Abstract
Purpose
Anterior cruciate ligament (ACL) reconstruction is a common surgical procedure, yet failure still largely occurs due to nonanatomically positioned grafts. The purpose of this study was to retrospectively evaluate patients with torn ACLs before and after reconstruction via 3D MRI and thereby assess the accuracy of graft position on the femoral condyle.
Methods
Forty-one patients with unilateral ACL tears were recruited. Each patient underwent 3D MRI of both knees before and after surgery. The location of the reconstructed femoral footprint relative to the patient’s native footprint was compared.
Results
Native ACL anatomical location of the native ACL had a significant impact on graft position. Native ACLs that were previously more anterior yielded grafts that were more posterior (3.70 ± 1.22 mm, P = 0.00018), and native ACL that were previously more proximal yielded grafts that were more distal (3.25 ± 1.09 mm, P = 0.0042). Surgeons using an independent drilling method positioned 76.2% posteriorly relative to the native location, with a mean 0.1 ± 2.8 mm proximal (P = 0.8362) and 1.8 ± 3.0 mm posterior (P = 0.0165). Surgeons using a transtibial method positioned 75% proximal relative to the native location, with a mean 2.2 ± 3.0 mm proximal (P = 0.0042) and 0.2 ± 2.6 mm posterior (P = 0.8007). These two techniques showed a significant difference in magnitude in the distal–proximal axis (P = 0.0332).
Conclusion
The femoral footprint position differed between the native and reconstructed ACLs, suggesting that ACL reconstructions are not accurate. Rather, they are converging to a normative reference point that is neither anatomical nor isometric.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
The anterior cruciate ligament (ACL) is the most commonly injured ligament in the human body with over 175,000 reconstructive procedures performed annually in the USA [1, 2]. Only 82% of athletes return to their sport after surgery with a nonanatomically positioned graft being one of the most common causes of clinical failure after ACL reconstruction [3,4,5,6,7,8]. To improve the surgical outcome, extensive research has been performed to investigate the shape and the characteristics of ACL bundles [9,10,11,12,13]. In recent years, restoring patients’ native ACL anatomy by placing the graft within the femoral and tibial footprint has been emphasized [14].
While surgeons’ predilections may differ in terms of graft selection, surgical technique, and fixation method [11], there is a general consensus that the goal of surgery is an anatomical reconstruction of the ACL [5, 11, 15,16,17]. Restoring the patient’s native ACL anatomy has been shown to improve knee kinematics [18] and to improve anteroposterior and tibial rotational stability [5, 18]. Graft placement at the appropriate location can sometimes be challenging to the surgeon [19]. As ACL rupture often occurs at the femoral attachment site, identification of the optimal femoral graft position for recreating the footprint has been heavily scrutinized in the literature [5, 19,20,21]. Several techniques and anatomical landmarks are described, such as the lateral intercondylar ridge [9, 17, 22, 23], bifurcate ridge [9, 24], apex of the deep cartilage (ADC) [25], and clockface [13, 26], to guide surgeons in femoral tunnel placement. However, the anatomical ridges can sometimes be hard to distinguish, especially in cases with distorted anatomy. In addition, some femoral guides, like posterior offset guides, have been shown to be inaccurate [27,28,29] and unable to reach the center of the anatomical femoral footprint [24, 30]. Moreover, patient anatomy and morphology is more variable than previously assumed, rendering clinical application difficult [24, 31].
The objective of this study is to retrospectively compare the location of the femoral tunnel produced via two different surgical techniques to the native footprint using three-dimensional magnetic resonance imaging (3D MRI) obtained pre- and post-ACL reconstruction. We hypothesized that both surgical techniques would accurately and precisely re-approximate graft placement within the native femoral footprint.
Materials and methods
Study protocol
Institutional review board approval was obtained prior to the onset of this study. A study previously performed by Hart et al. [32] used a similar protocol and the same data but examined different outcomes. Patients between the ages of 16 and 60 years that were suspected of having an ACL tear were recruited into the study. Patients with previous knee pathology, including previous surgery, previous ligamentous injury, inflammatory arthropathy, or osteoarthritis, and patients with a suspected multi-ligamentous knee injury were excluded from the study. As part of the normal preoperative work-up, a conventional two-dimensional (2D) MRI was performed to confirm the diagnosis of an ACL tear. If the scan did not demonstrate evidence of a tear, the patient was excluded from the study. Afterward, a 3D MRI was performed on both the injured knee and the contralateral knee.
