Abstract
Grafts placed too anteriorly on the femur are reportedly a common cause of failure in anterior cruciate ligament reconstruction. Some studies suggest more anatomic femoral tunnel placement improves kinematics. The ability of the transtibial technique and a tibial tunnel-independent technique (placed transfemorally outside-in) to place the guide pin near the center of the femoral attachment of the anterior cruciate ligament was compared in 12 cadavers. After arthroscopic placement of the guide pins, the femur was dissected and the three-dimensional geometry of the femur, anterior cruciate ligament footprint, and positions of each guide pin were measured. The transtibial guide-pin placement was 7.9 ± 2.2 mm from the center of the footprint (near its anterior border), whereas the independent technique positioned the guide pin 1.9 ± 1.0 mm from the center. The center of the footprint was within 2 mm of an anteroposterior line through the most posterior border of the femoral cartilage in the notch and a proximodistal line through the proximal margin of the cartilage at the capsular reflection. More accurate placement of the femoral tunnel might reduce the incidence of graft failure and might reduce long-term degeneration observed after reconstruction although both would require clinical confirmation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although many patient followup studies report good outcomes in the short-term after anterior cruciate ligament (ACL) reconstruction, some problems remain. Long-term studies have reported a high incidence of joint degeneration (as much as 52% to 56% 12 to 13 years after surgery) [36, 42] and an estimated 8% to 10% of ACL reconstructions result in recurrent instability and graft failure [18, 23, 51]. Furthermore, studies have suggested that the ability of current reconstruction techniques to prevent degenerative changes compared with nonoperative treatment is limited [11, 13–15, 36]. Although many mechanisms likely contribute to this degeneration (for example, traumatic injury to other soft tissues at the time of ACL rupture), numerous authors suggest the inability of the reconstruction to restore normal joint kinematics is an important factor [17, 34, 39, 50, 53, 54].
Many authors believe improper femoral tunnel placement is a common reason for failure of an ACL reconstruction [3, 19, 21, 25, 29, 37, 46, 54]. Frequently, the grafts are placed too far anteriorly on the femur, resulting in a vertically oriented graft [5, 9, 16, 25, 29, 56]. Several in vitro studies suggest a graft placed too far anteriorly on the femur results in excessive tension in the graft in flexion, which could lead to graft failure [8, 56]. Furthermore, a vertically oriented graft does not reproduce the oblique orientation of the ACL, which could limit the ability of the reconstruction to restore the abnormal kinematics observed after ACL deficiency [9, 30, 44]. Grafts placed near the center of the ACL attachment site on the femur more closely restore normal knee translation and rotation than a more vertically oriented graft [30, 35, 44].
The transtibial technique, in which the femoral tunnel is drilled through the tibial tunnel, is commonly used in ACL reconstruction [5, 19, 29, 46]. However, several studies have suggested that the transtibial technique might not be able to center the graft near the anatomic center of the ACL, owing to constraints imposed by the tibial tunnel [5, 9, 29]. Techniques where the femoral tunnel is placed independently of the tibial tunnel might allow for better placement of the femoral tunnel. Therefore, the objective of this study was to assess the ability of different reconstruction techniques to place the femoral guide pin near the center of the ACL attachment on the femur in a cadaver model. Two different techniques of guide-pin placement were compared: a single-incision transtibial technique in which the femoral guide pin was placed through a tibial tunnel (transtibial technique) and a two-incision technique [54] in which the femoral guide pin was placed transfemorally from the outside, independently of the tibial tunnel (independent technique, Fig. 1).
In the independent technique, a two-incision technique was used to place the femoral guide pin transfemorally from the outside-in, independently of the tibial tunnel. The target of the independent guide was placed at the center of the anterior cruciate ligament (ACL), as judged visually by the surgeon.
We hypothesized the independent technique would allow for closer placement of the guide pin to the center of the ACL compared with the transtibial technique. In addition, because the independent technique used in this study relies on visually identifying the center of the ACL stump, our secondary objective was to quantify the location and shape of the ACL on the femur, so anatomic landmarks could be identified for placing the femoral tunnel in cases in which the remnant of the ACL is missing.
