Abstract
Ion pair solid phase extraction was applied to the simultaneous preconcentration of iron and antimony. The ion pairs consisting of FeCl4 − or SbCl4 − anions and the benzyldimethyltetradecyl ammonium cation were formed on the surface of multi-walled carbon nanotubes, then eluted with nitric acid, and the elements finally quantified by ETAAS. The adsorption capacities of the impregnated MWCNTs are 9.2 mg g−1 for iron and 27.5 mg g−1 for antimony. The following analytical figures of merit were determined for iron and antimony, respectively: Enrichment factors of 210 and 230, assay precisions of ±5.3 % and ±4.8 %, linear calibration plots from 0.7 to 9.4 and 13.0 to 190 ng L−1, and detection limits of 0.17 and 3.5 ng L−1. The method was applied to the determination of iron and antimony in human hair, synthetic sample, and to the certified reference materials gold ore (MA-1b) and trace elements in water (SRM 1643d).

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron is the most important transition element involved in living system, being vital to both plants and animals. Its versatility is unique. It is at the active center of molecules responsible for oxygen transport and electron transport and is found in such diverse metalloenzyme as nitrogenase, various oxidases, hydrogenases, reductases, dehydrogenases, deoxygenases and dehydrases. Iron involved in enormous range of function and the whole gamut of life forms, from bacteria to man [1]. Total iron concentration should not exceed 5 mg L−1 in order to meet EU legislation [2].
Antimony is a toxic element which has received relatively little environmental attention, with only few studies in soils, sediments and biological materials. This is due to the fact that antimony is recognized as a non-essential element for life and also because its content in most environmental matrices is very low, implying the use of very sensitive analytical techniques for its determination [3]. The US Environmental Protection Agency (EPA) considers antimony and its compounds as priority pollutants and the Council of European Community established the maximum admissible concentration of antimony in surface and drinking water at 10 μg L−1. Its importance is due to a wide range of industrial applications, including use in glass making and ceramics, lead-antimony alloyed materials in batteries, ball bearings and ammunition, automobile brake linings as well as increased application to the development of microelectronics [4].
All these information emphasizes the importance of identifying and quantifying the amount of iron and antimony to provide comprehensive information about their properties and human health relevance. For this purpose, several analytical methods such as inductively coupled plasma-optical emission spectrometry, spectrophotometry, atomic fluorescence spectrometry, inductively coupled plasma-atomic emission spectrometry and inductively coupled plasma-mass spectrometry have been developed to measure iron and antimony in the clinical, environmental, industrial and pharmaceutical samples.
Antimony metal is a partially purified form of the element antimony. In modern commerce and industry, antimony typically contains of impurities, which include primarily iron, sulfur, zinc and arsenic. Moreover, there are some minerals such as; Pb4FeSb6S14, FeSb2S4, FeSb2O4 and etc. that have the iron and antimony simultaneously. So, simultaneous determination of iron and antimony is important. In present investigation, a new and green separation method based on ion pair formation-solid phase extraction (IP-SPE) combined with ETAAS was developed for the first time to the simultaneous preconcentration and determination of iron and antimony in several real samples. It was used the ion pairs consisting of FeCl4 − or SbCl4 − anions and the benzyldimethyltetradecyl ammonium (BTDA) cation were formed on the surface of multi-walled carbon nanotubes.
Experimental
Reagents and standards
All chemical reagents were of analytical-reagent grade and deionized water was used for preparation of the sample solutions. Multi-walled carbon nanotubes (MWCNTs) with 3–20 nm diameter, core diameter: 1–10 nm, SBET: 350 m2 g−1 and 95 % purity were purchased from Plasma Chem (www.plasmachem.com). The iron stock solution (100.0 mg L−1) was prepared by dissolving appropriate amount of Fe(NO3)3.9H2O (Sigma-Aldrich, www.sigmaaldrich.com) into a 100.0 mL volumetric flask and diluting to the mark with deionized water. A stock standard solution (100.0 mg L−1) of antimony was prepared by dissolving proper amount of SbCl3 (Merck, http://www.merck.com) in 10.0 mL of H2SO4 (98 %) and diluting to 100.0 mL with deionized water in a volumetric flask. Working standard solutions of iron and antimony were prepared freshly at various concentrations by diluting the stock solutions with deionized water. The ion pair forming agent (2 % w/v) solution was prepared by dissolving appropriate amount of benzyldimethyltetradecyl ammonium chloride dihydrate (Merck, http://www.merck.com) in deionized water. Acetone, methanol, ethanol, H2SO4, HF, HClO4, CH3COOH, HCl and HNO3 were purchased from Merck (http://www.merck.com). Deionized water was used throughout. The laboratory glassware was kept at 10 % HNO3 for 24 h and before using, was washed with deionized water and dried.