Following the preoperative diagnostic imaging, all patients underwent anatomical ACL reconstruction by one of four sports fellowship-trained orthopedic surgeons at our institution. The surgeons were blinded to the 3D MRIs but were allowed access to the conventional 2D MRIs for preoperative planning as it is standard procedure. The preferred surgical techniques are highlighted in Table 1. Surgeons 1 and 2 employed an independent drilling technique with flexible or rigid reamers, whereas surgeons 3 and 4 utilized a modified transtibial technique to perform anatomical ACL reconstruction. A quadrupled hamstring autograft with the same cortical button-type fixation (Endobutton; Smith & Nephew) on the femoral side and interference screw fixation on the tibial side (BIORCI; Smith & Nephew) was used by all four surgeons. The reconstructed knee underwent reimaging with 3D MRI at a minimum of 6 weeks post-surgery.
The imaging protocol for this study was a previously validated isotropic 3D MRI protocol [32,33,34]. Both 2D and 3D MRIs were performed using the same 1.5 T TwinSpeed Excite high-definition MRI scanner (GE Medical Systems). In the scanner, the knees were placed in near full extension, and an 8-channel high-definition surface coil was applied. For the 3D MRIs, an oblique-coronal proton density sequence along the plane of the ACL with slice gaps of 0.6 mm was obtained [32, 33]. The mean scanning times for all 3 image acquisitions (2D MRI of injured knee and 3D MRI of bilateral knees) was 45 min.
Femoral footprint analysis
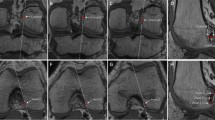
The use of 3D MRI allows for multiplanar reconstruction of the knee. Thus, it is possible to observe the ACL along its course in the coronal-oblique plan. Multiplanar reconstructions and measurements were performed on a PACS workstation with embedded multiplanar software (InteleViewer; Intelerad Medical Systems). Native ACL boundaries of the uninjured knee (the femoral footprint was not viewable in 28 of 41 injured knees) and the postoperative ACL graft were visualized with coronal-oblique and sagittal-oblique scans (Fig. 1).
Using the center of the footprints, pre- and postoperative coordinates were defined in relation to the apex of the deep cartilage and recorded on all 3D MRI scans. Measurement techniques did not differ in cases whereby the ACL remnant was preserved as measurements were made based on the footprint of the drilling hole. Remnants located outside the hole that could be seen on MRI were not considered in the footprint measurement. The primary outcome was to determine the accuracy and precision of the graft positions relative to the native ACL. The second outcome was comparison of other factors affecting the position of the graft, such as true anatomical location of the native ACL and the isometric point. We used the point defined by Zavras et al. [35] due to its ease of measurement in InteleViewer, its acceptance in the community, and its accuracy. Using a mid-sagittal MRI plane of the distal femur, the isometric point was determined to be three millimeters distal from the posterior aspect of Blumensaat’s line at the 10:30–11:00 o’clock position in right knees and 1:00–1:30 o’clock position in left knees.
Statistical analysis
Descriptive statistics, Student t test, and directional analysis of circular uniform distribution were used to quantify the position of the reconstructed femoral graft in relation to the native graft. Naming convention [9, 36, 37] and schematic diagram [37, 38] were consistent with the literature. A K-means algorithm on a statistical software (MATLAB, Mathworks) was used to investigate the effect of the native ACL on the position of the graft. For all statistical analysis, a P value of < 0.05 deemed statistically significant for comparative data.
Results
Forty-five total patients were recruited into the study from November 2014 to May 2016. Four patients were excluded from the study due to a normal ACL scan, significant motion artifact on the 3D MRI, and incomplete imaging. The 41 remaining patients had complete imaging and underwent ACL surgery performed by one of the four fellow trained surgeons at our institution. The mean age was 31 years, and 13 patients were females.