Materials and Methods
This study used a repeated-measures design in which the guide-pin placement of the independent technique and the transtibial technique were compared in the same cadaveric knee. We used 12 fresh-frozen cadaver knees (six left, six right; mean age, 66 ± 17 years) in this study. Knees with excessive osteoarthritis, obvious cartilage defects, or history of trauma or surgery were excluded from the study. The cruciate ligaments of all of the specimens were intact during arthroscopic inspection.
An a priori power analysis was performed to determine the appropriate number of specimens required to detect a difference in the distance from the center of the ACL to each of the guide pins. We assumed that a difference in guide-pin placement less than 2 mm would not be clinically meaningful, and assumed a standard deviation of 2 mm in the difference between the tunnel placements. With a repeated measures design and α = 0.05, at least 10 specimens were needed to provide 80% power.
We performed arthroscopy using standard anteromedial and anterolateral portals. The ACL was identified and transected, leaving the femoral and tibial attachments intact. We performed minimal notchplasty only when necessary to observe the entire undisturbed ACL femoral footprint. A 30° arthroscope was placed in both portals to provide a detailed view of the ACL footprint, aiding in better estimation of the size and shape of the footprint.
Next, we performed the independent technique using a 90°-femoral guide (Fig. 1) (RetroDrill; Arthrex, Naples, FL) [54]. With the knee at 90° flexion, the femoral guide was inserted through the anteromedial portal and positioned as closely as possible to the center of the anatomic footprint of the entire ACL femoral origin as assessed visually by the surgeon. A small stab incision on the lateral thigh just anterior to the lateral epicondyle and through the iliotibial band allowed insertion of the guide-pin sleeve to the bone. Then we advanced a guide pin inside the joint until it reached the 90°-femoral guide at the center of the ACL stump. The guide pin was withdrawn until the inner end was no longer visible inside the joint and the retrograde system was removed. This system used no hooks to constrain the position of the target point of the guide.
Next, we used a transtibial reconstruction guide (Arthrex) to place the femoral tunnel site. A PCL Oriented Placement Marking Hook (Arthrex) was attached to the Adapter C-Ring (placed in 50°) [6] and inserted through the anteromedial portal with the knee in 90° flexion. We placed the 7-mm tip extension of the marking hook against the base of the posterior cruciate ligament. The guide was angled to position the guide sleeve 1 cm above the pes anserinus and 2 cm medial of the tibial tubercle [38] to obtain sufficient tunnel length for the graft and form an approximately 65° angle [28, 45] with the tibial plateau in the coronal plane. The guide pin exited through the marking hook 7 mm from the posterior cruciate ligament. We removed the guide sleeve, marking hook, and guide and then confirmed correct pin positions. An 8-mm-diameter full-thickness cannulated drill was used to create the tibial tunnel. We used a 7-mm offset guide to identify the posterior wall of the lateral femoral condyle. The guide pin was inserted through the offset guide into a flexed knee as close as possible to the center of the ACL and advanced with the drill to exit the anterolateral femoral cortex. We left the independent and the transtibial pins in their respective femoral tunnels.
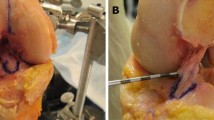
Then the femur and tibia were dissected of all soft tissues, except for cartilage and the attachment site of the ACL. The tibial tunnel was visually inspected to ensure that it was placed appropriately. We withdrew the guide pins from the femur completely and sutures were passed through each hole to identify the two tunnels. The specimen was then rigidly fixed in a clamp (Fig. 2), and we recorded the bony and cartilage geometry using a three-dimensional (3-D) digitizing stylus with an accuracy of 0.1 mm (Microscribe; Immersion Corp, San Jose, CA). Evenly spaced points on the bony and cartilage surfaces were recorded in 3-D in solid modeling software (Rhinoceros; McNeel and Associates, Seattle, WA). We also carefully recorded the border of the cartilage and bone. This cloud of points then was used to generate a 3-D surface model of each knee (Fig. 2).
A 3-D digitizing stylus (top) was used to generate 3-D models of the femur, including the cartilage border and ACL attachment site (bottom). In this specimen, the independent technique placed the guide pin near the centroid of the ACL, whereas the transtibial technique resulted in placement anterior and proximal to the center of the ACL.
We then used the stylus to reproduce the attachment site of the ACL. The outside border of the ligament attachment site was measured first. Next, we measured evenly spaced points across the surface of the ACL. A surface was fit to these points to define the attachment site of the ACL. Next, the geometric center of the entire ACL attachment site was calculated in 3-D using the solid modeling software. We then recorded points around the circumference of the hole formed by the guide pin using the stylus. The geometric centroid then was calculated and was used to define the location of the guide pin in 3-D space (Fig. 2).
