and energy dispersive X-ray spectroscopy. Preconcentration is based on the adsorption of the cationic Cd(II)-1,10-phenanthroline complex on the negatively charged m-NC. Parameters that affect complex formation and subsequent adsorption and desorption were optimized. The preconcentration factor is 50. Cd(II) was then quantified by flame AAS. The calibration graph is linear in the 0.5 to 50 μg∙L‾1concentration range, and the detection limit is 0.27 μg∙L−1. The method was applied to the determination of traces of Cd(II) in spiked waters, nail and hair samples. Recoveries ranged from 96.7 to 104.2 %.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is one of the most toxic heavy metal elements which occurs in the earth’s crust and ocean water. It is introduced into the environment by natural phenomena like volcanic eruptions and forest fires and anthropogenic sources like manufacture and application of phosphate fertilizers and fossil fuel. Cadmium from polluted soil and water can accumulate in plants and organisms, thus entering the food supply. Eating food or drinking water with high cadmium levels severely irritates the stomach, leading to vomiting and diarrhea, and sometimes death. Uptake of lower levels of cadmium over a long period of time can lead to a build-up of cadmium in the kidneys that will damage the kidneys and also cause bones to become fragile and break easily. Cadmium levels in hair, nail, blood, urine, kidney and other tissues have been used as biological indicators of exposure to cadmium. The normal concentration of Cd in biological samples ranges from subnanograms per milliliter to a few nanograms per milliliter in biological fluid and up to micrograms-per-gram level in some tissues. For example the reference values reported for hair, nails and kidney are in the ranges of 0.025–8.73, 0.08–2.6 and 1.67–22 μg g−1, respectively [1]. EPA requires water suppliers to limit cadmium concentration in water to <5 μg L−1 [2]. Therefore, there is a permanent demand for reliable analytical methods for determination of ultra-traces of cadmium in natural and biological samples.
Flame atomic absorption spectrometry (FAAS) is the most commonly-employed technique for determination of trace elements due to its high selectivity, speed and low operational cost. However, direct determination of metal ions in complex matrices is limited due to their low concentrations in a complicated matrix, so enrichment and separation procedures are often required before determination by FAAS [3]. There are many methods for separation and preconcentration of metal ions, which among them, solid phase extraction (SPE) is commonly used, and is a very favorable technique. The choice of appropriate sorbent is a critical factor to obtain selective adsorption, good recovery and high enrichment factor in SPE procedure.
Recently, magnetic solid-phase extraction (MSPE) has attracted significant attentions due to the advantages of both nano-sized materials and magnetic separation techniques [4]. Common magnetic nano-sized materials include magnetite [5] (Fe3O4) and maghemite [6] (γ-Fe2O3). Incorporation of these particles with other functionalized materials such as multi-walled carbon nanotubes [7], zeolites [8] and activated carbon [9] leads to the effective sorbents for the removal of both organic and inorganic pollutants. The unique properties of magnetic nano-sized materials including extremely small size, high surface area-to-volume ratio and the absence of internal diffusion resistance, provide better kinetics for adsorption of metal ions from aqueous solution [10, 11]. In MSPE, the sorbents are dispersed into sample solutions to achieve extraction and are easily isolated from suspension by the application of an external magnetic field.
Halloysite nanotubes (HNTs), (Al2Si2O5(OH)4 .2H2O) a type of naturally occurring clay minerals with nanotubular structures, are increasingly becoming the focus of investigations [12]. The outer surface of the HNTs has properties similar to SiO2 while the inner cylinder core is related to Al2O3. HNTs like other clays can adsorb cationic species via ion exchange reactions and by formation of inner-sphere and outer-sphere complexes through Si-O− and Al-O− groups at the clay structure [13]. Magnetic HNT composites have been applied for removal of some dyes from aqueous solution [14, 15]. However, their application to preconcentration of metal ions has not been reported till now.
The present paper reports the efficient preconcentration of trace cadmium as cationic complex of 1,10-phenanthroline (phen) on magnetic HNTs. Various influencing factors on the preconcentration process were studied and the applicability of the method for determination of trace cadmium in water and food samples was examined.
Materials and methods
Reagents and solutions
All reagents were of analytical grade and doubly distilled de-ionized water (obtained from Ghazi Serum Co., Tabriz, Iran) was used for the preparation of all solutions. Working solution of cadmium was prepared by diluting of 1000 mg L−1 standard atomic absorption solution (Merck, Darmstadt, Germany; www.merck-chemicals.com). pH adjustments were performed with 0.1 M HNO3 and NaOH (Merck). A 0.3 % solution of phen was prepared by dissolving 0.3 g of 1,10-phenanthroline (Merck) in 2.0 mL methanol and diluting to 100 mL with water. Halloysite nanotubes were purchased from Sigma-Aldrich (St. Louis, USA; www. sigmaaldrich.com). FeCl3.6H2O and FeSO4 · 7H2O were prepared from Merck. A 1.0 M HNO3 solution was prepared by appropriate dilution of concentrated HNO3 (Merck).