A scaled schematic diagram was used to evaluate the position of the reconstructed graft relative to the native ACL (Fig. 2). Surgeons using an independent drilling method positioned 76.2% posteriorly relative to the native location, with a mean 0.1 ± 2.8 mm proximal (P = 0.8362) and 1.8 ± 3.0 mm posterior (P = 0.0165). Surgeons using a transtibial method positioned 75% proximal relative to the native location, with a mean 2.2 ± 3.0 mm proximal (P = 0.0042) and 0.2 ± 2.6 mm posterior (P = 0.8007). These two techniques showed a significant difference in magnitude in the distal–proximal axis (P = 0.0332) but not in the anteroposterior axis (P = 0.0579). Overall, among the 41 participants, 5 had the graft positioned in the distal-anterior quadrant (Fig. 2), 11 in the proximal-anterior quadrant, 17 in the proximal-posterior quadrant, and 8 in the distal-posterior quadrant.
Scaled schematic diagram of reconstructed (squares) graft with relation to native (circle) ACL. The results are grouped by surgeon in which each patient is represented by a colored square. Surgeons 1 and 2 used an independent drilling technique, whereas surgeons 3 and 4 used a transtibial technique. The schematic diagram is a sagittal-plane anatomical drawing of the lateral wall of the femoral intercondylar notch with the Blumensaat line at an angle of 33° to the femoral anatomical axis and a posterior femoral condylar radius of curvature of 22.4 mm, in flexion
Evaluating the difference in orientation between the independent drilling and the transtibial technique showed a significant difference (P = 0.008) using a circular analog to the Kruskal–Wallis test. Assessing the accuracy from an orientation perspective showed that both independent drilling (P = 0.0395) and transtibial (P = 0.0129) techniques are not accurate as they do not respect a circular uniformity around the target point (Table 2, Figs. 3, 4). Only one surgeon was able to be precise using the independent drilling technique (k = 0.652, a Kurtosis close to one indicates a strongly peaked distribution) but was not accurate (P = 0.021).
Schematic representation of the accuracy and precision concept. Based on ISO 5725 definition, accuracy refers to the closeness of a measurement to the “true” value, whereas precision refers to how close the measurements are to each other. The four targets above describe the four different possible combination
Polar histogram of the circular distribution of the reconstructed ACL. The center point represents the native ACL. Zero degrees represent a full distal direction, 90 degrees a full anterior direction, 180 degrees a full proximal direction and 270 degrees posterior direction. The radial values in the graph signify the number of reconstructed ACLs within that range of angles. Here, both techniques were neither accurate, nor precise
To analyze the effect of the true anatomical location of the native ACL on the tunnel position created by the surgeons, an optimized k-means algorithm was used in MATLAB to generate clusters based on graft position relative to the native ACL. Based on the true anatomical location, the graft’s position was significantly different. More anterior native ACLs yielded more posterior grafts (3.70 ± 1.22 mm, P = 0.00018) (Table 3, Fig. 5). Moreover, more distal native ACLs produced more proximal grafts (4.22 ± 2.16 mm, P = 0.0013) while more proximal ACLs yielded more distal grafts (3.25 ± 1.09 mm, P = 0.0042). Conversely, native ACLs close to the typical anatomical location produced grafts at a similar location, with a P value of 0.3678 and 0.1966 for the distal and anterior axis, respectively.
Scaled schematic diagram representing a cluster analysis done using a k-means algorithm. Clusters are created randomly and optimized to form group of points sharing the same location. The position of the points is referenced from the apex of the deep cartilage. “typical location” is defined as the mean position
Additionally, the correlation between the graft position and the isometric point was evaluated. The position of the isometric points was significantly different than the graft position, with a P value of less than 0.0005 for all the surgeons (Fig. 6).