These 3-D models were used to establish anatomic coordinate systems to measure the position of the guide pins relative to the center of the ACL. We determined the proximodistal direction from a cylinder fit to the long axis of the femoral shaft. The transverse plane was defined as a plane perpendicular to the proximodistal direction. The anterior direction then was drawn perpendicular to a line tangent to both femoral condyles in the transverse plane. We calculated the distance between each tunnel site and the intact ACL using this coordinate system.
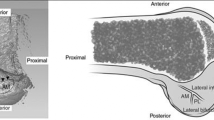
Next, we determined the average position and shape of the ACL attachment. The position of the center of the ACL was determined relative to an anatomic landmark that we defined as the intersection of the most posterior point on the cartilage bone border and the most proximal point on the cartilage visible in the notch (Fig. 3). We determined the average shape of the ACL in the sagittal plane by aligning the center of the footprint of each ACL. Radial lines intersecting at the center of the ACL footprint were drawn in 6° increments. We averaged the distance from the center of the ACL to the intersection of the radial lines with the border of each ACL to form an average ACL border (Fig. 3).
Position of an anatomic reference point relative to the center of the ACL was measured. The anatomic landmark was defined as the intersection of the most posterior point on the cartilage-bone border and the most proximal point on the cartilage visible in the notch. In addition, the shape and position of the ACL was measured in the sagittal plane (mean ± standard deviation).
All data were checked for normality using the Shapiro-Wilk test. The 3-D distances from the location of the guide pins placed using the transtibial and independent techniques to the center of the ACL attachment site were compared using a paired t-test. In addition, a paired t-test was used to compare the components of the distance between the guide pins in the anteroposterior and proximodistal directions.
Results
The independent technique allowed for placement of the guide pin closer (p = 0.00001) to the center of the ACL compared with the transtibial technique (Fig. 4). The transtibial technique placed the guide pin 7.9 ± 2.2 mm (mean ± standard deviation) from the center of the ACL, whereas the independent technique was 1.9 ± 1.0 mm from the center of the ACL. The transtibial technique resulted in more anterior (p = 0.006) and proximal (p = 0.01) placement of the guide pin compared with the transfemoral technique (Fig. 5). The guide pin placed using the transtibial technique was anterior to the center of the ACL by 5.1 ± 1.7 mm, whereas the transfemoral technique resulted in a position that was anterior by 0.3 ± 1.4 mm. The guide pin placed using the transtibial technique was 3.6 ± 3.9 mm proximal to the center of the ACL, whereas the transfemoral technique was 1.0 ± 1.1 mm distal to the center of the ACL.
The transtibial technique placed the guide pin anterior (p = 0.006) and proximal (p = 0.01) to the independent technique. The anatomic reference point, defined as the position of the intersection of an anteroposterior line through the most posterior border of the femoral cartilage and a proximodistal line through the proximal margin of the cartilage at the capsular reflection, was within 2 mm of the center of the ACL.
On average, the center of the ACL was within 2 mm from the anatomic reference point. This reference point was 1.8 ± 0.9 mm posterior and 0.2 ± 1.4 mm distal to the center of the ACL in the sagittal plane (Fig. 5). The average shape of the ACL in the sagittal plane was not elliptical (Fig. 3). Instead, it widened in the anteroposterior direction, moving proximally to distally. Its widest dimension in the anteroposterior direction was an average of 11.6 mm and 16.8 mm in the proximodistal direction. The ACL was close to the cartilage border with synovial lining between the fibers of the ACL and the cartilage border. The border of the ACL was 2.6 ± 0.7 mm from the posterior border of the cartilage in the anteroposterior direction and 4.5 ± 1.0 mm from the most proximal point on the cartilage visible from the notch. The center of the ACL was 8.6 ± 0.8 mm and 11.7 ± 1.2 mm from these two points, respectively.