Instrumentation
The iron and antimony measurements were performed with a Varian Spectra AA 220 atomic absorption spectrometer (Australia, http://www.varianinc.com) with a deuterium lamp background correction, equipped with graphite furnace (GTA-110 series).
Preparation of oxidized and modified MWCNTs
Raw MWCNTs were heated at 300 °C for 40 min to remove amorphous carbon. Prior to use, MWCNTs were oxidized with concentrated HNO3 in order to create binding sites onto the surface of MWCNTs. The treatment was carried out by dispersing 30.0 mL of HNO3 (63 %) to 2.5 g of MWCNTs and then refluxing for 8 h. Subsequently, the oxidized MWCNTs were washed with deionized water until elimination of any excess HNO3 (neutral pH of eluent water). A total of 2.5 g of oxidized MWCNTs was suspended in 50.0 mL of BTDA solution (2 % w/v) and the mixture was stirred for 24 h. The solid was filtered and dried at 50 °C. These modified MWCNTs (MMWCNTs) were used as adsorbent for simultaneous preconcentration of iron and antimony ions.
Preparation of column
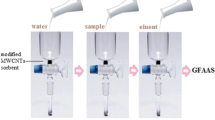
A total of 30.0 mg of MMWCNTs was slurred in water and then poured into a funnel-tipped glass tube (length; 80 mm, diameter; 5 mm). The column was conditioned with 2.0 mol L−1 of HCl. A piece of cotton was placed at the bottom of the column for allowing the adsorbent to settle properly.
Recommended procedure
For IP-SPE, a 50.0 mL standard solution containing 1.5 ng of Fe and 30.0 ng of Sb and 2.0 mL of HCl (10.0 mol L−1) was passed through the column with desired flow rate (3.0 mL min−1). Then, the formed ion pairs that settled on the column were desorbed with 1.5 mL of 2.0 mol L−1 HNO3 (flow rate; 4.0 mL min−1). Finally, 20 μL of eluate solution automatically injected by the autosampler into the graphite tube and then the absorbance of iron and antimony were measured under the operating conditions.
Samples preparation
Human hair
The human hair sample was immersed in acetone for 30 min, washed with water and then dried. 1.0 g of sample was weighed accurately, digested by 30.0 mL of a mixture solution of HClO4 and HNO3 (1:8 v/v) at low temperature. The digested solution was dried under elevated temperature and then, several drops of H2SO4 (1:1 v/v) were added to the residue [5]. After leaching with water, the residue was transferred to a 0.5 L measuring flask and diluted to the mark with deionized water.
For Fe, 0.5 mL of this solution was diluted to 500.0 mL and for Sb, no dilution is required. Then an aliquot of these solutions was selected and the experiment was carried out according to the procedure mentioned earlier.
Certified reference material
In order to confirm the validity of the developed procedure, this method has also been applied for the determination of the content of iron and antimony in Certified Reference Materials; (MA-1b reference gold ore) and (SRM 1643d-Trace Elements in Water).
-
MA-1b reference gold ore: A 100.0 mg sample was taken and dissolved completely in a mixture of HNO3, HCl and HF (2:4:1 volume ratio) with heating. The solution was cooled, diluted and filtered. The volume of the filtrated was raised to 1.0 L with deionized water in a volumetric flask. For Fe, 50.0 mL of this solution was diluted to 1.0 L and for Sb, no dilution is needed. Then an aliquot of these sample solutions was taken individually and the iron and antimony contents were determined by the recommended procedure.
-
SRM 1643d-Trace Elements in Water: A proper volume of solution (100.0 μL for iron and 1.0 mL for antimony) was poured into a 0.5 L measuring flask and diluted to the mark with deionized water. Then, an aliquot of these sample solutions was taken and the iron and antimony contents were determined by the suggested method.