Discussion
While surgical techniques have improved drastically over the last few decades, anatomical graft positioning remains challenging [32, 39]. Surgeons using an independent drilling method positioned 76.2% posteriorly relative to the native location (P = 0.0165) compared to a transtibial method that positioned 75% of the grafts proximally relative to the native location (P = 0.0042). Our transtibial results are consistent with those reported by Scanlan et al. [39]. Although a mean of 1.8 ± 3.0 mm may appear small, placing a graft more posteriorly, such as in the posterolateral bundle, can create great tension forces and even graft failure [40, 41]. It is reported in the literature that independent drilling offers a more anatomical reconstruction compared to transtibial drilling but no significant difference clinically [30, 42, 43]. In our findings, both techniques differ significantly in magnitude in the distal–proximal axis (P = 0.0332) compared to the anterior–posterior axis (P = 0.0579), suggesting that the femoral tunnel position is partially technique dependent.
The 3D MRI protocol used in this study has been previously validated and applied with great success [32,33,34]. The same dataset of 41 patients was previously evaluated by Hart et al. [32] using a similar protocol. In their analysis, the position of the reconstructed graft was significantly different in magnitude than the native ACL position, which was both proximal (mean 1.2 ± 3 mm; P = 0.02) and posterior (mean 1.0 ± 2.9 mm; P = 0.01). These results suggested that the surgeons were not recreating the native ACL location with the graft. Yet, this analysis was solely based on magnitude of error and did not consider orientation. For our study, both concepts of accuracy and precision were investigated (Fig. 3). We showed that the position of the graft relative to the native ACL does not follow a uniform circular distribution (Table 2, Fig. 4). These results suggest that both the independent drilling and the transtibial methods are not accurate. However, one surgeon using independent drilling was precise with his technique, implying that precision is surgeon-dependent. This has great implication as Kato et al. [44] reported inferior kinematics in a porcine model when the femoral tunnel was not placed in the appropriate anatomical location. Despite technological advancement, it remains extremely challenging to replicate the native ACL with single bundle reconstruction, especially given the relatively wide nature of the femoral footprint. However, graft placement in proximity to the native anatomical footprint optimizes stability and functionality compared to nonanatomically positioned grafts [5, 18, 45].
A lot of literature has attempted to define the most isometric graft location to allow it to maintain its function throughout the range of motion [35, 46,47,48]. Multiple authors have described the location of the isometric point to be on the femur [48,49,50,51], with small differences between each point, as isometry is defined as a zone [46, 47]. In our study, the point defined by Zavras et al. [35] was chosen as the isometric point due to its ease of measurement in InteleViewer, its acceptance in the community, and its accuracy. Moreover, it represents the previous preferred location for ACL graft placement [50]. Interestingly, a graft placed in the center of the femoral footprint is less isometric than a tunnel in the more anterior region [40, 50, 52]. As reported by Hefzy et al. [50], the anterior position demonstrated minimal anisometry with 1–4 mm of length change throughout the range of motion. In comparison, a graft positioned in the center of the footprint would be expected to demonstrate a length change of 5–7 mm throughout the range of motion [40, 52]. However, the native ACL does not have a full isometric behavior in the last 30° of extension [53]. Yet, better rotational stability can be obtained by placing the graft within the anatomical femoral footprint versus an isometric femoral tunnel placed outside the femoral footprint [45]. Thus, placing the graft within the most isometric region within the anatomical footprint is more desirable for the patient than maximizing the isometric position alone [54]. This statement is consistent with our findings as no significant correlation was found between the graft location and the most isometric point (P < 0.0005), suggesting that the surgeons were effectively not placing the femoral tunnel in the isometric location.
With the desire to reproduce a more anatomical ACL [55], a renewed interest in ACL anatomy has shown that morphology, size, and location are quite variable [13, 31, 39, 56]. In a study of 137 patients undergoing ACL reconstruction, Kopf et al. [31] reported width variability in the footprint ranging from 12 to 22 mm. Both Scanlan et al. [39] and Edwards et al. [13] reported large morphological variation in the footprint between specimen. These findings are consistent with the results obtained from Hart et al. [32] on the same dataset as this study. Surgeons are positioning the graft at a location that is more comfortable for them (Fig. 5). When the native ACL feels too anterior, the surgeons positioned the graft more posterior (P = 0.00018). The same conclusion can be reached when the native ACL is too proximal (P = 0.0013) or distal (P = 0.0042). However, when the native ACL is close to the anatomical location defined by Hart et al. [25] (12 mm distal and 3 mm anterior), the graft was positioned at a similar location as the native ACL (P value of 0.3678 and 0.1966 for the distal and anterior axis, respectively). These results suggest that surgeons are not accounting for anatomical variations and therefore not performing patient-specific anatomical ACL construction [5]. Instead, they are correcting toward a normative population reference value, which is not the true anatomical location. To help surgeons perform a true patient-specific anatomical reconstruction, 3D MRI could be used as a preoperative tool to visualize the anatomy and be more precise and accurate.