Discussion
Inaccurate femoral tunnel placement is a common cause of failure in ACL reconstruction [3, 18, 19, 21, 25, 29, 46] with the graft often being placed too far anteriorly. An anteriorly oriented graft does not restore the oblique orientation of the ACL and has limited ability to restore normal knee stability [30, 44]. Recent cadaver studies have suggested a graft placed near the center of the ACL more closely restores knee kinematics than an anteriorly placed graft [35, 44]. Therefore, anatomic graft placement is likely important given the inability of current reconstruction techniques to restore normal kinematics and is believed an important factor contributing to the long-term development of osteoarthritis after ACL reconstruction [17, 39, 48, 50]. We compared the ability of the transtibial technique and a tibial tunnel independent technique to anatomically place the femoral tunnel near the center of the ACL footprint and hypothesized the independent technique would allow for closer placement of the guide pin to the center of the ACL compared with the transtibial technique. Because the independent technique relies on visually identifying the center of the ACL stump, we quantified the location and shape of the ACL on the femur, so anatomic landmarks could be identified for placing the femoral tunnel in cases in which the remnant of the ACL is missing.
This study has some limitations. One limitation was that it was performed in older cadavers, with transtibial and independent guide-pin placement performed in the same knee. However, every effort was made to make the surgery as realistic as possible. Surgery was performed using a full arthroscopic surgical setup, using the same approaches as used clinically. After placing the independent guide pin, it was withdrawn until no longer visible, so that its placement did not bias placement of the transtibial guide pin. Therefore, we do not believe that our conclusions regarding tunnel placement would change during clinical ACL surgery. We also did not divide the ACL into functional bundles [20]. From the stump of the ACL, it was difficult to distinguish two or three distinct functional bundles. Instead, the ACL appeared to be a continuous structure consisting of many different fiber bundles of different lengths and orientations. Although we did not separate the ACL stump into two or three bundles, the data generated from this study are important, as anatomic femoral tunnel placement has been a concern in single- and double-bundle ACL reconstructions [16, 55], and whether a single- or double-bundle reconstruction should be performed remains controversial [1, 10, 26, 41, 43, 47, 49, 52] and is outside the scope of this study. Finally, we do not know how much difference in placement will substantially alter the risk of either graft failure or long-term degeneration; both would require clinical confirmation.
Although we aimed for placement of the guide pin in the middle of the attachment site [7, 16, 54], optimal graft placement for restoring normal motion under physiologic loading conditions is unknown. Cadaver studies suggest that placing the graft closer to the center of the attachment site might improve knee kinematics compared with an anterior graft placement. For example, several cadaver studies reported placement of the graft in a more oblique or lateral position improves rotatory knee stability [35, 44, 52]. One of these studies reported that under rotary loads, an oblique, anatomically placed single-bundle graft reproduced intact knee kinematics, whereas a more vertically oriented graft did not [44]. Both grafts reproduced anterior laxity under anterior tibial loads [44]. In another study under combined rotary loads, no differences in tibial rotation and anterior translation were detected between a double-bundle reconstruction and a laterally placed single-bundle reconstruction [52]. Under anterior tibial loads, no differences in anterior laxity were detected below 60° [52], a range of flexion where the quadriceps load the ACL [27, 33]. Although more patient studies are needed to evaluate the effects of ACL reconstruction on kinematics under physiologic loading conditions, these cadaver studies suggest that more anatomic placement of the femoral tunnel might help to more closely restore normal knee motion. This is likely important because abnormal knee kinematics generally are believed an import factor in the degenerative changes observed after ACL injury [17, 34, 39, 50, 52, 54].
Difficulties placing the femoral tunnel in the ACL footprint using single-incision transtibial tunnel techniques have been reported [5, 9, 29]. Arnold et al. [5] reported the anatomic attachment site of the ACL could not be reached with a femoral aiming guide through a standard tibial tunnel. The closest position that could be reached was at the margin of the anatomic attachment site, deep and high in the notch [5]. In the current study, we also had difficulty placing the guide pin near the center of the ACL attachment using the transtibial technique in every specimen. The transtibial tunnel was drilled at 65° in the coronal plane [28, 45] to enable placement of the guide pin lower on the wall of the notch. Using the offset guide in the over-the-top position allowed the femoral guide pin to be positioned at a desired distance from the posterior cortex but did not define the femoral tunnel location completely. We rotated the guide to place the tunnel lower on the lateral wall of the notch. However, on average, guide pins placed using the transtibial technique were within 1 mm of the anterior border of the ACL. The limited ability of the transtibial technique to place the guide pin in the footprint is likely the result of the constraints of the tibial tunnel and also partly attributable to constraints imposed by the guide hooks used.