Instruments
An Analytik Jena flame atomic absorption spectrometer model Nov. 400 (Jena, Germany; www.analytik-jena.de) furnished with an air–acetylene flame and a cadmium hollow cathode lamp, operated at 3.0 mA, was used for Cd determination. The instrument was set at a wavelength of 228.8 nm and slit width of 1.2 nm. A Metrohm model 654 was used for pH measurements. An ultrasonic bath (FALC, Italy; www.falcinstruments.it) was used for agitating the solutions in extraction processes (40 kHz, 100 W). The size and structure of HNT–Fe3O4 were characterized by scanning electron microscope (SEM) and energy dispersive X-ray spectroscopy (EDX) (Mira 3 FEG, Tescan Co., Czech Republic; www.tescan.com). Fourier transform infrared (FTIR) spectra were recorded by a Bruker Tensor-27 spectrometer (Germany; www.bruker.com) using KBR pellet. An X-ray diffractometer (Siemens D500, Germany; www.labx.com) equipped with Cu Kα (λ = 0.154 nm) was used to acquire the XRD patterns of powdered samples. The magnetic properties of nanoparticles were characterized by means of a vibrating sample magnetometer (Meghnatis Kavir Co., Kashan, Iran) at room temperature.
Preparation of HNT–Fe3O4
The HNT–Fe3O4 composite were synthesized according to the literature with some modifications [15]. A suspension of 0.5 g of HNT in a 200 mL of solution containing 0.582 g FeCl3 · 6H2O and 0.300 g FeSO4.7H2O refluxed at 70 °C for 1.5 h in an oil bath under N2 atmosphere. Then NH3 solution (25 mL, 4 mol L−1) was added dropwise to prepare iron oxide. The mixtures were aged at 70 °C for 1.5 h and then washed 3 times with distilled water. The obtained composites were dried at room temperature for 24 h.
MSPE procedure
An aliquot of 100 mL of aqueous sample or standard solution was transferred to a beaker and 3.0 mL of 0.3 % phen solution was added to form the cadmium chelate and the pH was adjusted to 6.0 with 1.0 mL phosphate buffer (0.2 M). Then, 0.05 g of magnetic HNTs was added to the solution and placed in an ultrasonic bath and sonicated for 5 min at 25 °C. Afterwards, a strong magnet (with strength of ~ 0.4 Tesla) was positioned at the bottom of the beaker and magnetic HNTs were isolated from the suspension (which takes about 7 min). The preconcentrated target analyte was desorbed with 2.0 mL of 0.1 M HNO3 under sonication for 1 min. The nanoparticles were isolated by the magnet in a very short time (less than 1 min) and the final solution was analyzed by FAAS.
Sample preparation
Water samples
Three water samples including tap water, spring water and well water were selected and the developed method was applied to determine their cadmium content. Tap water was collected from our laboratory (University of Tabriz, Tabriz, Iran) and spring and well water was collected from environs of Tabriz, Iran. The water samples were filtered through a Millipore 0.45 μm pore-size membrane into polyethylene bottles. Their pH values were adjusted to 6.0 by addition of phosphate buffer and analyzed according to the general procedure. Recovery experiments were also conducted by spiking the samples with appropriate amounts of cadmium, and determining their cadmium concentration by this method.
Biological samples
Human hair and fingernail samples were selected as typical biological samples and digested according to the given procedure [16]. The nail samples were scraped and the hair samples were cut into pieces so as to ensure feasible and fast digestion of the samples. Then, they were washed with nonionic detergent (Triton X-100) and soaked in deionized water for 10 min. It was followed by soaking in acetone to remove external contamination. Finally the samples were washed with deionized water and dried at 60 °C overnight in a drying oven.
The dried samples (0.2 g) were digested with 10 mL of 8:2 mixture of concentrated nitric acid and perchloric acid. The mixture was heated until complete evaporation to obtain a clear solution. Each digested sample was transferred into a 100 mL volumetric flask and made up to the mark with distilled water. Recovery tests were also performed by spiking the dried samples with a known amount of cadmium standard solution before sample digestion. After drying the samples in an oven at 50 °C, the digestion procedure was conducted and cadmium content was determined by the developed method.