The present study has shown the limitations of current surgical techniques to reproduce the native femoral footprint accurately and precisely. Nevertheless, it was limited by the fact that it is a radiographic study and that the clinical outcomes were not investigated. Moreover, this study utilized a centroid approach to define the location of the ACL within the footprint. As shown by Kopf et al. [31], there is a wide variation in the femoral ACL footprint, and using a centroid approach might not totally reflect the patient’s anatomy. Furthermore, our study used an isometric point defined in the literature by Zavras et al. [35]. While this point is widely accepted among the orthopedic community, it was originally described in cadaveric specimens. Hence, it may not be generalizable to living tissue. In addition, the low sample size of the study prevented the use of one-factor ANOVA for circular distribution between surgeons.
Conclusion
Despite modern surgical techniques, the femoral footprint position differed between the native and reconstructed ACLs, suggesting that ACL reconstructions are not accurate. Instead of reproducing true anatomy, ACL reconstructions performed today converge to a normative reference point that is nor anatomic, nor isometric. In the last 10 years, vast research has raised concerns over the body of the literature called anatomic. A new nomenclature might help differentiate these terms: isometric being the most isometric point, anatomical being a point that is not isometric and does change with respect to the patient anatomy, and true anatomical being patient-specific ACL location. Overall, true anatomical patient-specific reconstruction should be the goal moving forward.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gottlob CA, Baker JC, Pellissier JM, Colvin L (1999) Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res 367:272–282
Spindler KP, Wright RW (2008) Anterior cruciate ligament tear. N Engl J Med 359(20):2135–2142. https://doi.org/10.1056/NEJMcp0804745
Anderson AF, Snyder RB, Lipscomb AB (2001) Anterior cruciate ligament reconstruction: a prospective randomized study of three surgical methods. Am J Sports Med 29(3):272–279. https://doi.org/10.1177/03635465010290030201
Ardern CL, Webster KE, Taylor NF, Feller JA (2011) Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med 45(7):596–606. https://doi.org/10.1136/bjsm.2010.076364
Bedi A, Altchek DW (2009) The “footprint” anterior cruciate ligament technique: an anatomic approach to anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg 25(10):1128–1138. https://doi.org/10.1016/j.arthro.2009.03.008
Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR (2003) Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med 31(1):2–11. https://doi.org/10.1177/03635465030310011501
Aune AK, Holm I, Risberg MA, Jensen HK, Steen H (2001) Four-strand hamstring tendon autograft compared with patellar tendon-bone autograft for anterior cruciate ligament reconstruction: a randomized study with two-year follow-up. Am J Sports Med 29(6):722–728. https://doi.org/10.1177/03635465010290060901
Barrett GR, Noojin FK, Hartzog CW, Nash CR (2002) Reconstruction of the anterior cruciate ligament in females. Arthrosc J f Arthrosc Relat Surg 18(1):46–54. https://doi.org/10.1053/jars.2002.25974
Ferretti M, Ekdahl M, Shen W, Fu FH (2007) Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthrosc J Arthrosc Relat Surg 23(11):1218–1225. https://doi.org/10.1016/j.arthro.2007.09.008
Farrow LD, Chen MR, Cooperman DR, Victoroff BN, Goodfellow DB (2007) Morphology of the femoral intercondylar notch. JBJS 89(10):2150–2155. https://doi.org/10.2106/JBJS.F.01191