Tunnels placed too far anteriorly on the femur reportedly have poor clinical outcomes [2, 21, 25, 44, 54]. Anteriorly placed grafts may lead to notch impingement or graft stretching [25, 37, 56]. Several biomechanical studies show tunnels placed at the 11 o’clock position restore anterior tibial stability under anterior tibial loads but fail to control rotational stability [35, 44]. In addition, a graft placed too far anteriorly could result in a vertically oriented graft [5, 9, 16, 25, 29, 56]. A vertically oriented graft is unlikely to restore the oblique orientation of the ACL [9, 32, 44, 54] and might provide inadequate restraint to the increased internal rotation [4, 12, 17] and medial tibial translation [12, 31] observed in patients with ACL deficiency. Altered kinematics after ACL injury are thought to predispose the knee to degenerative changes [4, 17, 39, 40, 50].
The independent, two-incision technique allowed for placement of the guide pin within 2 mm of the center of the ACL footprint. The center of the ACL was assessed visually by the surgeon and was placed independently of a tibial tunnel. This technique has the advantage that the tunnel can be centered in the ACL footprint, low on the wall of the notch, without the constraint of a guide through the tibial tunnel. Although we used outside-in placement through the lateral femur in this study, other approaches, including that from a medial anterior portal, front entry through an anterior portal, or rear entry through a posterior approach, can be used to achieve independent placement. All of these aim for a position chosen by the surgeon to optimize tunnel location with respect to ACL anatomy.
Although the independent technique could place the guide pin near the center of the ACL footprint with a visible remnant, it may be difficult to observe the ACL remnant in some patients (eg, patients with chronic injuries or in the case of revision ACL surgery) [22]. Previous studies have used radiographic techniques to reference the location of the ACL [7, 21, 46]. In the current study, we found the ACL attachment site was centered near the intersection of an anteroposterior line passing through the most posterior point on the cartilage-bone border and a proximodistal line passing the most proximal corner of the cartilage visible in the notch (Fig. 3). These landmarks are visible during standard arthroscopy, are independent of knee flexion angle, and potentially allow for placement of the guide pin within 2 mm of the ACL. Others have used a clock-face analogy to describe placement of the graft arthroscopically [9, 52, 56]. Despite its widespread use, the clock-face description might be difficult to reproduce because of the 3-D geometry of the ACL and variability associated with fitting a circular clock face to the noncircular intercondylar notch [54].
Anatomic studies have indicated a high variability in the shape of the ACL attachments [22]. Previous studies describe the femoral ACL footprint as an oval [47], semicircular [20], rounded triangular [5], or a more circular [24] shape. Accounting for the 3-D geometry of the ACL footprint, as performed in our study, might provide a more realistic description of the ACL because of its attachment on the curved surface of the intercondylar notch. We observed shape qualitatively more similar to that depicted by Harner et al. [24]. The ACL footprint was not strictly elliptical; instead, it widened in the anteroposterior direction moving proximally to distally.
With an intact ACL footprint, the ACL femoral guide pin can be placed within 2 mm of the center of the ACL using the independent technique. The transtibial technique was less consistent, with the guide pin being placed too far anteriorly, approximately 7 mm from the center of the ACL attachment site. If there is no ACL stump, the femoral guide pin can be placed to within 2 mm of the geometric center of the ACL using the intersection of a vertical line through the most posterior edge of the cartilage border and a horizontal line through the most proximal point of the cartilage border in the notch. More anatomic placement of the femoral tunnel might help reduce the incidence of graft failure and might prevent the long-term degenerative changes observed after ACL reconstruction, although these would require clinical confirmation.
References
Adachi N, Ochi M, Uchio Y, Iwasa J, Kuriwaka M, Ito Y. Reconstruction of the anterior cruciate ligament: single- versus double-bundle multistranded hamstring tendons. J Bone Joint Surg Br. 2004;86:515–520.
Aglietti P, Buzzi R, Giron F, Simeone AJ, Zaccherotti G. Arthroscopic-assisted anterior cruciate ligament reconstruction with the central third patellar tendon: a 5-8-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1997;5:138–144.
Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;6 Suppl 1:S2–12.
Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44.
Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9:194–199.
Arthrex. Transtibial ACL reconstruction with soft tissue grafts surgical technique. Available at: www.arthrex.com. Accessed August 18, 2007.