Synthetic sample
Since no standard samples were accessible for simultaneous determination of iron and antimony with developed method (According to iron and antimony linear ranges), a synthetic mixture containing of different cations was prepared, aliquots of it were taken and the general procedure was applied.
Results and discussion
It was observed in the preliminary experiments that when MWCNTs are added to the solution of BTDA, this reagent is directly adsorbed and immobilized into the surface of multi-walled carbon nanotubes. BTDA is an ion pair forming agent which has been used for the extraction and determination of some metal ions [6]. The retention of the BTDA on the surface of MWCNTs was then confirmed by the FT-IR analysis. Comparing the FT-IR spectra of BTDA, bare MWCNTs and MMWCNTs revealed that MMWCNTs contains the peaks corresponding to the MWCNTs matrix and some of the bands of the BTDA (2852.20 and 2921.75 cm−1 of C–H stretching (chain), 3019.34 and 3041.45 cm−1 of C–H of aromatic ring, 655.67 and 778.21 cm−1 of monofunctionalized aromatic ring and 1559.28 of C=C stretching). Thus, it suggests that MWCNTs are successfully modified with the BTDA. Furthermore, it was confirmed that the MMWCNTs have the capability of sorption and desorption of iron and antimony from the aqueous solutions. So, a combination of IP-SPE with ETAAS was developed for simultaneous preconcentration and determination of ultra-trace amounts of iron and antimony ions from different matrices.
Effect of HCl concentration
Iron and antimony in hydrochloric acid solution form the chloro-complexes (FeCl4 − and SbCl4 −), which are extracted on modified adsorbent in solid phase extraction. So, HCl volume is an important parameter on the formation of the FeCl4 − and SbCl4 − complexes prior to the ion pair formation. Therefore, a low pH medium is necessary for the extraction of ion pairs. The results show that the extraction efficiency increased with increasing the HCl (10.0 mol L−1) volume up to 2.0 mL and remaining constant for more volumes. So further experiments were carried out with 2.0 mL of HCl 10.0 mol L−1.
Effect of type, concentration and volume of eluent
A variety of solvents were tested in order to elute the adsorbed ion pairs from the column. In order to choose the most effective eluent for quantitative recovery, acetone, methanol, ethanol, CH3COOH, H2SO4 and HNO3 were studied. The results showed that the extraction recovery of Fe and Sb ions were quantitative with ethanol, methanol and HNO3 (2.0, 4.0 and 5.0 mol L−1) as the eluting agent, but due to the high solubility of ion pair forming agent in methanol and ethanol and decreasing the reusability of the column, HNO3 (2.0 mol L−1) was selected as eluent.
The volume of HNO3 (2.0 mol L−1) as the eluting agent was varied in the range 0.5–5 mL. As depicted from Fig. 1, it was observed that maximum recovery of Fe and Sb was observed with 1.0 and 1.5 mL of HNO3 (2.0 mol L−1), respectively. So, 1.5 mL of HNO3 (2.0 mol L−1) was selected as optimum eluent volume.
Effect of HNO3 volume as eluent on the extraction efficiency of iron and antimony. Experimental conditions were the following: amount of Fe and Sb, 1.5 ng and 30.0 ng; HCl concentration; 0.4 mol L−1; sample volume 50.0 mL; sample solution flow rate; 3.0 mL min−1; eluent flow rate; 4.0 mL min−1, extraction temperature, room temperature
Effect of adsorbent type
A series of selected adsorbents, such as carbon nanotube, active carbon and Fullerene C60 were used in order to find the best adsorbent for the adsorption of iron and antimony from the sample solution. Between these adsorbent, carbon nanotube was found to give the best extraction efficiency (>96 % for both of Fe and Sb), whereas the active carbon (44 % & 59 %) and C60 (29 % & 13 %) did not show the quantitative extraction recovery for iron and antimony, respectively. Thus, in the present study, carbon nanotube was selected as adsorbent.
Effect of sample volume
The preconcentration capability of IP-SPE system was further considered by studying the effect of aqueous volume on the extraction recovery of iron and antimony from different sample volumes (50–500 mL). The results showed that the extraction was quantitative with the aqueous phase volume up to 320 mL of iron and 350 mL of antimony (Fig. 2). Based on the final extraction volume (1.5 mL) and the maximum sample volume that the extraction was quantitative (320 mL for Fe and 350 mL for Sb), a preconcentration factor of 213 for iron and 233 for antimony were determined.