Anderson MJ, Browning WM III, Urband CE, Kluczynski MA, Bisson LJ (2016) A systematic summary of systematic reviews on the topic of the anterior cruciate ligament. Orthop J Sports Med 4(3):2325967116634074. https://doi.org/10.1177/2325967116634074
Edwards A, Bull AM, Amis AA (2007) The attachments of the anteromedial and posterolateral fibre bundles of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 15(12):1414–1421. https://doi.org/10.1007/s00167-007-0417-6
Edwards A, Bull AM, Amis AA (2008) The attachments of the anteromedial and posterolateral fibre bundles of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 16(1):29–36. https://doi.org/10.1007/s00167-007-0410-0
van Eck CF, Kopf S, Irrgang JJ, Blankevoort L, Bhandari M, Fu FH, Poolman RW (2012) Single-bundle versus double-bundle reconstruction for anterior cruciate ligament rupture: a meta-analysis—does anatomy matter? Arthrosc J Arthrosc Relat Surg 28(3):405–424. https://doi.org/10.1016/j.arthro.2011.11.021
Cain JE, Clancy JW (2002) Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am 33(4):717–725. https://doi.org/10.1016/s0030-5898(02)00026-3
Fu FH, van Eck CF, Tashman S, Irrgang JJ, Moreland MS (2015) Anatomic anterior cruciate ligament reconstruction: a changing paradigm. Knee Surg Sports Traumatol Arthrosc 23(3):640–648. https://doi.org/10.1007/s00167-014-3209-9
Fu FH, Jordan SS (2007) The lateral intercondylar ridge—a key to anatomic anterior cruciate ligament reconstruction. JBJS. https://doi.org/10.2106/JBJS.G.00851
Sadoghi P, Kröpfl A, Jansson V, Müller PE, Pietschmann MF, Fischmeister MF (2011) Impact of tibial and femoral tunnel position on clinical results after anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg 27(3):355–364. https://doi.org/10.1016/j.arthro.2010.08.015
Lo IK, de Maat GH, Valk JW, Frank CB (1999) The gross morphology of torn human anterior cruciate ligaments in unstable knees. Arthrosc J Arthrosc Relat Surg 15(3):301–306. https://doi.org/10.1016/s0749-8063(99)70039-3
Marchant BG, Noyes FR, Barber-Westin SD, Fleckenstein C (2010) Prevalence of nonanatomical graft placement in a series of failed anterior cruciate ligament reconstructions. Am J Sports Med 38(10):1987–1996. https://doi.org/10.1177/0363546510372797
Morgan JA, Dahm D, Levy B, Stuart MJ, Group MS (2012) Femoral tunnel malposition in ACL revision reconstruction. J Knee Surg 25(5):361 https://doi.org/10.1055/s-0031-1299662
Xerogeanes JW, Hammond KE, Todd DC (2012) Anatomic landmarks utilized for physeal-sparing, anatomic anterior cruciate ligament reconstruction: an MRI-based study. JBJS 94(3):268–276. https://doi.org/10.2106/JBJS.J.01813
Hutchinson MR, Ash SA (2003) Resident’s ridge: assessing the cortical thickness of the lateral wall and roof of the intercondylar notch. Arthroscopy 19(9):931–935. https://doi.org/10.1016/j.arthro.2003.09.002
Kopf S, Musahl V, Tashman S, Szczodry M, Shen W, Fu FH (2009) A systematic review of the femoral origin and tibial insertion morphology of the ACL. Knee Surg Sports Traumatol Arthrosc 17(3):213–219. https://doi.org/10.1007/s00167-008-0709-5
Hart A, Han Y, Martineau PA (2015) The apex of the deep cartilage: a landmark and new technique to help identify femoral tunnel placement in anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg 31(9):1777–1783. https://doi.org/10.1016/j.arthro.2015.03.026
Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SLY (2003) Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. Arthrosc J Arthrosc Relat Surg 19(3):297–304. https://doi.org/10.1053/jars.2003.50084
Ducsharm M, Banaszek D, Hesse D, Kunz M, Reifel C, Bardana D (2014) Assessing the accuracy of femoral tunnel placement in anatomic ACL reconstruction (913.13). FASEB J 28(1_supplement):913–1013. https://doi.org/10.1096/fasebj.28.1_supplement.913.13