Bernard M, Hertel P, Hornung H, Cierpinski T. Femoral insertion of the ACL: radiographic quadrant method. Am J Knee Surg. 1997;10:14–21; discussion 21–22.
Bylski-Austrow DI, Grood ES, Hefzy MS, Holden JP, Butler DL. Anterior cruciate ligament replacements: a mechanical study of femoral attachment location, flexion angle at tensioning, and initial tension. J Orthop Res. 1990;8:522–531.
Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33:717–725.
Crawford C, Nyland J, Landes S, Jackson R, Chang HC, Nawab A, Caborn DN. Anatomic double bundle ACL reconstruction: a literature review. Knee Surg Sports Traumatol Arthrosc. 2007;15:946–964; discussion 945.
Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: a prospective outcome study. Am J Sports Med. 1994;22:632–644.
DeFrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34:1240–1246.
Fink C, Hoser C, Hackl W, Navarro RA, Benedetto KP. Long-term outcome of operative or nonoperative treatment of anterior cruciate ligament rupture: is sports activity a determining variable? Int J Sports Med. 2001;22:304–309.
Fithian DC, Paxton EW, Stone ML, Luetzow WF, Csintalan RP, Phelan D, Daniel DM. Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament-injured knee. Am J Sports Med. 2005;33:335–346.
Fithian DC, Paxton LW, Goltz DH. Fate of the anterior cruciate ligament-injured knee. Orthop Clin North Am. 2002;33:621–636, v.
Garofalo R, Mouhsine E, Chambat P, Siegrist O. Anatomic anterior cruciate ligament reconstruction: the two-incision technique. Knee Surg Sports Traumatol Arthrosc. 2006;14:510–516.
Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75–79.
Getelman MH, Friedman MJ. Revision anterior cruciate ligament reconstruction surgery. J Am Acad Orthop Surg. 1999;7:189–198.
Gill TJ, Steadman JR. Anterior cruciate ligament reconstruction the two-incision technique. Orthop Clin North Am. 2002;33:727–735, vii.
Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint: anatomical, functional and experimental analysis. Clin Orthop Relat Res. 1975;106:216–231.
Giron F, Buzzi R, Aglietti P. Femoral tunnel position in anterior cruciate ligament reconstruction using three techniques: a cadaver study. Arthroscopy. 1999;15:750–756.
Giron F, Cuomo P, Aglietti P, Bull AM, Amis AA. Femoral attachment of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14:250–256.
Grossman MG, ElAttrache NS, Shields CL, Glousman RE. Revision anterior cruciate ligament reconstruction: three- to nine-year follow-up. Arthroscopy. 2005;21:418–423.
Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15:741–749.
Harner CD, Giffin JR, Dunteman RC, Annunziata CC, Friedman MJ. Evaluation and treatment of recurrent instability after anterior cruciate ligament reconstruction. Instr Course Lect. 2001;50:463–474.
Harner CD, Poehling GG. Double bundle or double trouble? Arthroscopy. 2004;20:1013–1014.
Hirokawa S, Solomonow M, Lu Y, Lou ZP, D’Ambrosia R. Anterior-posterior and rotational displacement of the tibia elicited by quadriceps contraction. Am J Sports Med. 1992;20:299–306.
Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29:567–574.
Kohn D, Busche T, Carls J. Drill hole position in endoscopic anterior cruciate ligament reconstruction: results of an advanced arthroscopy course. Knee Surg Sports Traumatol Arthrosc. 1998;6 Suppl 1:S13–15.
Lee MC, Seong SC, Lee S, Chang CB, Park YK, Jo H, Kim CH. Vertical femoral tunnel placement results in rotational knee laxity after anterior cruciate ligament reconstruction. Arthroscopy. 2007;23:771–778.
Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826–1834.
Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. Comparison of the ACL and ACL graft forces before and after ACL reconstruction: an in-vitro robotic investigation. Acta Orthop. 2006;77:267–274.
Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SL. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32:395–400.
Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32:984–992.
Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19:297–304.
Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152.
Moisala AS, Jarvela T, Harilainen A, Sandelin J, Kannus P, Jarvinen M. The effect of graft placement on the clinical outcome of the anterior cruciate ligament reconstruction: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2007;15:879–887.
Morgan CD, Kalman VR, Grawl DM. Definitive landmarks for reproducible tibial tunnel placement in anterior cruciate ligament reconstruction. Arthroscopy. 1995;11:275–288.
Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34:2006–2012.
Ristanis S, Giakas G, Papageorgiou CD, Moraiti T, Stergiou N, Georgoulis AD. The effects of anterior cruciate ligament reconstruction on tibial rotation during pivoting after descending stairs. Knee Surg Sports Traumatol Arthrosc. 2003;11:360–365.
Rue JP, Ghodadra N, Bach BR. Jr. Femoral tunnel placement in single-bundle anterior cruciate ligament reconstruction: a cadaveric study relating transtibial lateralized femoral tunnel position to the anteromedial and posterolateral bundle femoral origins of the anterior cruciate ligament. Am J Sports Med. 2008;36:73–79.
Salmon LJ, Russell VJ, Refshauge K, Kader D, Connolly C, Linklater J, Pinczewski LA. Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft: minimum 13-year review. Am J Sports Med. 2006;34:721–732.
Sasaki SU, da Mota e Albuquerque RF, Pereira CA, Gouveia GS, Vilela JC, Alcaras Fde L. An in vitro biomechanical comparison of anterior cruciate ligament reconstruction: single bundle versus anatomical double bundle techniques. Clinics. 2008;63:71–76.
Scopp JM, Jasper LE, Belkoff SM, Moorman CT 3rd. The effect of oblique femoral tunnel placement on rotational constraint of the knee reconstructed using patellar tendon autografts. Arthroscopy. 2004;20:294–299.
Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am. 2003;85:1018–1029.
Sommer C, Friederich NF, Muller W. Improperly placed anterior cruciate ligament grafts: correlation between radiological parameters and clinical results. Knee Surg Sports Traumatol Arthrosc. 2000;8:207–213.
Steiner ME, Murray MM, Rodeo SA. Strategies to improve anterior cruciate ligament healing and graft placement. Am J Sports Med. 2008;36:176–189.
Stergiou N, Ristanis S, Moraiti C, Georgoulis AD. Tibial rotation in anterior cruciate ligament (ACL)-deficient and ACL-reconstructed knees: a theoretical proposition for the development of osteoarthritis. Sports Med. 2007;37:601–613.
Streich NA, Friedrich K, Gotterbarm T, Schmitt H. Reconstruction of the ACL with a semitendinosus tendon graft: a prospective randomized single blinded comparison of double-bundle versus single-bundle technique in male athletes. Knee Surg Sports Traumatol Arthrosc. 2008;16:232–238.
Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983.
Wolf RS, Lemak LJ. Revision anterior cruciate ligament reconstruction surgery. J South Orthop Assoc. 2002;11:25–32.
Yamamoto Y, Hsu WH, Woo SL, Van Scyoc AH, Takakura Y, Debski RE. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32:1825–1832.
Yoo JD, Papannagari R, Park SE, DeFrate LE, Gill TJ, Li G. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med. 2005;33:240–246.
Yu J, Garrett WE. Femoral tunnel placement in anterior cruciate ligament reconstruction. Oper Tech Sports Med. 2006;14:45–49.
Zantop T, Diermann N, Schumacher T, Schanz S, Fu FH, Petersen W. Anatomical and nonanatomical double-bundle anterior cruciate ligament reconstruction: importance of femoral tunnel location on knee kinematics. Am J Sports Med. 2008; Feb 22 [Epub ahead of print].
Zavras TD, Race A, Amis AA. The effect of femoral attachment location on anterior cruciate ligament reconstruction: graft tension patterns and restoration of normal anterior-posterior laxity patterns. Knee Surg Sports Traumatol Arthrosc. 2005;13:92–100.
Acknowledgments
We gratefully acknowledge the advice of Dean C. Taylor, MD throughout this project. We thank Richard R. Glisson and Clinton A. Leiweke for technical assistance. Arthrex kindly provided the cadavers and surgical equipment used in this study. The financial support of the Division of Orthopaedics and the Sports Medicine Center is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
The institution of the authors has received funding from Arthrex.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
About this article
Cite this article
Kaseta, M.K., DeFrate, L.E., Charnock, B.L. et al. Reconstruction Technique Affects Femoral Tunnel Placement in ACL Reconstruction. Clin Orthop Relat Res 466, 1467–1474 (2008). https://doi.org/10.1007/s11999-008-0238-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-008-0238-z