Results and discussions
HNTs are two-layered aluminosilicates, chemically similar to kaolin, which have a predominantly hollow tubular structure in the submicron range. Chemically, the outer surface of this nanoclay has properties similar to tetrahedral SiO2 which is negatively charged above pH 2.4 while the inner region of the cylinder is related to octahedral Al2O3 which is positive below pH 8.5 [17]. Since the surface is mainly silica, its charge will be negative over a wide range of pH, as a result, halloysite tends to have a polyanionic surface, except at very low pH values, and should readily bind cationic complexes. 1,10- phenanthroline is a complexing agent which forms a cationic complex with cadmium (Cd(phen)3 2+) [18]. The reported stability constant (logβ) for this complex is 15.3 [19] and the pK a of phen is about 5.0 [19], so the complex formation can be complete at pH values higher than 5. This cationic complex can be efficiently adsorbed on negatively charged HNTs. We used magnetic HNTs instead of HNTs as sorbent. In MSPE, the sorbents are dispersed into sample solutions and so the equilibrium between the sorbents and sample solution can reach quickly which is beneficial to achieve high extraction efficiency in a short time. Moreover, after extraction step, magnetic sorbents enriched with analytes are easily isolated from suspension by the application of an external magnetic field without the need for column passing operations and additional centrifugation or filtration procedures.
Characterization of HNTs–Fe3O4
The morphology and particle size of original HNTs and HNTs–Fe3O4 are shown in Fig. 1. As can be seen from Fig. 1b the Fe3O4 nanoparticles were attached on the wall of HNTs. During SEM observation, we also determined the content of constituents by EDX (Fig. 1c, d). The amount of Fe element in the HNTs–Fe3O4 sample was obtained as 25 wt%.
Fig. 2a exhibits the FTIR spectra of Fe3O4, HNT and HNT–Fe3O4. In the FT-IR spectrum of HNTs, the peaks at 3696.78 and 3624.62 cm−1 were attributed to the stretching vibrations of inner-surface Al–OH. The bands at 3455.76 and 1641.36 cm−1 were assigned to O-H stretching vibration and O-H bending vibration of water, respectively. The bands at 1036.11, 794.76, 753.52 and 689.10 cm−1 were assigned to the stretching vibrations of Si-O. The peak at 911.42 cm−1 was attributed to the bending vibration of Al-OH. The bands observed at 536.99 and 468.90 cm−1 were attributed to the bending vibration of Al-O-Si and Si-O-Si, respectively [20]. The above mentioned peaks also appeared in FTIR of HNT–Fe3O4, in which the broad and intense band at 3424.98 cm−1 was due to stretching vibrations of hydroxyl groups from iron oxide. Finally the band related to Al-O-Si of HNT at 536 cm−1 and the Fe3O4 characteristic peak at around 575.54 cm−1 overlap in HNT–Fe3O4 [15].
The XRD patterns of the HNT and HNTs–Fe3O4 are shown in Fig. 2b. In the powder XRD patterns of HNT–Fe3O4, there are distinct peaks at 12.15°, 20.11° and 24.60° which can be indexed to halloysite nanotubes. Moreover, the new diffraction peaks at 30.29°, 35.62°, 43.26°, 57.87° and 62.67° can be identified as Fe3O4 which illustrates that magnetic Fe3O4 nanoparticles are successfully installed on the surface of HNTs.
Fig. 2c shows the magnetization of HNTs–Fe3O4 as a function of the applied magnetic field at 298 K. As seen, magnetization increased with an increase in the magnetic field. HNTs–Fe3O4 possessed good magnetic properties with the saturation magnetization of about 25.38 emu g−1 and exhibited an extremely small hysteresis loop and low coercivity, as a typical characteristic of superparamagnetic particles.
Optimization of MSPE procedure
The method was optimized in terms of pH value, amounts of adsorbent, loading with phen, sample volume, and extraction volume. The respective data are given in the Electronic Supporting Information. The optimized conditions are (1) a pH value of 6.0, (2) use of phosphate buffer; (3) a sonication time of 5 min, (4) use of 3 mL phen solution, (5) use of 100 mL sample volume and (6) elution with 2.0 mL HNO3 together with 60 s sonication. The sorbent can be reused after being regenerated with 10 mL distilled water and 10 mL phosphate buffer (pH = 6) respectively, and are stable up to five adsorption-elution cycles without a significant decrease in the recovery for cadmium.