Han Y, Hart A, Martineau PA (2014) Is the clock face an accurate, precise, and reliable measuring tool for anterior cruciate ligament reconstruction? Arthrosc J Arthrosc Relat Surg 30(7):849–855. https://doi.org/10.1016/j.arthro.2014.03.007
Fu FH (2008) The clock-face reference: simple but nonanatomic. Arthrosc J Arthrosc Relat surg 24(12):1433. https://doi.org/10.1016/j.arthro.2008.09.003
Kaseta MK, DeFrate LE, Charnock BL, Sullivan RT, Garrett WE (2008) Reconstruction technique affects femoral tunnel placement in ACL reconstruction. Clin Orthop Relat Res 466(6):1467–1474. https://doi.org/10.1007/s11999-008-0238-z
Kopf S, Pombo MW, Szczodry M, Irrgang JJ, Fu FH (2011) Size variability of the human anterior cruciate ligament insertion sites. Am J Sports Med 39(1):108–113. https://doi.org/10.1177/0363546510377399
Hart A, Sivakumaran T, Burman M, Powell T, Martineau PA (2018) A prospective evaluation of femoral tunnel placement for anatomic anterior cruciate ligament reconstruction using 3-dimensional magnetic resonance imaging. Am J Sports Med 46(1):192–199. https://doi.org/10.1177/0363546517730577
Han Y, Kurzencwyg D, Hart A, Powell T, Martineau PA (2012) Measuring the anterior cruciate ligament’s footprints by three-dimensional magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc 20(5):986–995. https://doi.org/10.1007/s00167-011-1690-y
Kijowski R, Davis KW, Woods MA, Lindstrom MJ, De Smet AA, Gold GE, Busse RF (2009) Knee joint: comprehensive assessment with 3D isotropic resolution fast spin-echo MR imaging—diagnostic performance compared with that of conventional MR imaging at 3.0 T. Radiology 252(2):486–495. https://doi.org/10.1148/radiol.2523090028
Zavras TD, Race A, Amis AA (2005) The effect of femoral attachment location on anterior cruciate ligament reconstruction: graft tension patterns and restoration of normal anterior–posterior laxity patterns. Knee Surg Sports Traumatol Arthrosc 13(2):92–100. https://doi.org/10.1007/s00167-004-0541-5
Giron F, Cuomo P, Aglietti P, Bull AM, Amis AA (2006) Femoral attachment of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 14(3):250–256. https://doi.org/10.1007/s00167-005-0685-y
Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH (2012) Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy 28(6):872–881. https://doi.org/10.1016/j.arthro.2011.11.026
Siebold R, Axe J, Irrgang JJ, Li K, Tashman S, Fu FH (2010) A computerized analysis of femoral condyle radii in ACL intact and contralateral ACL reconstructed knees using 3D CT. Knee Surg Sports Traumatol Arthrosc 18(1):26–31. https://doi.org/10.1007/s00167-009-0969-8
Scanlan SF, Lai J, Donahue JP, Andriacchi TP (2012) Variations in the three-dimensional location and orientation of the ACL in healthy subjects relative to patients after transtibial ACL reconstruction. J Orthop Res 30(6):910–918. https://doi.org/10.1002/jor.22011
Lubowitz JH (2014) Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc 22(5):1190–1195. https://doi.org/10.1007/s00167-013-2694-6
Markolf KL, Park S, Jackson SR, McAllister DR (2009) Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. JBJS 91(1):107–118. https://doi.org/10.2106/JBJS.G.01215
Riboh JC, Hasselblad V, Godin JA, Mather RC III (2013) Transtibial versus independent drilling techniques for anterior cruciate ligament reconstruction: a systematic review, meta-analysis, and meta-regression. Am J Sports Med 41(11):2693–2702. https://doi.org/10.1177/0363546513506979
Dhawan A, Gallo RA, Lynch SA (2016) Anatomic tunnel placement in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg 24(7):443–454. https://doi.org/10.5435/JAAOS-D-14-00465
Kato Y, Ingham SJ, Kramer S, Smolinski P, Saito A, Fu FH (2010) Effect of tunnel position for anatomic single-bundle ACL reconstruction on knee biomechanics in a porcine model. Knee Surg Sports Traumatol Arthrosc 18(1):2–10. https://doi.org/10.1007/s00167-009-0916-8