Effect of sample volume on the extraction efficiency of iron and antimony. Experimental conditions were the same as in Fig. 1 except the sample volume. (Eluent volume, 1.5 mL)
Effect of sample and eluent flow rates
In a SPE system, the flow rate of sample solution and eluent through the column is a very important parameter for controlling the time of adsorption and analysis. To optimize the sample solution flow rate, a 50.0 mL solution of 1.5 ng of iron and 30.0 ng of antimony ions was passed through the column at flow rates in the range of 0.5–5 mL min−1. The results show that the quantitative recoveries for Fe and Sb were achieved in 3.0 and 3.5 mL min−1 of the sample solution, respectively. However, when the sample loading rate was higher than 3.0 mL min−1 for Fe and 3.5 mL min−1 for Sb, low recoveries of analytes were obtained. To decrease the analysis time for the experiments, a sample flow rate of 3.0 mL min−1 was selected for subsequent experiments.
After adsorption process, the column was then washed with eluent to remove metal ions. For this purpose, 1.5 mL of HNO3 (2.0 mol L−1) was passed to desorb the bounded Fe and Sb ions from MMWCNTs. The results demonstrate that the eluent flow rate variation in the ranges of 0.5–4.0 mL min−1 had no effect on the recovery of iron and antimony ions from the column. Therefore 4.0 mL min−1 of HNO3 (2.0 mol L−1) was selected and used for eluent flow rate.
Adsorption capacity
The adsorption capacity of MMWCNTs was determined by passing 30.0 mL of solution containing 1.0 mg of iron and antimony from 20.0 mg of sorbent. The amounts of Fe and Sb ions adsorbed on to the MMWCNTs were calculated by the difference between the initial and the final concentrations of analyte ions in the solution. The maximum adsorption capacity has been found to be 9.2 and 27.5 mg g−1 for iron and antimony, respectively. The difference between the adsorption capacities of two metal ions can be due to their size, degree of hydration, charge/radius ratio and electrostatic force magnitude between ion pairs.
In order to see the role of BTDA in the extraction process, the suggested method was done using unmodified MWCNTs. The maximum adsorption capacity of unmodified MWCNTs has been found to be 1.65 mg g−1 for Fe and 8.61 mg g−1 for Sb, which implies that the adsorption capacity of BTDA-MWCNTs is much higher than unmodified MWCNTs.
Reusability of modified adsorbent
In order to investigate the effectiveness of modified adsorbent, the BTDA-MWCNT was reused in IP-SPE. This adsorbent was recycled by washing with HNO3 and deionized water before the reuse. The experimental results indicate that the recoveries of analytes decrease only slightly when the adsorbent is reused nine times for iron and six times for antimony.
Effect of interfering ions
The interference of several anions and cations those are abundant in real samples and can influence the performance of the system was studied by using a solution containing 5.0 ng L−1 of Fe and 85.0 ng L−1 of Sb and adding various concentrations of the potential interferences. The tolerance level was defined as the maximum amount of potentially interfering species producing an error of ±5 % in Fe and Sb determination. The tolerance level of each potentially interfering ion was tested and if interference occurred, the ratio was reduced until it ceased. Table 1 shows the substances studied and their maximum amounts tolerable. As can be seen, several species did not interfere even at high concentrations, and this method is applicable to the analysis of Fe and Sb in different samples.
Performance characteristics
Under the optimum conditions, performance characteristics of the recommended IP-SPE method were obtained by processing standard solutions of iron and antimony. This method permits the determination of Fe and Sb over a concentration range of 0.7–9.4 ng L−1 (R 2 = 0.9983) and 13.0–190 ng L−1 (R 2 = 0.9985), respectively. The detection limit based on 3Sb/m (where Sb is the standard deviation of the blank signals and m is the slope of the calibration curve after extraction) and relative standard deviation (R.S.D.) for seven replicate measurements at 5.0 ng L−1 of Fe and 85.0 ng L−1 of Sb were found to be 0.17 ng L−1 and ±5.3 % for Fe and 3.5 ng L−1 and ±4.8 % for Sb, respectively. The enrichment factor, which was calculated based on the slopes of the calibration curves with or without the extraction, was 210 for iron and 230 for antimony. Also, the preconcentration factors are 213 and 233 for Fe and Sb, respectively.