Adsorption capacity
In order to determine the adsorption capacity of the magnetic HNTs, 10 mg of the sorbent was added to 25 mL of an aqueous solution containing 25 mg L−1 Cd(II), 3 mL of 0.3 % phen and phosphate buffer (pH = 6.0). After sonicating for 45 min and separation of the sorbent by applying an external magnetic field, the remained cadmium ions in the supernatant solution were determined by using FAAS. The adsorption capacity was found to be 11.4 ± 0.4 mg g−1 (n = 3).
Analytical figures of merit
The analytical performances of the developed methods were evaluated under the conditions already defined. While the linear range without preconcentration was 0.02–3.0 mg L−1, the calibration graph after preconcentration by using the developed MSPE was linear in the range of 0.5–50 μg L−1 with a correlation coefficient of 0.9995. The regression equation was y = 0.006× + 0.0082, where y is the absorbance and x is the concentration of Cd in μg L−1. The detection limit according to the definition of IUPAC (3Sb/b, where Sb is the standard deviation of blank and b is the slope of calibration graph [21]) was 0.27 μg L−1. Considering the volumes of sample and eluent, the maximum preconcentration factor for this method was 50. A study of precision was performed by carrying out five independent measurements of solutions of Cd(II) at 20.0 μg L−1 level and gave a relative standard deviation of 2.3 %.
The solid phase extraction of Cd(II) in presence of different amounts of potentially interfering ions was investigated and the results are shown in Table 1. An ion was considered to interfere when its presence produced a variation of more than 5 % in the absorbance of the sample. It is clear that none of the tested species interfere with the determination of Cd(II) in the biological and water samples.
Accuracy of the method was checked by analyzing a standard reference material (water sample NIST SRM 1643e) with a certified Cd content of 6.56 ± 0.073 μg L−1. The obtained value by using the developed method was 6.13 ± 0.33 μg L−1 (mean of three determinations ± standard deviation), which is in good agreement with the certified concentration. It can be concluded that the proposed method is accurate and free from systematic errors.
The characteristics of the developed MSPE procedure were compared with those of some other reported methods in Table 2. As can be seen, our method is comparable with most of other MSPE methods in terms of detection limit and linear range, though its adsorption capacity is lower. However, it should be mentioned that the synthesis procedure for our sorbent is very simpler and faster than the reported procedures for other sorbents, since almost all of those procedures are multi-step and need functionalization of magnetic Fe3O4, while our method is one-step without functionalization process. Finally, compared to conventional SPE, the MSPE procedure is much faster and simpler since there is no need for column preparation, centrifugation or filtration process.
Analytical applications
In order to confirm the applicability of the method, it was applied to the determination of cadmium in several water and biological samples. The suitability of the developed method was checked by spiking samples with a known amount of cadmium before sample digestion. The analytical results and the recoveries were given in Table 3. As can be seen in all cases, the cadmium recovery for the spiked samples is quantitative.
Conclusions
The magnetic nano-composite HNTs-Fe3O4, was successfully applied as an adsorbent for solid-phase extraction and preconcentration of Cd(II) as Cd-phen complex. The cationic complex of cadmium with 1,10-phenanthroline can be efficiently adsorbed on negatively charged HNTs. The main characteristics of this new sorbent is using of inexpensive and natural material, its magnetic feature which simplifies extraction process and re-usability and applicability for several types of samples. Moreover, its preparation procedure is quite simple and fast.
References
Tsalev D (1984) Atomic absorption spectrometry in occupational and environmental health practice. CRC Press, p 40
Water: basic information about regulated drinking water contaminants. http://water.epa.gov/drink/contaminants/basicinformation/cadmium.cfm#four.