Musahl V, Plakseychuk A, VanScyoc A, Sasaki T, Debski RE, Mcmahon PJ, Fu FH (2005) Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med 33(5):712–718. https://doi.org/10.1177/0363546504271747
Dabirrahmani D, Hogg MC, Walker P, Biggs D, Gillies RM (2013) Comparison of isometric and anatomical graft placement in synthetic ACL reconstructions: a pilot study. Comput Biol Med 43(12):2287–2296. https://doi.org/10.1016/j.compbiomed.2013.10.008
Kernkamp W, Varady N, Li J, Tsai T, Asnis P, Nelissen R, Gill T, de Velde VS, Li G (2018) An in vivo prediction of anisometry and strain in anterior cruciate ligament reconstruction-a combined magnetic resonance and dual fluoroscopic imaging analysis. Arthrosc J Arthrosc Relat Surg. https://doi.org/10.1016/j.arthro.2017.10.042
Zavras T, Race A, Bull A, Amis A (2001) A comparative study of’isometric’points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc 9(1):28–33. https://doi.org/10.1007/s001670000170
Friederich N, O’Brien W (1992) Functional anatomy of the cruciate ligaments. In: The knee and the cruciate ligaments. Springer, pp 78–91
Hefzy MS, Grood ES, Noyes FR (1989) Factors affecting the region of most isometric femoral attachments: part II: the anterior cruciate ligament. Am J Sports Med 17(2):208–216. https://doi.org/10.1177/036354658901700210
Sidles JA, Larson RV, Garbini JL, Downey DJ, Matsen FA (1988) Ligament length relationships in the moving knee. J Orthop Res 6(4):593–610. https://doi.org/10.1002/jor.1100060418
Pearle AD, Shannon FJ, Granchi C, Wickiewicz TL, Warren RF (2008) Comparison of 3-dimensional obliquity and anisometric characteristics of anterior cruciate ligament graft positions using surgical navigation. Am J Sports Med 36(8):1534–1541. https://doi.org/10.1177/0363546508315536
Markolf KL, Burchfield DM, Shapiro MM, Davis BR, Finerman GA, Slauterbeck JL (1996) Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft: part I insertion of the graft and anterior-posterior testing. JBJS 78(11):1720–1727. https://doi.org/10.2106/00004623-199611000-00013
Pearle A, McAllister D, Howell S (2015) Rationale for strategic graft placement in anterior cruciate ligament reconstruction: IDEAL femoral tunnel position. Am J Orthop 44(6):253–258
Yagi M, Wong EK, Kanamori A, Debski RE, Fu FH, Woo SL-Y (2002) Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med 30(5):660–666. https://doi.org/10.1177/03635465020300050501
Lu W, Zhu W, Peng L, Fen W, Li H, Ou Y, Liu H, Wang D, Zeng Y (2015) Femoral footprint variation of the posterolateral bundle of the anterior cruciate ligament and double-bundle reconstruction. Knee 22(3):169–173. https://doi.org/10.1016/j.knee.2014.10.009
Acknowledgements
The authors thank Janet Faith who was the research coordinator, Lyne Santello for coordinating MRI, and Dr Eric Lenczner, Dr Mark Burman and Dr Robert Marien for participating in this study.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
AH, PAM, and MB contributed to study conception and design; AH contributed to data acquisition; CL contributed to analysis and data interpretation; and CL, MT, and JL contributed to manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent data
The McGill Institutional Review Board approved the study, and consent was obtained by the research investigator.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Legler, J., Laverdiere, C., Boily, M. et al. Evaluating femoral graft placement using three-dimensional magnetic resonance imaging in the reconstruction of the anterior cruciate ligament via independent or transtibial drilling techniques: a retrospective cohort study. Eur J Orthop Surg Traumatol 34, 1297–1306 (2024). https://doi.org/10.1007/s00590-023-03788-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-023-03788-4