Analytical applications
In order to establish the validity of the procedure, the suggested method was applied to the simultaneous extraction of iron and antimony in human hair. The reliability of the method was checked by the analysis of the samples spiked with the known amount of iron and antimony in human hair sample. The results presented in Table 2 reveal that recovery at 95 % confidence level is satisfactory.
To verify the accuracy of the method, this procedure was also applied to the determination of iron and antimony in two different certified reference alloys; MA-1b reference gold ore and SRM 1643d-Trace Elements in Water and a synthetic sample. The analytical results are given in Table 3. As can be seen, the obtained results are in good agreement with the reference values and no significant difference between the results and the accepted values. Thus, the procedure is reliable for analysis of a wide range of samples.
Comparison with other methods
Table 4 compares the characteristic data of the suggested sorbent with other sorbents for determination of iron and antimony which have been reported in the literature [7–24]. As can be seen from Table 4, for both elements, the recommended method possesses a wide linear dynamic range, high sampling volume, high preconcentration factor and high sensitivity. The recommended method has also the lowest detection limit except the results reported in the following literature [23, 24] and best enrichment factor for simultaneous extraction of iron and antimony ions. Moreover, because of non-toxicity of this method (without using of any organic and toxic solvent), it is proper to mention this method as a green and environmentally-friendly method.
Conclusion
In this study, a new method including Ion Pair formation-Solid Phase Extraction (IP-SPE) in combination with ETAAS was applied for the first time to the simultaneous preconcentration and determination of iron and antimony. It has been shown that iron and antimony ions form the negative complexes (FeCl4 − and SbCl4 −) in the presence of appropriate concentration of HCl and then, these complexes can be adsorbed on the surface of modified MWCNTs (BTDA-MWCNTs) via ion pair formation prior to determination by ETAAS. In Comparison with the liquid–liquid extraction, this method doesn’t use any organic and toxic solvent and the interfacial area between the sorbent and the aqueous phase is large.
Besides considerably high preconcentration ability, some other benefits of the system are simultaneous determination of two elements, enhancement of ETAAS sensitivity, environmentally-friendly property, its simplicity and speed of analysis.
References
Shakerian F, Dadfarnia S, Shabani AMH (2009) Separation, preconcentration and measurement of inorganic iron species by cloud point extraction and flow injection flame atomic absorption spectrometry. J Iran Chem Soc 6:594–601
Tašev K, Karadjova I, Arpadjan S, Cvetković J, Stafilov T (2006) Liquid/liquid extraction and column solid phase extraction procedures for iron species determination in wines. Food Control 17:484–488
Lepp NW, Edwards R, Jones KC (1995) Heavy metal in soil, 2nd ed. Blackie Academic and Professional, Glasgow
Toghill KE, Lu M, Compton RG (2011) Electroanalytical determination of antimony. Int J Electrochem Sci 6:3057–3076
Jahandari S, Taher MA, Fazelirad H, Sheikhshoai I (2013) Anodic stripping voltammetry of silver(I) using a carbon paste electrode modified with multi-walled carbon nanotubes. Microchim Acta 180:347–354
Fazelirad H, Taher MA (2013) Ligandless, ion pair-based and ultrasound assisted emulsification solidified floating organic drop microextraction for simultaneous preconcentration of ultra-trace amounts of gold and thallium and determination by GFAAS. Talanta 103:375–383
Yalcinkaya O, Kalfa OM, Turker AR (2010) Chelating agent free solid phase extraction (CAF-SPE) method for separation and/or preconcentration of iron(III) ions. Turk J Chem 34:207–217