Camel V (2003) Solid phase extraction of trace elements. Spectrochim Acta Part B At Spectrosc 58:1177–1233
Hao L, Wang C, Wu Q, Li Z, Zang X, Wang Z (2014) Metal–organic framework derived magnetic nanoporous carbon: novel adsorbent for magnetic solid-phase extraction. Anal Chem 86:12199–12205
Baghban N, Haji Shabani AM, Dadfarnia S (2012) Solid phase extraction of trace amounts of cadmium with cetyltrimethylammonium bromide-coated magnetic nanoparticles prior to its determination by flame atomic absorption spectrometry. J Chin Chem Soc 59:782–787
Giakisikli G, Anthemidis AN (2013) Automated magnetic sorbent extraction based on octadecylsilane functionalized maghemite magnetic particles in a sequential injection system coupled with electrothermal atomic absorption spectrometry for metal determination. Talanta 110:229–235
Daneshvar Tarigh G, Shemirani F (2013) Magnetic multi-wall carbon nanotube nanocomposite as an adsorbent for preconcentration and determination of lead (II) and manganese (II) in various matrices. Talanta 115:744–750
Oliveira LCA, Petkowicz DI, Smaniotto A, Pergher SBC (2004) Magnetic zeolites: a new adsorbent for removal of metallic contaminants from water. Water Res 38:3699–3704
Bystrzejewski M, Pyrzyńska K, Huczko A, Lange H (2009) Carbon-encapsulated magnetic nanoparticles as separable and mobile sorbents of heavy metal ions from aqueous solutions. Carbon 47:1201–1204
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D (2010) Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci 349:293–299
Girginova PI, Daniel-da-Silva AL, Lopes CB, Figueira P, Otero M, Amaral VS, Pereira E, Trindade T (2010) Silica coated magnetite particles for magnetic removal of Hg2+ from water. J Colloid Interface Sci 345:234–240
Kamble R, Ghag M, Gaikawad S, Panda BK (2012) Halloysite nanotubes and applications. J Adv Sci Res 3:25–29
Zhao Y, Abdullayev E, Vasiliev A, Lvov Y (2013) Halloysite nanotubule clay for efficient water purification. J Colloid Interface Sci 406:121–129
Duan J, Liu R, Chen T, Zhang B, Liu J (2012) Halloysite nanotube-Fe3O4 composite for removal of methyl violet from aqueous solutions. Desalination 293:46–52
Xie Y, Qian D, Wu D, Ma X (2011) Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chem Eng J 168:959–963
Abdulrahman FI, Akan JC, Chellube ZM, Waziri M (2012) Levels of heavy metals in human hair and nail samples from Maiduguri metropolis, Borno State, Nigeria. World Environ 2:81–89
Lvov YM, Shchukin DG, Möhwald H, Price RR (2008) Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2:814–820
Marczenko Z (1986) Separation and spectrophotometric determination of elements. E Horwood
Irving H, Mellor DH (1962) 1002 The stability of metal complexes of 1,10-phenanthroline and its analogues. Part I. 1,10-Phenanthroline and 2,2′-bipyridyl. J Chem Soc 5222
Luo P, Zhao Y, Zhang B, Liu J, Yang Y, Liu J (2010) Study on the adsorption of neutral red from aqueous solution onto halloysite nanotubes. Water Res 44:1489–1497
(1996) Guidance for industry: Q2B validation of analytical procedures: methodology. Rockville, MD
Taghizadeh M, Asgharinezhad AA, Pooladi M, Barzin M, Abbaszadeh A, Tadjarodi A (2013) A novel magnetic metal organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim Acta 180:1073–1084
Davudabadi Farahani M, Shemirani F (2012) Supported hydrophobic ionic liquid on magnetic nanoparticles as a new sorbent for separation and preconcentration of lead and cadmium in milk and water samples. Microchim Acta 179:219–226
Alvand M, Shemirani F (2014) Preconcentration of trace cadmium ion using magnetic graphene nanoparticles as an efficient adsorbent. Microchim Acta 181:181–188
Sohrabi MR, Matbouie Z, Asgharinezhad AA, Dehghani A (2013) Solid phase extraction of Cd(II) and Pb(II) using a magnetic metal-organic framework, and their determination by FAAS. Microchim Acta 180:589–597
Taghizadeh M, Asgharinezhad AA, Samkhaniany N, Tadjarodi A, Abbaszadeh A, Pooladi M (2014) Solid phase extraction of heavy metal ions based on a novel functionalized magnetic multi-walled carbon nanotube composite with the aid of experimental design methodology. Microchim Acta 181:597–605
Behzad SK, Balati A, Amini MM, Ghanbari M (2014) Triazine-modified magnetite nanoparticles as a novel sorbent for preconcentration of lead and cadmium ions. Microchim Acta 181:1781–1788
Sharma RK, Puri A, Monga Y, Adholeya A (2014) Newly modified silica-based magnetically driven nanoadsorbent: a sustainable and versatile platform for efficient and selective recovery of cadmium from water and fly-ash ameliorated soil. Sep Purif Technol 127:121–130
Ebrahimzadeh H, Kasaeian M, Khalilzadeh A, Moazzen E (2014) New magnetic polymeric nanoparticles for extraction of trace cadmium ions and the determination of cadmium content in diesel oil samples. Anal Methods 6:4617–4624
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 65 kb)
Rights and permissions
About this article
Cite this article
Amjadi, M., Samadi, A. & Manzoori, J.L. A composite prepared from halloysite nanotubes and magnetite (Fe3O4) as a new magnetic sorbent for the preconcentration of cadmium(II) prior to its determination by flame atomic absorption spectrometry. Microchim Acta 182, 1627–1633 (2015). https://doi.org/10.1007/s00604-015-1491-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1491-y