Mirzajani R, Pourreza N, Kiasat A, Abdollahzadeh R (2013) Solid phase extraction and determination of Fe(III) in some vegetables and natural water samples using a new inorganic/organic hybrid adsorbent. Int J Environ Anal Chem. doi:10.1080/03067319.2013.823490
Pourreza N, Rastegarzadeh S, Kiasat AR, Yahyavi H (2012) Spectrophotometric determination of iron(II) after solid phase extraction of its 2,2′ bipyridine complex on silica gel-polyethylene glycol. J Spectrosc 2013:1–6
Khayatian G, Kolaie HRAV, Nasiri F, Atashkar B, Hassanpoor S (2010) Determination of total iron in environmental samples by solid phase extraction with dimethyl(E)-2-(2-methoxyphenoxy)-2-butenedioate. J Chin Chem Soc 57:118–123
Kassem MA, Amin AS (2013) Spectrophotometric determination of iron in environmental and food samples using solid phase extraction. Food Chem 141:1941–1946
Cui Y, Hu Z-J, Yang J-X, Gao H-W (2012) Novel phenyl-iminodiacetic acid grafted multiwalled carbon nanotubes for solid phase extraction of iron, copper and lead ions from aqueous medium. Microchim Acta 176:359–366
Shakerian F, Dadfarnia S, Shabani A, Rohani M (2008) MultiSimplex optimization of on-line sorbent proconcentration and determination of iron by FI-AAS and microcolumn of immobilized ferron. Talanta 77:551–555
Amin AS, Gouda AA (2008) Utility of solid-phase spectrophotometry for determination of dissolved iron(II) and iron(III) using 2,3-dichloro-6-(3-carboxy-2-hydroxy-1-naphthylazo)quinoxaline. Talanta 76:1241–1245
Zeng C, Yang F, Zhou N (2011) Hollow fiber supported liquid membrane extraction coupled with thermospray flame furnace atomic absorption spectrometry for the speciation of Sb(III) and Sb(V) in environmental and biological samples. Microchem J 98:307–311
Ozdemir N, Soylak M, Elci L, Dogan M (2004) Speciation analysis of inorganic Sb(III) and Sb(V) ions by using mini column filled with Amberlite XAD-8 resin. Anal Chim Acta 505:37–41
Zhang L, Morita Y, Sakuragawa A, Isozaki A (2007) Inorganic speciation of As(III, V), Se(IV, VI) and Sb(III, V) in natural water with GF-AAS using solid phase extraction technology. Talanta 72:723–729
Zhang L, Ishi D, Shitou K, Morita Y, Isozaki A (2005) Simultaneous multi-element analysis of total As, Se and Sb on titanium dioxide by slurry sampling-graphite furnace atomic absorption spectrometry. Talanta 68:336–342
Garbos S, Rzepecka M, Bulska E, Hulanicki A (1999) Microcolumn sorption of antimony(III) chelate for antimony speciation studies. Spectrochim Acta B 54:873–881
Menegário AA, Smichowski P, Tonello PS, Polla G, Oliveira EP, Santelli RE (2008) On-line redox speciation analysis of antimony using l-proline immobilized on controlled pore glass and hydride generation inductively coupled plasma optical emission spectrometry for detection. Anal Chim Acta 625:131–136
Rojas FS, Ojeda CB, Pavón JMC (2007) Preconcentration of inorganic antimony(III) in environmental samples by PSTH-Dowex microcolumn and determination by FI-ETAAS. Talanta 72:951–956
Menegário AA, Silva AJ, Pozzi E, Durrant SF, Abreu CH (2006) On-line determination of Sb(III) and total Sb using baker’s yeast immobilized on polyurethane foam and hydride generation inductively coupled plasma optical emission spectrometry. Spectrochim Acta B 61:1074–1079
Wu H, Wang X, Liu B, Liu Y, Li S, Lu J, Tian J, Zhao W, Yang Z (2011) Simultaneous speciation of inorganic arsenic and antimony in water samples by hydride generation-double channel atomic fluorescence spectrometry with on-line solid-phase extraction using single-walled carbon nanotubes micro-column. Spectrochim Acta B 66:74–80
Yu C, Cai Q, Guo Z-X, Yang Z, Khoo SB (2002) Antimony speciation by inductively coupled plasma mass spectrometry using solid phase extraction cartridges. Analyst 127:1380–1385
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fazelirad, H., Taher, M.A. Preconcentration of ultra-trace amounts of iron and antimony using ion pair solid phase extraction with modified multi-walled carbon nanotubes. Microchim Acta 181, 655–662 (2014). https://doi.org/10.1007/s00604-014-1169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1169-x